Mechanistic studies of Ni-catalyzed electrochemical homo-coupling reactions of aryl halides†
Abstract
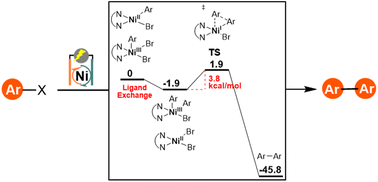
Ni-catalyzed electrochemical arylation is an attractive, emerging approach for molecular construction as it uses air-stable Ni catalysts and efficiently proceeds at room temperature. However, the homo-coupling of aryl halide substrates is one of the major side reactions. Herein, extensive experimental and computational studies were conducted to examine the mechanism of Ni-catalyzed electrochemical homo-coupling of aryl halides. The results indicate that an unstable NiII(Ar)Br intermediate formed through oxidative addition of the cathodically generated NiI species with aryl bromide and a consecutive chemical reduction step. For electron-rich aryl halides, homo-coupling reaction efficiency is limited by the oxidative addition step, which can be improved by negatively shifting the redox potential of the Ni-catalyst. DFT computational studies suggest a NiIII(Ar)Br2/NiII(Ar)Br ligand exchange pathway for the formation of a high-valent NiIII(Ar)2Br intermediate for reductive elimination and production of the biaryl product. This work reveals the reaction mechanism of Ni-catalyzed electrochemical homo-coupling of aryl halides, which may provide valuable information for developing cross-coupling reactions with high selectivity.
- This article is part of the themed collection: Electrosynthesis


 Please wait while we load your content...
Please wait while we load your content...
