Open Access Article
Open Access ArticleCurrent status and future prospects of bioactive molecules delivered through sustainable encapsulation techniques for food fortification
Divakar
Dahiya
a,
Antonia
Terpou
b,
Marilena
Dasenaki
 c and
Poonam S.
Nigam
c and
Poonam S.
Nigam
 *d
*d
aWexham Park Hospital, Wexham Street, Slough SL2 4HL, UK
bDepartment of Agricultural Development, Agrofood and Management of Natural Resources, School of Agricultural Development, Nutrition & Sustainability, National and Kapodistrian University of Athens, 34400 Psachna, Greece
cLaboratory of Food Chemistry, Department of Chemistry, National and Kapodistrian University of Athens, Panepistimiopolis Zographou, 15771 Athens, Greece
dBiomedical Sciences Research Institute, Ulster University, Coleraine BT52 1SA, UK. E-mail: p.singh@ulster.ac.uk
First published on 31st May 2023
Abstract
In a world of growing population and climate changing, health and sustainable food production are nowadays considered the most pressing challenges. Foods enriched with bioactive compounds provide numerous health benefits to consumers. For instance, essential oils and extracts prepared from several natural edible resources including herbs, spices, fruits, seeds, flowers and medicinal plants are selected to obtain bioactive compounds. These ingredients have been selected based on their active components providing antioxidant activity, antiinflammation activity, aroma, and various other health-promoting attributes. Although having applications in foods and nutraceuticals, certain bioactive compounds are found susceptible to oxidative degradation, while some are known to be chemically unstable, which results in minimizing their health-promoting effects. Similarly, the instability of bioactive compounds during several stages of food manufacturing and storage, in conjunction with poor bioavailability, fast release, low solubility, and chemical instability when exposed to different conditions in the gastrointestinal tract, significantly compromise their anticipated benefits. Therefore, effective vehicles and sustainable techniques are essential for the delivery of bioactive compounds in foods to retain requisite benefits. The encapsulation process is one of the most popular techniques applied for the protection and controlled release of bioactive compounds, as well as for masking undesirable odours and bitter tastes of certain food ingredients. This review gives insight into studies published on bioactive preparations and the techniques of encapsulation for their integration to obtain enhanced and sustainable health-promoting food products. The benefits achieved include the protection of bioactive compounds against adverse environmental conditions, improvement of their physicochemical functionalities, stability during processing and storage, and enhanced bioavailability, providing health-promoting and anti-disease activities to the consumers.
1. Introduction
Food affects human health in many ways besides providing the human body with energy mainly provided by macronutrients (carbohydrates: 4 kcal per gram, proteins: 4 kcal per gram, and fat: 9 kcal per gram) as food contains other ingredients such as micronutrients essential for healthy functioning and development.1 In addition to ingredients essential to the human body, providing a stable supply of nutritious food, there are functional ingredients that can enhance human well-being. Specifically, functional foods containing bioactive compounds and other bio-sourced ingredients have attracted the attention of researchers and the food industry in recent years.2The need for research and formulations for functional healthy foods is mainly driven by consumers' demand for well-being, health improvement and disease prevention through the intake of a diet enriched with bioactive ingredients.3,4 These biologically active compounds can include dietary compounds and metabolites, living probiotic cells, dietary fibers, and active ingredients from different plant resources such as phenolic compounds, antioxidants, bioactive peptides, polyunsaturated fatty acids (e.g., omega-3), and vitamins. For instance, fruits and vegetables being rich in phenolic compounds, terpenes, terpenoids, alkaloids, etc. also include dietary fibers providing unique beneficial effects to the consumer. These biologically active compounds can be isolated and are widely used for food fortification.5–7
Functional foods i.e., foods that have a potentially positive effect on health beyond basic nutrition, as well as bioactive supplements have become popular because of their nutraceutical benefits.6,8,9 Different preparations of functional foods and beverages enhanced with bioactive compounds have been studied and designed to meet the requirement of a large group of consumers, including vegans, vegetarians, and people with a compromised digestibility for dairy-based products.10,11 The presence of bioactive compounds in the diet is linked with beneficial effects on specific human body functions, beyond the adequate nutritional effects, which mainly depend on the biochemical state of bioactive compounds in a product at the time of consumption, reaching the consumer’s bloodstream.9,12 Specifically, the health effects from the intake of functional foods have been established to confer anti-tumour, anti-inflammatory, anti-oxidative, anti-hypertensive, and anti-hyperlipidemic effects, in addition to their basic nutritional functions.10,13
The constituents of food intake especially bioactive constitutes positively affect the function of the gastro-intestinal tract (GIT) as well as human metabolism; however, bioactives are known to be sensitive to a variety of environmental factors resulting in low bioavailability.13–15 As a result, encapsulation is presented as a viable vehicle for ensuring bioactive stability and viability through food storage as well as though food passage in the GIT.16 Moreover, bioactive encapsulation can also be applied for masking unwilling aromas derived from bioactive ingredients. For instance, functional chemicals, such as polyphenols when administrated in foods at high content may confer bitter taste and astringency while encapsulated polyphenols mask any unpleasant bitterness from the consumer.17 Likewise, microalgae when administrated in foods they may provide fishy and/or marine odors while the density is mainly attributed to the food matrix, highlighting the need for their encapsulation beyond the stability and control release needs.18,19
The reasons to use encapsulation applications span across diverse sectors, including food, pharmaceutical, cosmetic, metrology, and analytical chemistry industries as well as research. Central to most dietary and health applications is the protection of bioactive components in food systems and their effective delivery to the consumer. Thus, this review will discuss reports on the sources of bioactive molecules, which have been isolated, extracted, and studied for their bioactivities (antioxidants, proteins, lipids, polysaccharides, flavoring, and colour), targeting their sustainable integration into food products through the application of encapsulation techniques.
