Density functional theory based computational investigations on the stability of highly active trimetallic PtPdCu nanoalloys for electrochemical oxygen reduction†
Abstract
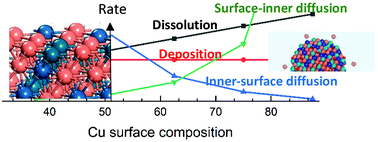
Activity, cost, and durability are the trinity of catalysis research for the electrochemical oxygen reduction reaction (ORR). While studies towards increasing activity and reducing cost of ORR catalysts have been carried out extensively, much effort is needed in durability investigation of highly active ORR catalysts. In this work, we examined the stability of a trimetallic PtPdCu catalyst that has demonstrated high activity and incredible durability during ORR using density functional theory (DFT) based computations. Specifically, we studied the processes of dissolution/deposition and diffusion between the surface and inner layer of Cu species of Pt20Pd20Cu60 catalysts at electrode potentials up to 1.2 V to understand their role towards stabilizing Pt20Pd20Cu60 catalysts. The results show there is a dynamic Cu surface composition range that is dictated by the interplay of the four processes, dissolution, deposition, diffusion from the surface to inner layer, and diffusion from the inner layer to the surface of Cu species, in the stability and observed oscillation of lattice constants of Cu-rich PtPdCu nanoalloys.
- This article is part of the themed collection: Nanoalloys: recent developments and future perspectives


 Please wait while we load your content...
Please wait while we load your content...