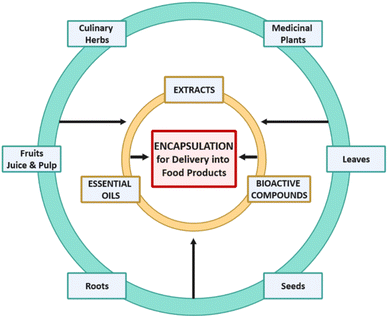
2. Sources of bioactive compounds
Nowadays, various chronic diseases tend to rise including obesity, diabetes, cancer, hypertension, and cardiovascular diseases most likely as a result of modern lifestyle and bad human dietetic habits.13 As a result of the increased interest from consumers globally, novel food products and nutraceuticals are being developed containing bioactive compounds obtained from several sources. These bioactives can be incorporated into food matrices in order to improve their nutritional value and provide health benefits.13 Researchers have investigated different natural resources, and optimized chemical and physical bioprocesses to yield bioactive compounds possessing different characteristics. The relevant references on the isolation and extraction of bioactive compounds originating from several natural edible resources including herbs, spices, fruits, seeds, flowers, and medicinal plants are summarised below:1. Essential oils have been recovered from culinary and medicinal herbs, like basil. The plants belonging to the Ocimum genus of the Lamiaceae family are considered to be a rich source of essential oils, and have expressed biological activity and use in different areas of human activity.20 The biologically active compounds present in volatile oils of different species of Ocimum are studied as a source of natural antibacterial ingredients while the plant has been traditionally consumed for the treatment of digestive, respiratory, and sedative disorders.21,22 A representative example of the species is Ocimum americanum which contains several phytochemical components, such as phenolic acids, flavonoids, tannins, terpenoids, alkaloids, saponin, steroids and glycosides.22 The abundance of these bioactive compounds in Ocimum essential oils can provide antimicrobial, antioxidant and anti-inflammatory effects to the consumer.22,23
2. Essential oils were isolated from several Mentha species, such as spearmint, peppermint, and garden mint, which are used as an aroma additive in several commercial products.24 These oils have been characterized for several bioactivities.24 Particularly, seasonal and climatic variations have an effect on the content and chemical composition of bioactive compounds in Mentha plants. Therefore, the activities of essential oils recovered from different Mentha species could be different, as this criterion has been studied by Hussain et al. analysing the oils obtained from four species of Mentha.25
3. Rosemary leaves are reported to be a natural source of bioactive compounds. Essential oils have been extracted from the aromatic evergreen leaves of this culinary herb (Rosmarinus officinalis). Rosemary is a potent antioxidant herb rich in polyphenols belonging to the family Lamiaceae, and is popularly used as a spice and medicine.26 Sharma et al. reported an efficient method of extraction yielding a high concentration of rosmarinic acid up to 33.49 mg g−1, which was found to contribute substantially to the high antioxidant potential of the extracts.27 Though this plant is grown for its aromatic and unusual flowers, researchers have reported its antiproliferative, antioxidant, and antibacterial activities.28
4. Pomegranate (Punica granatum L.) possesses different bioactive compounds, and belongs to the family Punicaceae. Pomegranate juice is known for its high levels of bioactive compounds such as flavonoids and other phenolics, exhibiting antioxidant, antimicrobial, and antimutagenic properties.6 Significantly, all parts of the pomegranate plant; the seed, the peel, the juice, and leaves are known to be rich in bioactive compounds including anthocyanin, gallic acid, catechin, quercetin, alkaloids, flavonoids, etc.6,29 Many methods target the extraction of polyphenols from red-coloured natural fruit juices like pomegranate (Punica granatum) or juice by-products targeting application in fortified novel products.30,31 Agro-industrial by-products are of significant importance as many studies reveal their valorization potential which in many cases exceeds the original product value. For instance, recent studies reveal that the extract of pomegranate peels show significantly higher antioxidant capacity than pomegranate seeds or juice31
5. Bioactive metabolites have been found in endophytes, like Endolichenic fungi.32 Endolichenic microorganisms are an intriguing source of bioactive compounds with pharmacological potential. For instance, polysaccharides, sterols, and alkaloids have been reported for their biosynthesis employing a bioagent sourced from a medicinal plant Swertia chirayita.33
6. Extracts from several Centaurea species (family Asteraceae, tribe Centaureinae) such as Polyclada Dc. belonging to family Asteraceae, are a source of novel bioactive compounds. The Centaurea genus includes approximately 500 species mainly distributed in the Mediterranean region.34 Phytochemical investigations on this genus generally revealed the isolation of sesquiterpene lactones, flavonoids, phenyl propanoids, lignans and phenolic compounds. These compounds are reported for their anti-inflammatory, antimicrobial, antidiabetic and anticancer activities.34,35 Associated with this exuberant chemodiversity, a plethora of herbal remedies have been valorized through the years to treat abscesses, asthma, haemorrhoids, peptic ulcers, malaria, common cold, stomach upset and abdominal pain.
7. Pumpkin (Cucurbita pepo), watermelon (Citrullus lanatus), and melon (Cucumis melo) belong to the Cucurbitaceae family and they can be also mentioned as cucurbits growing extensively in tropical and subtropical regions. The bioactive composition and health benefits have also been studied in the fruits of the Cucurbitaceae family (Momordica charantia L.).36 In addition, bioactive compounds possessing antioxidant activity have been extracted from the edible seeds of the Cucurbitaceae family. Cucurbitaceae seeds are an alternative source of plant oil, which is used as a raw material for certain food applications. Pumpkin (Cucurbita pepo) seeds are reported as nutraceutics for their bioactive properties mainly retrieved from their phenolic content.36,37
8. Endophytes are microbes that asymptomatically colonize the biotopes reported to survive in a symbiosis relationship with host plants having medicinal properties. Endophytes promote plant growth, development and defence by the production of metabolites.38 Endophytes have been studied as a promising source of novel bioactive compounds, highlighting the potential to obtain targeted bioactive compounds faster through laboratory production than by bioactive producing plants.39 Significantly, endophytes are emerging as an eco-friendly candidate producing bioactive compounds with therapeutic use while promoting crop yield productivity as biostimulants.39 This approach can address environmental and agricultural concerns, producing high-value metabolites within a sustainable agriculture perspective.
9. Compounds rich in potent anti-inflammatory and antioxidant properties, such as curcumin have been isolated from different spices such as turmeric. The rhizomes of turmeric (Curcuma longa L.) are rich in essential oils (5–10%, v/w) and curcuminoids (1–2%, w/w).40 Turmeric is a widely used spice in the Indian subcontinent and its extracts have been widely used in traditional medicine and Ayurveda.40 A combination of curcumin isolated from turmeric and alpha-linolenic acid have been studied for its beneficial properties against cancer cells.41 Likewise, saffron (Crocus sativus L.), which is a perennial herb belonging to the family Iridaceae, is a widely popular product of Greece (Kozanis Crocus; “red gold”) providing a significant proportion of antioxidants, with more than 300 volatile and non-volatile compounds. The main constituents of saffron are carotenoids, glucosides, flavonoids and monoterpenes while its most significant bioactive components are apocarotenoids such as safranal, picrocrocin, and crocins known for their beneficial effects.42,43
10. Vegetative sources rich in antioxidant properties have been investigated, such as the leaves of the plant Camellia sinensis (popular as tea). Antioxidant properties were assayed as the free radical quenching capacity of several types of commonly used tea leaves (Earl grey, black tea, Ceylon tea, & green tea). The infusions of tea leaves were investigated for antioxidant activity using ascorbic acid, trolox or gallic acid as reference antioxidants. The radical quenching capacity of tea infusions were expressed as trolox equivalent antioxidant capacity (TEAC) or ascorbic acid equivalent antioxidant capacity (AAEAC).44
11. Plants of Origanum species like common oregano and wild marjoram were used to isolate essential oils. The essential oil of oregano (Origanum vulgare) presents antioxidant and antimicrobial activities, mainly due to the presence of carvacrol and thymol. The chemical composition of the essential oils distilled from the extract of Origanum species may provide antiproliferative, antioxidant, anti-inflammatory, and antidiabetic activities, and more recently, cancer suppressive activity has been reported.45
12. Rubia cordifolia L. (commonly known as madder) is a species of a flowering herbal plant of the coffee family, Rubiaceae, utilized for ages in China as a medicinal plant.46R. cordifolia is usually cultivated for a red pigment derived from its roots employed to treat hematemesis, haemorrhage, rheumatism, metrorrhagia, contusion, and chronic bronchitis. Various studies have also demonstrated the anti-inflammatory, antioxidant, anticancer, and antimicrobial effects of the aqueous root extract of R. cordifolia.47,48
13. Ashwagandha or the “Indian Ginseng” (Withania somnifera) is a medicinal herb, and the bioactivity of its root extracts is being used since ancient times for health restoration in therapeutic Ayurveda.49 Recent scientific reports show that the aqueous extracts of its roots possess antioxidant, anti-osteoporotic, anti-arthritic, anti-epilepsy, anti-Alzheimer, anticancer, and antimicrobial activities mainly attributed to the bioactive compound triterpenoid steroidal lactone.49 Unfortunately, the yield of secondary metabolites retrieved from cultivated plants is not always produced in adequate amounts.
14. Extracts in different solvents, polar and non-polar, were prepared from the dark orange-coloured petals of Calendula officinalis, a flowering plant in the daisy family Asteraceae. Efstratiou et al. studied different extracts of Calendula and reported its bioactivity against broad spectrum pathogens.50
15. Extracts were obtained from Stachys schtschegleevii, an endemic species of Iran. Stachys is a genus of plants, one of the largest in the mint family Lamiaceae; it is considered a valuable medicinal plant that is widely used in herbal medicines. Its different preparations have been studied for the presence of free-radical-scavenging and antibacterial properties.51
3. Protection of bioactivities in ingredients and products
Food functionality can be claimed when health beneficial effects are provided to the consumer through food intake. Likewise, food fortification with bioactive compounds targets the production of novel foods with improved beneficial characteristics.8,13 The direct fortification of food products with bioactive compounds is a complex procedure as in many cases the bioactives may become unstable depending on the food matrix and negative effects on the quality of the final product (e.g., sensory properties such as weird taste, appearance, and bad odor) may also occur.52One of the most imperative challenges of foods enhanced with bioactive compounds is the controlled release of the compound of interest from the food matrix to the gastrointestinal tract while retaining its bioactivities.13 Bioactive compounds can be present in food matrices but do not always reach the bloodstream as in many cases they are decomposed, losing their beneficial value. Moreover, the application of bioactive compounds in the food industry remains limited up until nowadays. The loss/reduction of functionality of these health promoting bioactives remains a serious hazard in food industrial production as the environmental conditions (e.g., oxygen, heat, and light) and food manufacturing conditions (e.g., high temperatures and high pressure) can cause serious effects on the activity of bioactives.52 To overcome these shortcomings, different food grade matrices have been proposed by researchers to encapsulate bioactive compounds in order to enhance their stability, bioactivity and bioavailability while increasing their concentration in the produced novel foods. The desired characteristics of different biocatalysts applied as encapsulation matrices are presented, highlighting sustainable production and high bioavailability.53
Encapsulation is a technique that involves the introduction of bioactive compounds in a secure way into the matrix of products. More specifically, using an effective vehicle, the ingredients and additives with the required bioactivity can be entrapped within or coated with another material, or system (encapsulating agent).16 This strategy targets the protection of the activity of additive compounds from external conditions, restraining their direct contact with the conditions of the surrounding environment, and/or controlling their release (Fig. 1).
The coated material is called the core material while the material used for coating is called the shell, carrier, or encapsulant. Moreover, encapsulation can also be used to mask any undesirable strong aroma and bitter taste of additives and food ingredients.54 The entrapment of bioactive molecules protects them against adverse environmental conditions, improves stability during processing and storage, and allows the controlled release of bioactive compounds. Encapsulation technology is applied in the pharmaceutical, chemical, cosmetic, and food industries. Several encapsulation technologies have been studied that include emulsification, entrapment, spray chilling and cooling, spray drying, fluidized bed coating, extrusion, inclusion complexation, and coacervation.54–56
4. Encapsulation techniques
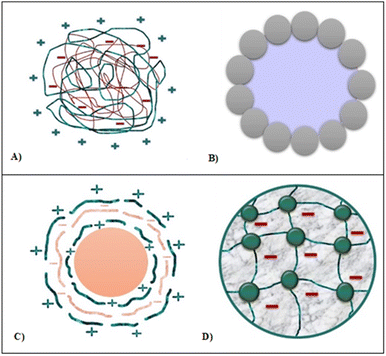
Encapsulation can be defined as a process of holding active agents (bioactive compounds) within a food-grade carrier/matrix to improve the delivery of these active compounds into food products.Among encapsulation techniques, Pickering emulsions (Fig. 2C), cross-linked polymer gels (Fig. 2D), complex coacervates (Fig. 2A), core-shell structure microcapsules and self-assembled structures (Fig. 2B) are the most popular delivery systems to enhance the bioavailability and stability of bioactive compounds (Fig. 2).
Carrier agents, biopolymer microparticles or nanoparticles are applied to sustain the functional characteristics of bioactive compounds within the food matrix.75 Likewise, microencapsulation can be defined as a process where bioactive compounds are contained within carrier agents acting as droplets surrounded by a coating or embedded in a homogeneous or heterogeneous matrix to produce effective capsules protecting the bioactive compounds. In general, core materials (essential oils, vitamins, flavonoids, polyunsaturated fatty acids, probiotics, etc.) selected as bioactive compounds are blended within the matrix and encapsulated by using a wall material (Table 1).
| Technique of encapsulation | Bioactive compound | Material of encapsulation | Ref. |
|---|---|---|---|
| Spray drying | Spirulina sp. LEB-18 | Maltodextrin and soy lecithin | 19 |
| Spray drying | Pomegranate seed oil | Succinylated taro starch and β-cyclodextrin | 57 |
| Spray drying | Anthocyanin | Gum arabic, maltodextrin, and gelatin | 58 |
| Freeze drying | Probiotic cells | Pistacia lentiscus resin | 5 |
| Freeze drying | Sour cherry pomace extract | Whey and soy proteins | 59 |
| Freeze-drying | L. casei ATCC393 | Wheat bran | 60 |
| Cross-linked biopolymer | Lactoferrin (glycoprotein) | Calcium alginate | 61 |
| Spray-drying and ionic gelation | Anthocyanins | Maltodextrin, whey protein, and gum arabic | 62 |
| Complex coacervation | Apple polyphenols | Cyclodextrin | 63 |
| Complex coacervation | Probiotic cells | Whey protein isolate and gum arabic | 64 |
| Complex coacervation | Flaxseed oil | Gelatin-gum Arabic | 65 |
| Complex coacervation | β-Carotene | Palm oil with chitosan/carboxymethylcellulose | 66 |
| Complex coacervation and spray drying | Peppermint oil | Albumin, gum acacia and an oxidized starch crosslinker | 67 |
| Cross-linking and microemulsification | Gallic acid | Whey protein hydrolysates | 68 |
| Microemulsification | Caffeine | Whey protein isolate | 69 |
| Self-assembled nanoparticle microcapsules | Vitamin D3 | Whey protein isolate | 70 |
| Core-shell structure microcapsules/freeze-drying | Yeast | Pine sawdust | 71 |
| Pickering emulsion | Thymol | Zein/gum Arabic nanoparticle | 72 |
| Pickering emulsion | β-Carotene | Hydrolyzed soya protein isolate | 73 |
| Pickering emulsion | Chlorophyll | Gelatin, agar, oil phase, and water | 74 |
Selection of the wall material is of paramount importance and mainly depends on the chemical characteristics of the produced food as well as the inserted bioactives (Table 1). The wall material must have low production costs and provide sustainability and high encapsulation efficiency, retaining its characteristics throughout food production and storage.5,71,75
5. Bioactive compounds encapsulated for enhanced food quality
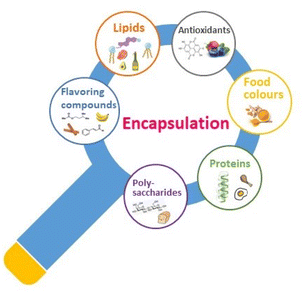
Encapsulation is considered a significant method for improving bioactive distribution in food as well as helping in the delivery of bioactives to the gastrointestinal tract. Encapsulation or microencapsulation is actually a method that encloses bioactive molecules either by coating or insertion in a complex matrix targeting the creation of capsules which contain molecules storing beneficial features.8 The most frequently encapsulated bioactive molecules are lipids, antioxidants, proteins, carbohydrates, food colours and various flavoring compounds (Fig. 3).Overall, encapsulation techniques increase the stability of bioactive compounds, enable them to resist low pH environments and enzymatic activity of the gastrointestinal tract, promote targeted delivery of active compounds, improve viscosity, promote low water solubility, and facilitate their incorporation in many food products.52,76
5.1 Flavoring compound encapsulation
Flavors are known as the essence of foods and play important roles in consumer acceptance and consumption of foods. Most flavoring compounds that exist in foods are volatile and chemically unstable in the presence of air, light, moisture, and high temperatures; therefore, encapsulation provides the means for stabilization when exposed to air, light, heat, and moisture.75 Microencapsulation can also be used to mask unpleasant flavors and odors as well as to provide barriers between sensitive bioactive materials and the environment. Moreover, the controlled release of flavour ingredients according to their threshold values can cover the effects of other unpleasant odours in food products. For instance, below threshold levels, organic acids contribute to the complexity of wine aroma, but very high concentrations may pose a negative effect on the final products.12,77,78 For example, Shinohara (1985) reported that the presence of organic acids (C6–C10) in wine at regular concentrations (4–10 mg L−1) contributes to a pleasant aromatic profile while a higher than 20 mg L−1 organic acid concentration can negatively affect the final aromatic profile of wine.77 In the wine industry it has been well documented that esters are characterized by low flavour thresholds, and are known to pose a major impact on the flavour of alcoholic beverages.12 Specifically, ethyl esters of fatty acids are associated with flowery or fruity aromatic profiles and are highly desirable in wine and other alcoholic products. Likewise, the acetates of higher alcohols and terpenes (e.g., D-limonene, β-myrcene, linalool, α-terpineol, and β-citronellol) are significant volatiles that contribute to wine aroma, imparting flowery notes. Thus, encapsulation and controlled release of flowery or fruity aromatic volatiles can mask unpleasant effects posed by a high organic acid concentration of wine products.12 Volatile compound encapsulation prior to use in foods or beverages may limit aroma degradation or loss during processing and storage.79Another mechanism of action in the application of encapsulation techniques in food ingredients containing off-flavors is by targeting deodorization.80 For instance, algae and microalgae contain off-flavors and odors (e.g., fishy and/or marine odor caused by the production of dimethyl sulfide) which affect the quality of the final product and make it less desirable to the consumers.18 Algae-fortified new food products can be implemented with other flavouring agents that mask these off-flavor compounds.18,19,81 Microencapsulation of food ingredients containing off-flavors like microalgae may mask the characteristic flavour providing novel food with accepted organoleptic characteristics.19
Several methods have been reported for flavor microencapsulation. Usually, spray drying of flavor components dominates the techniques used for the production of flavor powders.82 Spray drying is one of the most frequently used encapsulation techniques for thermosensitive bioactive components as it promotes faster drying and short-term exposure to heat, it is easy to apply, it is cost-effective, and it creates high-quality microcapsules.19,83 Complex coacervation, however, is a new promising low cost technique for flavor compound encapsulation providing high encapsulation yields (up to 99%) and controlled release of flavoring compounds.84 Even though it has been previously reported that coacervation showed limited application in flavour encapsulation such as evaporation of volatiles, dissolution of an active compound into the processing solvent and oxidation of the product, recent reports provide us with more promising results.85
The protein–polysaccharide combinations that have been reported for flavor encapsulation by complex coacervation include gelatin/gum Arabic, gum Arabic/albumin and xanthan gum/gelatin.86 In cases where a flavor is being encapsulated by a process based on complex coacervation, water-miscible or partially water-soluble components present in the flavor can affect the coacervation process and nature of the formed coacervate.75,79 Gelatin-based complex coacervation systems for flavor compounds introduce protocols depending mainly on the type of gelatin applied for coacervation. In many cases a crosslinking factor can be added to improve the stability of the microcapsules. Numerous variations of this process have been reported throughout the literature because of the different polyanions that can be applied to produce complex coacervates suitable for microcapsule formation. A representative example is the microencapsulation of garlic oil via complex coacervation using gelatin and gum acacia as e cell wall material in which the cross-linked garlic oil contained in coacervates allowed the controlled release of garlic oil in pepsin solution within 5 h. The studied systems remained stable and effective protecting garlic oil against primary and secondary oxidation throughout storage (45 °C) compared to free garlic oil which showed high oxidation under the same conditions.87 Another study targeted the formation of heat-resistant jasmine oil applying complex coacervation microcapsules of gelatin and gum Arabic. Their heat-resistance test showed that the nano-capsules cross-linked under alkaline conditions showed sustainability while chromatographic analysis showed a destruction in the fractions of free jasmine essential oil.88 In conclusion complex coacervation can be noted as a very promising low-cost microencapsulation technology showing optimum characteristics in flavor compouns encapsulation as it provides high encapsulation yields (up to 99%) verifying the controlled release and stability of flavor compounds.75,79
5.2 Lipid encapsulation
Lipids belong to a broad category of food compounds that include different types of molecules such as carotenoids, fatty acids, oil-soluble vitamins, antioxidants, phospholipids, and phytosterols. Lipids are known to have low water solubility and the majority of them are highly susceptible to oxidation; therefore, encapsulation offers an alternative to the food industry to facilitate their incorporation via sustained product quality, storage, and protection against oxidation in a large variety of food products.83Several encapsulation methods and wall materials have been tested for lipid encapsulation depending on rheological properties, achievement of controlled release, dispersion and stabilization of the encapsulated oil, and the ability to hold the core within the capsule.67,83 The most widely applied method to increase their stability is the use of microencapsulation. A combination of wall materials such as pectin, gum arabic, and protein isolate has been proved to improve lipid stability while utilization of sustainable and other renewable resources is also considered very promising according to recent data. The main strategies for lipid encapsulation include spray drying, freeze-drying, complex coacervation, extrusion, spray-chilling/cooling, and ionic gelation.57,67
As lipids are known to be susceptible to oxidation, encapsulation by spray-drying is proposed as a technique to protect them against harsh environmental factors.83 Even though many studies have highlighted the successive effect of this technique on lipid encapsulation, more recent data provide a controversial input. For instance, according to Łozińska et al. (2020), the best wall materials for fish oil micro-encapsulation are protein + lipid + carbohydrate and protein + lipid, the result in this case highlighted that spray-drying methods were of lower expectations.89 Another study by Linke et al. (2020) evaluated the rate of oxidation of encapsulated and non-encapsulated fish oil highlighting that the encapsulated oil could be oxidized even when incorporated within the protective matrix.90 In addition, the oxidative stability of the encapsulated fish oil was determined by its oxidation behavior, and as a result, the solid protective matrix was not proved sufficient to obstruct the penetration of environmental oxygen. Therefore, more complex materials need to be studied targeting efficient protection of lipids from oxidation.
Flaxseed oil, for example, is a significant source of omega-3 fatty acids and has been acknowledged for its role in disease prevention and human health.91 Due to its incompatibility with many food systems, encapsulation has been studied as an alternative option for food industries. Liu et al. (2010) encapsulated flaxseed oil within gelatin–gum arabic (GA) complex coacervates. The produced capsules showed high encapsulation efficiency (∼84%) providing in parallel a protective effect against oxidative products when compared to the original oil.65 Consequently, coacervates can be proposed as successful encapsulation materials when applied as lipid protective capsules.
5.3 Encapsulation of antioxidants
Antioxidants as food preservatives have gained exquisite attention in recent years as they may prevent food deterioration in parallel with preserving their nutritional food value. Antioxidant therapy has been proved as a powerful tool against oxidative stress as bioactive antioxidant compounds can reduce or terminate chain reactions caused by free radicals. Specifically, natural antioxidants are mostly preferred as they possess enhanced thermal stability and antioxidation ability compared to synthetic antioxidants providing in parallel potential health benefits and reinsuring safety in food applications.92Food antioxidants can be classified into different categories based on their properties including water soluble compounds like phenolic compounds, citrates, flavonoids, and anthocyanins, and lipid soluble compounds like terpenoids, carotenoids, tocopherols, and vitamins.93 In general, for antioxidant compounds to be characterized as efficient they need to confer high reactivity, biological availability, ubiquity, versatility and the ability to cross physiological barriers. More importantly, bioactivity of antioxidants may be influenced by oxygen, light, temperature and moisture. Thus, encapsulation techniques have been extensively studied targeting their efficient stabilization thought-out food processing and storage.94 More specifically, microencapsulation has been proved to promote the delivery of vitamins and minerals to foods mainly by preventing their interaction with other food components; for example, iron bioavailability can be severely affected by interaction with food ingredients (e.g. tannins, polyphenols & phytates).7 Apart from oxygen, acids can also cause problems in conjunction with other food ingredients, such as a decrease in flavour, providing undesirable odors and providing undesirable changes in pH.95
Ascorbic acid, which is a water-soluble vitamin, is considered as one of the most important antioxidants. However, environmental factors, such as pH, temperature, oxygen, metal ions, UV light and X-rays can affect its stability.96–99 Therefore, several microencapsulation techniques have recently been utilized to reduce these problems.100 For example, complex coacervation of gelatin-A and sodium alginate as a new microencapsulating material has been applied using gelatin as the crosslinker and ascorbic acid as a model active agent.101 Likewise, to enhance microencapsulation, the use of the double emulsion technique made it possible to obtain microcapsules with a hydrophilic core providing high encapsulation efficiency. To conclude, incorporation of novel technologies seems to be very promising for revealing the incentive quality of processed substances, and tailor-made microcapsules need to be considered for specific applications.
5.4 Food colour encapsulation
Plant pigments are unique chemical substances considered as secondary compounds involved in plant photosynthesis, metabolism, light-harvesting, and protection from photo-oxidation while being responsible for the colourful appearance of fruits and plants.102 In particular, the most common plant pigments are carotenoids (yellow-orange), chlorophylls, anthocyanins (red-purple) and betalains (red-yellow). In most cases these colourants can also exceed antioxidant capacity when administrated in adequate amounts in foods. Natural colourants are being used as additives in foods targeting optimum organoleptic characteristics in addition to their medicinal effects.74 Unfortunately, natural colourants show low bioavailability with regard to their beneficial effects and in many cases, a lack of stability, showing less attractive colours which are not attractive to the consumer. As a result, encapsulation of colour pigments has been applied to reinsure colour sustainability.74,102 Targeting a more sustainable point of view, agricultural residues can become a significant source of natural pigments with low costs, reducing the environmental fingerprint during food supplement applications.Carotenoids can be easily oxidized under processing and storage conditions. For instance, lycopene, which is a carotenoid sensitive to oxidation, is usually introduced into foods to provide color. However, lycopene's application in the food industry is limited due to its poor solubility and low bio-accessibility. Several delivery systems have been employed for the delivery of lycopene including protein-based nanoparticles, emulsions, liposomes, etc. Protein-based spray-dried microcapsules have been prepared economically and widely used.103 Silva et al. (2012) encapsulated lycopene by complex coacervation using gelatin and pectin, and despite the high encapsulation efficiency, it did not provide sufficient protection of the lycopene during storage.104
Chlorophyll is also known as an unstable compound as its stability can be highly affected by heat, light, temperature, and pH. On the other hand, chlorophyll has been proven to be more thermolabile compared to carotenoids.74
Application of anthocyanins in the food and pharmaceutical industries is limited as they are prone to degradation, being extremely sensitive to oxygen, light, temperature, pH, and enzymes.58
5.5 Protein encapsulation
Bioactive proteins are also considered one of the most important families of bioactive molecules which are applied for encapsulation, as numerous food-derived peptides can act as antioxidants, growth factors, immune regulatory factors, or anti-hypertensive agents. In order to exert a beneficial health effect, some bioactive proteins have to reach the uptake site in the small bowel intact, but most proteins are highly susceptible to biodegradation during transit through the gastrointestinal tract. Other proteins may require hydrolysis in the stomach and small intestine in order to release specific bioactive peptides or amino acids.105 Thus, the encapsulation of proteins depends on the type of protein, its health effect, and the product that will serve as a vehicle for the bioactive protein.1065.6 Polysaccharide encapsulation
Another family of bioactive molecules that may benefit from encapsulation are carbohydrates, mainly referring to bioactive carbohydrates that are found in dietary fibers. These fibers are very heterogeneous and vary in the type and distribution of polysaccharides as well as in the quantity of monosaccharides. They are usually classified according to their molecular structure and physicochemical properties, their origin, or according to their physiological effects.105 Polysaccharides are mainly applied as vehicle matrices for the delivery of other bioactive molecules or microorganisms.8The fibers that benefit from encapsulation are the soluble non-digestible polysaccharides that have been used for cholesterol reduction, prevention of constipation, reduction of glycaemic fluctuations, prebiotic effects, and antioxidant effects.107 For instance, polysaccharides isolated from seaweed have shown rich antioxidant activity, and their encapsulation would provide food supplements and products of high standards.107 The main challenge for the encapsulation of these compounds is not the targeted release but the increment of the total fiber content in food to exert the aforementioned health benefits. Thus, most efforts are focusing on increasing the total fiber load in the food load by packing enough fibers in capsules without interfering with the product quality such as changes in texture, mouthfeel, or flavor.108
6. In vitro digestibility and release profile of encapsulated bioactive compounds
The successful application of encapsulation depends on the selection of food-grade carrier materials, which should possess the desired physicochemical and rheological properties. Such an encapsulation process is a necessary support to preserve the targeted bioactive compound within a specific food matrix. This strategy targets the protection of the activity of additive compounds from external conditions, restraining their direct contact with the conditions of the surrounding environment, and/or controlling their release. Studies have been designed to investigate the bioavailability and bioactivity of encapsulated phenolics and carotenoids isolated from red pepper waste during in vitro simulated gastrointestinal digestion. The in vitro digestion of freeze-dried encapsulates and spray-dried encapsulates was determined by simulation of digestion in gastric fluid and intestinal fluid. In another study, the effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of Carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity were investigated. The effects of in vitro gastrointestinal digestion on the release and bioactivity of encapsulated bioactives have been studied after each digestion step. The results obtained in in vitro tests showed that the release of bioactive molecules from encapsulated entities, phenolics and carotenoids, as well as the antioxidant, anti-hyperglycemic, and anti-inflammatory activities were influenced by the pH and level of intestinal fluid.109,1107. Conclusions
Bioactive compounds can be applied in food enrichment to produce functional foods, food preservative-antimicrobials, food colorants, anti-cancer agents, or anti-inflammatory agents. Beyond academic research and innovation, it is the industry that must focus their development on scaling up the production of foods fortified with bioactive compounds. The bioavailability of these compounds is the main obstacle that food producing companies will have to overcome in order to align with food authority regulations on health claims. The European Food Safety Authority (EFSA), for instance, only authorises health claims if they are based on sound scientific evidence and are easy to understand by consumers.111 Thus, ascertaining bioavailability is the main target in functional food production.The bioavailability of bioactive compounds depends on the matrix of food in which the bioactive delivery system is incorporated, and on other food ingredients consumed in the diet along with the enriched product. Encapsulation, a technique which involves the introduction of bioactive compounds into a matrix, has been proven as one of the most effective techniques for the delivery of health-promoting ingredients in adequate amounts to targeted regions after consumption. As the bioavailability varies between different individuals and within a single person, depending on the food composition, consumers' health condition, and other factors, a proper experimental design regarding the encapsulation of bioactive compounds is of paramount importance. Interestingly the main target to sustain the bioactivity, as well as the concentration of bioactive compounds in food products, has been proven successful in many cases. In conclusion, the incorporation of novel technologies seems to be very promising for revealing the incentive quality of processed substances, and tailor-made microcapsules need to be considered for their specific applications.
8. Future prospects
Future studies should emphasize on the limitations of encapsulation in meeting the commercial demands for industrial-scale production. From a more ambitious perspective in the future, the field of bioactive delivery may include smart selective targeted delivery systems designed to release bioactive compounds to particular regions or cells of the consumer, providing personalized delivery systems based on each individual's habits and health needs.Bioavailability is a key step in ensuring the bio-efficacy of bioactive compounds after consumption. The bioavailability of bioactive compounds mainly refers to the fraction of the ingested compound when it reaches the blood.112 As mentioned before, most of the bioactive compounds show low bioavailability which is the major concern. Numerous encapsulation techniques have been enlisted to enhance the bioavailability of bioactive compounds. The compounds which show instability during food processing and within the gastrointestinal tract should be encapsulated in food-grade carrier agents. This will allow their timely release after consumption, facilitating bioactive uptake. Controlled release and increased residence within the gastrointestinal tract can be achieved by encapsulation, but for a bioactive compound, a tailor-made bio-capsule is required. Finally, regarding the food sector, two main aspects for applying these insights need to be kept under consideration. In the first instance, the procedure is important, and the encapsulation material needs to be of low cost. Finally, and more importantly, the production industry must keep in mind the consumers' preference for natural ingredients used as additives.
Conflicts of interest
There are no conflicts to declare.References
- D. Dahiya and P. S. Nigam, Int. J. Mol. Sci., 2023, 24(6), 5748 CrossRef CAS PubMed.
- V. Ganatsios, P. Nigam, S. Plessas and A. Terpou, Beverages, 2021, 7(3), 48 CrossRef CAS.
- D. Dahiya and P. S. Nigam, Int. J. Mol. Sci. 2, 2023, 24(4), 3074 CrossRef CAS PubMed.
- D. Dahiya and P. S. Nigam, Fermentation, 2023, 9, 1–12 CrossRef CAS.
- A. Terpou, P. S. Nigam, L. Bosnea and M. Kanellaki, LWT, 2018, 97, 109–116 CrossRef CAS.
- I. Mantzourani, A. Terpou, A. Bekatorou, A. Mallouchos, A. Alexopoulos, A. Kimbaris, E. Bezirtzoglou, A. A. Koutinas and S. Plessas, Food Chem., 2020, 308, 125658 CrossRef CAS PubMed.
- Y. Olive Li, V. P. Dueik González and L. L. Diosady, in Microencapsulation in the Food Industry, ed. A. G. Gaonkar, N. Vasisht, A. R. Khare and R. Sobel, Academic Press, San Diego, 2014, pp. 501–522, DOI: DOI:10.1016/B978-0-12-404568-2.00038-8.
- A. Terpou, A. Papadaki, I. K. Lappa, V. Kachrimanidou, L. A. Bosnea and N. Kopsahelis, Nutrients, 2019, 11, 1591 Search PubMed.
- D. Dahiya and P. S. Nigam, Microorganisms, 2022, 10(3), 665 CrossRef CAS PubMed.
- D. Dahiya and P. S. Nigam, Foods, 2022, 11(18), 2760–2773 CrossRef CAS PubMed.
- D. Dahiya and P. S. Nigam, Fermentation, 2023, 9, 1–12 CrossRef CAS.
- V. Ganatsios, A. Terpou, A.-I. Gialleli, M. Kanellaki, A. Bekatorou and A. A. Koutinas, Food Bioprod. Process., 2019, 117, 373–379 CrossRef CAS.
- A. Terpou and A. K. Rai, in Current Developments in Biotechnology and Bioengineering, ed. A. K. Rai, S. P. Singh, A. Pandey, C. Larroche and C. R. Soccol, Elsevier, 2022, pp. 31–45, DOI: DOI:10.1016/B978-0-12-823506-5.00017-5.
- D. Dahiya and P. S. Nigam, Microorganisms, 2021, 9(12), 1–16 CrossRef.
- C. P. Champagne and P. Fustier, Curr. Opin. Biotechnol., 2007, 18, 184–190 CrossRef CAS PubMed.
- M. K. Purkait, D. Haldar and P. Duarah, in Advances in Extraction and Applications of Bioactive Phytochemicals, ed. M. K. Purkait, D. Haldar and P. Duarah, Academic Press, 2023, pp. 141–166, DOI: DOI:10.1016/B978-0-443-18535-9.00005-3.
- O. Aizpurua-Olaizola, P. Navarro, A. Vallejo, M. Olivares, N. Etxebarria and A. Usobiaga, Food Chem., 2016, 190, 614–621 CrossRef CAS PubMed.
- L. Bosnea, A. Terpou, E. Pappa, E. Kondyli, M. Mataragas, G. Markou and G. Katsaros, Proceedings, 2021, 70, 99 Search PubMed.
- T. T. Batista de Oliveira, I. Miranda dos Reis, M. Bastos de Souza, E. da Silva Bispo, L. Fonseca Maciel, J. I. Druzian, P. P. Lordelo Guimarães Tavares, A. de Oliveira Cerqueira, E. dos Santos Boa Morte, M. B. Abreu Glória, V. Lima Deus and L. R. Radomille de Santana, LWT, 2021, 148, 111674 CrossRef CAS.
- A. Avetisyan, A. Markosian, M. Petrosyan, N. Sahakyan, A. Babayan, S. Aloyan and A. Trchounian, BMC Complementary Altern. Med., 2017, 17, 60 CrossRef PubMed.
- A. I. Hussain, F. Anwar, P. S. Nigam, S. D. Sarker, J. E. Moore, J. R. Rao and A. Mazumdar, LWT--Food Sci. Technol., 2011, 44, 1199–1206 CrossRef CAS.
- H. D. A. Dharsono, S. A. Putri, D. Kurnia, D. Dudi and M. H. Satari, Molecules, 2022, 27, 6350 CrossRef CAS PubMed.
- A. Rakha, N. Umar, R. Rabail, M. S. Butt, M. Kieliszek, A. Hassoun and R. M. Aadil, Biomed. Pharmacother., 2022, 156, 113945 CrossRef CAS PubMed.
- F. Brahmi, A. Abdenour, M. Bruno, P. Silvia, P. Alessandra, F. Danilo, Y.-G. Drifa, E. M. Fahmi, M. Khodir and C. Mohamed, Ind. Crops Prod., 2016, 88, 96–105 CrossRef CAS.
- A. I. Hussain, F. Anwar, P. S. Nigam, M. Ashraf and A. H. Gilani, J. Sci. Food Agric., 2010, 90, 1827–1836 CrossRef CAS PubMed.
- I. Borrás-Linares, Z. Stojanović, R. Quirantes-Piné, D. Arráez-Román, J. Švarc-Gajić, A. Fernández-Gutiérrez and A. Segura-Carretero, Int. J. Mol. Sci., 2014, 15, 20585–20606 CrossRef PubMed.
- Y. Sharma, R. Velamuri, J. Fagan and J. Schaefer, Molecules, 2020, 25, 4599 CrossRef CAS PubMed.
- A. I. Hussain, F. Anwar, S. A. Chatha, A. Jabbar, S. Mahboob and P. S. Nigam, Braz. J. Microbiol., 2010, 41, 1070–1078 CrossRef CAS PubMed.
- I. Mantzourani, M. Daoutidou, M. Dasenaki, A. Nikolaou, A. Alexopoulos, A. Terpou, N. Thomaidis and S. Plessas, Foods, 2022, 11, 861 CrossRef CAS PubMed.
- W. Wu, S. Jiang, M. Liu and S. Tian, Ultrason. Sonochem., 2021, 80, 105833 CrossRef CAS PubMed.
- Z. Hayder, W. Elfalleh, K. B. Othman, M. A. Benabderrahim and H. Hannachi, Acta Phytoecol. Sin., 2021, 41, 150–156 CrossRef.
- S. Agrawal, S. K. Deshmukh, M. S. Reddy, R. Prasad and M. Goel, S. Afr. J. Bot., 2020, 134, 163–186 CrossRef CAS.
- H. Sharma, A. K. Rai, R. Chettri and P. S. Nigam, Arch. Microbiol., 2021, 203, 5173–5182 CrossRef CAS PubMed.
- E. Serino, G. Chianese, G. Musto, G. Zengin, D. Rigano, M. Stornaiuolo, C. Formisano and O. Taglialatela-Scafati, Phytochemistry, 2022, 199, 113189 CrossRef CAS PubMed.
- S. Demir, C. Karaalp and E. Bedir, Phytochemistry, 2017, 143, 12–18 CrossRef CAS PubMed.
- L. Rezig, M. Chouaibi, W. Meddeb, K. Msaada and S. Hamdi, Process Saf. Environ. Prot., 2019, 127, 73–81 CrossRef CAS.
- M. Yasir, B. Sultana, P. S. Nigam and R. Owusu-Apenten, Food Chem., 2016, 199, 307–313 CrossRef CAS PubMed.
- M. Jia, L. Chen, H. L. Xin, C. J. Zheng, K. Rahman, T. Han and L. P. Qin, Front. Microbiol., 2016, 7, 906 Search PubMed.
- P. Tiwari, S. Kang and H. Bae, Microbiol. Res., 2023, 266, 127241 CrossRef CAS PubMed.
- A. Sharma, A. Ray and R. S. Singhal, J. Cleaner Prod., 2023, 382, 135313 CrossRef.
- T. Aldhirgham, K. Henderson, P. S. Nigam and R. Owusu-Apenten, J. Appl. Life Sci. Int., 2017, 10, 1–12 CrossRef.
- N. Pitsikas, in Saffron, ed. M. Sarwat and S. Sumaiya, Academic Press, 2020, pp. 131–139, DOI: DOI:10.1016/B978-0-12-818462-2.00011-5.
- E. Karkoula, I.-V. Dagla, E. Baira, N. Kokras, C. Dalla, A.-L. Skaltsounis, E. Gikas and A. Tsarbopoulos, J. Pharm. Biomed. Anal., 2020, 177, 112878 CrossRef CAS PubMed.
- Y. M. Chan, N. K. Cheng, P. S. Nigam and R. K. Owusu-Apenten, 2016, 6, 2, pp. 1–8, DOI:10.9734/jalsi/2016/27235.
- N. Leyva-López, E. P. Gutiérrez-Grijalva, G. Vazquez-Olivo and J. B. Heredia, Molecules, 2017, 22(6), 989 CrossRef PubMed.
- C.-H. Shen, C.-T. Liu, X.-J. Song, W.-Y. Zeng, X.-Y. Lu, Z.-L. Zheng, P. Jie, R.-T. Zhan and Y. Ping, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2018, 1090, 73–80 CrossRef CAS PubMed.
- C. H. Shen, Y. Liu, X. W. Yu, X. J. Song, Z. C. Xiao, R. T. Zhan and P. Yan, Chin. J. Exp. Tradit. Med. Formulae, 2018, 24, 147–153 Search PubMed.
- X.-P. Gong, Y.-Y. Sun, W. Chen, X. Guo, J.-K. Guan, D.-Y. Li and G. Du, BMC Complementary Altern. Med., 2017, 17, 20 CrossRef PubMed.
- L. Sharma, B. Maurya and S. P. Rai, Ind. Crops Prod., 2023, 193, 116238 CrossRef CAS.
- E. Efstratiou, A. I. Hussain, P. S. Nigam, J. E. Moore, M. A. Ayub and J. R. Rao, Complementary Therapies in Clinical Practice, 2012, 18, 173–176 CrossRef PubMed.
- M. Abichandani, L. Nahar, P. Singh, R. R. Chitnis, H. Nazemiyeh, A. Delazar and S. D. Sarker, Arch. Biol. Sci., 2010, 62, 941–945 CrossRef.
- F. Adinepour, S. Pouramin, A. Rashidinejad and S. M. Jafari, Food Res. Int., 2022, 157, 111212 CrossRef CAS PubMed.
- D. Dahiya and P. Singh Nigam, Sustainability, 2022, 14(14) DOI:10.3390/su14148673.
- M. Saifullah, M. R. I. Shishir, R. Ferdowsi, M. R. Tanver Rahman and Q. Van Vuong, Trends Food Sci. Technol., 2019, 86, 230–251 CrossRef CAS.
- A. Terpou, L. Bosnea and M. Kanellaki, SCIOL Biomedicine, 2017, 1, 1–10 Search PubMed.
- B. Muhoza, B. Qi, J. D. Harindintwali, M. Y. Farag Koko, S. Zhang and Y. Li, Food Hydrocolloids, 2022, 124, 107239 CrossRef CAS.
- M. C. Cortez-Trejo, A. Wall-Medrano, M. Gaytán-Martínez and S. Mendoza, Food Biosci., 2021, 41, 100929 CrossRef CAS.
- S. Akhavan Mahdavi, S. M. Jafari, E. Assadpour and M. Ghorbani, J. Food Eng., 2016, 181, 59–66 CrossRef CAS.
- V. Tumbas Šaponjac, G. Ćetković, J. Čanadanović-Brunet, B. Pajin, S. Djilas, J. Petrović, I. Lončarević, S. Stajčić and J. Vulić, Food Chem., 2016, 207, 27–33 CrossRef PubMed.
- A. Terpou, A. Bekatorou, M. Kanellaki, A. A. Koutinas and P. Nigam, Process Biochem., 2017, 55, 1–10 CrossRef CAS.
- M. Raei, G. Rajabzadeh, S. Zibaei, S. M. Jafari and A. M. Sani, Int. J. Biol. Macromol., 2015, 79, 669–673 CrossRef CAS PubMed.
- O. Norkaew, P. Thitisut, S. Mahatheeranont, B. Pawin, P. Sookwong, S. Yodpitak and A. Lungkaphin, Food Chem., 2019, 294, 493–502 CrossRef CAS.
- T. Aree, Food Chem., 2023, 409, 135326 CrossRef CAS PubMed.
- L. A. Bosnea, T. Moschakis, P. S. Nigam and C. G. Biliaderis, LWT, 2017, 77, 282–289 CrossRef CAS.
- S. Liu, N. H. Low and M. Nickerson, J. Am. Oil Chem. Soc., 2010, 87, 809–815 CrossRef CAS.
- J. K. Rutz, C. D. Borges, R. C. Zambiazi, C. G. da Rosa and M. M. da Silva, Food Chem., 2016, 202, 324–333 CrossRef CAS PubMed.
- W. R. Glomm, P. P. Molesworth, E. M. Sandru, L. T. Truong, A. Brunsvik and H. Johnsen, Appl. Sci., 2021, 11, 3956 CrossRef CAS.
- H. Nourbakhsh, A. Madadlou, Z. Emam-Djomeh, Y.-C. Wang and S. Gunasekaran, Food Chem., 2016, 210, 317–324 CrossRef CAS PubMed.
- A. Madadlou, S. Jaberipour and M. H. Eskandari, LWT–Food Sci. Technol., 2014, 57, 725–730 CrossRef CAS.
- A. Abbasi, Z. Emam-Djomeh, M. A. E. Mousavi and D. Davoodi, Food Chem., 2014, 143, 379–383 CrossRef CAS PubMed.
- A. Terpou, V. Ganatsios, M. Kanellaki and A. A. Koutinas, Microorganisms, 2020, 8, 764 CrossRef CAS PubMed.
- J. Li, X. Xu, Z. Chen, T. Wang, Z. Lu, W. Hu and L. Wang, Carbohydr. Polym., 2018, 200, 416–426 CrossRef CAS PubMed.
- C. V. Molina, J. G. Lima, I. C. F. Moraes and S. C. Pinho, Food Sci. Biotechnol., 2019, 28, 59–66 CrossRef CAS PubMed.
- A. Raei, S. A. Yasini Ardakani and M. Daneshi, J. Food Process Eng., 2017, 40, e12529 CrossRef.
- N. Eghbal and R. Choudhary, LWT, 2018, 90, 254–264 CrossRef CAS.
- K. Liu, Y.-Y. Chen, L.-H. Pan, Q.-M. Li, J.-P. Luo and X.-Q. Zha, Food Res. Int., 2022, 155, 111073 CrossRef CAS PubMed.
- T. Shinohara, Agric. Biol. Chem., 1985, 49, 2211–2212 CAS.
- R. S. Jackson, in Wine Science, ed. R. S. Jackson, Academic Press, San Diego, 4th edn, 2014, pp. 831–888, DOI:10.1016/B978-0-12-381468-5.00011-7.
- T. Koupantsis, E. Pavlidou and A. Paraskevopoulou, Food Hydrocolloids, 2014, 37, 134–142 CrossRef CAS.
- B. S. O. Colonia, G. V. d. M. Pereira, J. C. de Carvalho, S. G. Karp, C. Rodrigues, V. T. Soccol, L. S. Fanka and C. R. Soccol, Food Chemistry Advances, 2023, 100270, DOI:10.1016/j.focha.2023.100270.
- A. Terpou, L. Bosnea, M. Mataragkas and G. Markou, Proceedings, 2021, 70, 97 Search PubMed.
- B. C. Carolina, S. Carolina, M. C. Zamora and C. Jorge, LWT--Food Sci. Technol., 2007, 40, 1792–1797 CrossRef CAS.
- M. Geranpour, E. Assadpour and S. M. Jafari, Trends Food Sci. Technol., 2020, 102, 71–90 CrossRef CAS.
- S. Gouin, Trends Food Sci. Technol., 2004, 15, 330–347 CrossRef CAS.
- R. J. Flores, M. D. Wall, D. W. Carnahan and T. A. Orofino, J. Microencapsulation, 1992, 9, 287–307 CrossRef CAS PubMed.
- L. A. Bosnea, T. Moschakis and C. G. Biliaderis, Food Bioprocess Technol., 2014, 7, 2767–2781 CrossRef CAS.
- L.-F. Siow and C.-S. Ong, J. Food Process. Technol., 2013, 4, 1–5 Search PubMed.
- Y. Lv, F. Yang, X. Li, X. Zhang and S. Abbas, Food Hydrocolloids, 2014, 35, 305–314 CrossRef CAS.
- N. Łozińska, A. Głowacz-Różyńska, W. Artichowicz, Y. Lu and C. Jungnickel, J. Food Eng., 2020, 268 Search PubMed.
- A. Linke, J. Weiss and R. Kohlus, Food Res. Int., 2020, 127, 108705 CrossRef CAS PubMed.
- Q. Zhou, L. Ma, W. Zhao, W. Zhao, X. Han, J. Niu, R. Li and C. Zhao, J. Funct. Foods, 2019, 103602, DOI:10.1016/j.jff.2019.103602.
- S. Sharma, S.-F. Cheng, B. Bhattacharya and S. Chakkaravarthi, Trends Food Sci. Technol., 2019, 91, 305–318 CrossRef CAS.
- G. Ozkan, P. Franco, I. De Marco, J. Xiao and E. Capanoglu, Food Chem., 2019, 272, 494–506 CrossRef CAS PubMed.
- J. Aguiar, B. N. Estevinho and L. Santos, Trends Food Sci. Technol., 2016, 58, 21–39 CrossRef CAS.
- M. Trindade and C. Grosso, J. Microencapsulation, 2000, 17, 169–176 CrossRef CAS PubMed.
- A. Alishahi, A. Mirvaghefi, M. Tehrani, H. Farahmand, S. Shojaosadati, F. Dorkoosh and M. Z. Elsabee, Food Chem., 2011, 126, 935–940 CrossRef CAS.
- C. Kirby, C. Whittle, N. Rigby, D. Coxon and B. Law, Int. J. Food Sci. Technol., 1991, 26, 437–449 CrossRef CAS.
- M.-L. Liao and P. A. Seib, Food Chem., 1988, 30, 289–312 CrossRef CAS.
- M. Uddin, M. Hawlader and H. Zhu, J. Microencapsulation, 2001, 18, 199–209 CrossRef CAS PubMed.
- K. G. H. Desai and H. J. Park, Drying Technol., 2005, 23, 1361–1394 CrossRef CAS.
- N. Devi, D. Hazarika, C. Deka and D. K. Kakati, J. Macromol. Sci., Part A: Pure Appl.Chem., 2012, 49, 936–945 CrossRef CAS.
- S. Ghosh, T. Sarkar, A. Das and R. Chakraborty, LWT, 2022, 153, 112527 CrossRef CAS.
- C. Jia, D. Cao, S. Ji, W. Lin, X. Zhang and B. Muhoza, LWT, 2020, 118, 108837 CrossRef CAS.
- D. F. Silva, C. S. Favaro-Trindade, G. A. Rocha and M. Thomazini, J. Food Process. Preserv., 2012, 36, 185–190 CrossRef CAS.
- D. J. McClements, E. A. Decker, Y. Park and J. Weiss, Crit. Rev. Food Sci. Nutr., 2009, 49, 577–606 CrossRef CAS PubMed.
- P. de Vos, M. M. Faas, M. Spasojevic and J. Sikkema, Int. Dairy J., 2010, 20, 292–302 CrossRef CAS.
- R. Raja Priya and S. S. Khora, in Marine Antioxidants, ed. S.-K. Kim, K.-H. Shin and J. Venkatesan, Academic Press, 2023, DOI: DOI:10.1016/B978-0-323-95086-2.00012-6, pp. 433–448.
- M. Cunningham, G. Vinderola, D. Charalampopoulos, S. Lebeer, M. E. Sanders and R. Grimaldi, Trends Food Sci. Technol., 2021, 112, 495–506 CrossRef CAS.
- J. Vulić, V. Šeregelj, A. Kalušević, S. Lević, V. Nedović, V. Tumbas Šaponjac, J. Čanadanović-Brunet and G. Ćetković, Molecules, 2019, 24, 2837 CrossRef PubMed.
- S. Ydjedd, S. Bouriche, R. Lopez-Nicolas, T. Sanchez-Moya, C. Frontela-Saseta, G. Ros-Berruezo, F. Rezgui, H. Louaileche and D. J. Kati, Agric. Food Chem., 2017, 65, 827–835 CrossRef CAS PubMed.
- D. Dahiya and P. S. Nigam, Fermentation, 2022, 8(7), 1–16 CrossRef.
- D. Dahiya and P. S. Nigam, Microorganisms, 2022, 10(9), 1–14 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2023 |