Open Access Article
Open Access ArticleImpact of dissolved sulfide on a hybrid membrane bioreactor treating the effluent of a mainstream up-flow anaerobic sludge blanket†
Tomás
Allegue
 *,
Adrián
Arias
*,
Adrián
Arias
 ,
Alberto
Liñares
,
Francisco
Omil
,
Alberto
Liñares
,
Francisco
Omil
 and
Juan Manuel
Garrido
and
Juan Manuel
Garrido

CRETUS, Department of Chemical Engineering, Universidade de Santiago de Compostela, 15782 Santiago de Compostela, Galicia, Spain. E-mail: tomas.martinez@ku.ac.ae; adrian.arias.bano@gmail.com; albertolinhareslamas@gmail.com; francisco.omil@usc.es; juanmanuel.garrido@usc.es
First published on 30th August 2023
Abstract
Despite being toxic to some microbes in wastewater treatment, sulfide can also promote nitrogen removal through sulfide-oxidizing bacteria. This study evaluates the dissolved sulfide impact on a hybrid MBR treating the effluent of a mainstream UASB. A UASB-MBR (176 L) was fed with synthetic domestic sewage and operated for 154 days. Two periods were distinguished, one without (Period I) and one with (Period II) sulfide dissolved in the UASB effluent. Dissolved methane, COD, nitrogen, and organic micropollutants (OMP)s removals accomplished in the MBR during both periods were compared. Initially, sulfide inhibited methane removal, but once fully oxidized into sulfate in the anoxic compartment, the efficiencies recovered to similar levels as without sulfide (>70%). Sulfide additions significantly enhanced the MBR denitrification potential through sulfide-oxidizing bacteria, with improved removals in Period II (63.4 TN Lfeed−1) compared to Period I (40 mg TN Lfeed−1). Most of the nitrogen removal occurred in the anoxic compartment of the MBR, however, up to 21% of the nitrogen was denitrified in the aerobic compartment within the biofilm carriers. Aerobic methane oxidation coupled with denitrification, heterotrophic denitrifiers, sulfide oxidation, and anammox processes were involved in the nitrogen removal. COD and OMPs removals were not affected by sulfide.
Water impactThis study assesses the impact of dissolved sulfide on a novel hybrid MBR (176 L) treating the effluent from a mainstream UASB. The key finding of the study is that dissolved sulfide not only did not hinder the MBR's denitrification potential but significantly improved it. Additionally, sulfide had no effect on the removal of COD, dissolved CH4, or micropollutants. |
1. Introduction
The up-flow anaerobic sludge blanket (UASB) technology is a popular choice for treating municipal wastewater in warm climates due to its benefits, such as energy recovery, and lower sludge production and operating costs compared to conventional aerobic processes. It is widely used in warm regions, where hundreds of full-scale UASB reactors are treating domestic sewage.1 Additional post-treatment is required due to the poor quality of UASB effluents with regards to organic matter, solids, ammonium, and dissolved methane. Despite the high methane concentration in the biogas,2 which ranges from 70 to 80%, not all the methane is present in the biogas, and between 20 and 60% could be present dissolved in the effluent.3 This dissolved methane can easily be released into the environment, leading to its contribution to climate change as methane is a potent greenhouse gas.4 Thus, there is an urgent need for methods to recover or remove the dissolved methane.Unlike conventional aerobic treatments, anaerobic processes are well-known for their negligible nitrogen removal capacity. Typically, UASB effluents contain concentrations ranging from 30 to 50 mg TN L−1.5 The presence of nitrogen in their effluents could lead to serious environmental problems such as eutrophication or toxicity in the receiving water bodies. In case the treated effluent is not used for reusing purposes (e.g., irrigation), nitrogen removal must be therefore addressed. Across the globe, discharge standards are becoming increasingly stringent, with limits for total nitrogen (TN) as low as 10 mg TN L−1 in sensitive areas of the European Union (Council Directive 91/271/ EEC). In Brisbane (Australia), the limits are even tighter at 3 mg TN L−1.6 To meet nitrogen discharge regulations, domestic sewage treatment plants often add external carbon sources, such as ethanol, methanol, or acetic acid, to enhance conventional denitrification processes. However, this approach has limitations, as the high cost of these carbon-based compounds and the resulting large amount of sludge production make it unsustainable and expensive. Additionally, the issue of dissolved methane in their effluent is not resolved.
Utilizing dissolved methane and nitrogen in UASB effluent offers a scope for creating innovative post-treatment strategies that employ methane as a potential electron donor for nitrogen removal through biological processes. Given the low C/N ratio in UASB effluents, using methane as an electron donor could be a highly appealing approach for removing nitrogen from wastewater without the need for costly external carbon sources, resulting in more environmentally sustainable and efficient sewage treatment processes.7–11
Various microbiological pathways exist for the coupling of methane oxidation with denitrification processes. In the aerobic methane oxidation coupled to denitrification (AMO-D), aerobic methanotrophs can convert methane into oxidation products (e.g., methanol), that can be used by conventional denitrifiers (eqn (1) and (2)).12 The removal of dissolved methane and nitrogen can also be achieved simultaneously under anaerobic conditions via nitrate/nitrite-dependent anaerobic methane oxidation bioprocesses, referred to as N-damo. This process involves two groups of microorganisms: N-damo archaea and N-damo bacteria. N-damo archaea can oxidize methane into carbon dioxide by reducing nitrate to nitrite,13 while N-damo bacteria can oxidize methane by reducing nitrite into N2.14
| 5CH4 + 5O2 + 4NO3− + 4H+ → 2N2 + 12H2O + 5CO2 | (1) |
| 3CH4 + 3O2 + 4NO2− + 4H+ → 2N2 + 8H2O + 3CO2 | (2) |
Besides methane, sulfide is present dissolved in effluents of anaerobic reactors with concentrations that could reach up to 97.5 mg S L−1.15 The sulfate ion is reduced to hydrogen sulfide in sewerage systems and anaerobic reactors through sulfate-reducing bacteria (SRB), using organic matter as the electron donor. The sulfide concentration mainly depends on the sulfate content in fresh sewage, which could have either an anthropic or natural origin16 or be caused by seawater intrusion in ashore sewerage systems.17 Sulfide is responsible for several environmental problems such as equipment corrosion, odor nuisances, deterioration of the receiving water bodies due to its chemical oxygen demand (COD) contribution, and inhibitory effect on biological processes.7 Sulfide concentrations of only 2.6 and 1.2 mg S L−1 caused 50% inhibition (IC50) towards ammonia (AOB) and nitrite-oxidizing bacteria (NOB) activity, respectively.18
In another study, sulfide concentrations of only 0.04 and 0.1 mg H2S–S L−1 inhibited the N2O reduction capacity of conventional heterotrophic denitrifiers by 50%.19 The first value was achieved using biomass unacclimated to environments with sulfide, while the latter was attained using biomass acclimated to sulfide, which could explain the higher tolerance. The inhibitions levels observed were strongly correlated with the concentration of H2S rather than the total sulfide. In the same study, a reduction of 50% in the nitrite reduction capacity was observed at 2 mg H2S–S L−1 for both, unacclimated and acclimated biomass.
The potential impact of sulfide on certain microorganisms could be significant in the case of implementing additional biological post-treatment systems to treat anaerobic effluent. However, sulfide may also be utilized by autotrophic sulfur-oxidizing bacteria (SOB) as an electron donor for denitrification, which could enhance the nitrogen removal capabilities of UASB post-treatment systems. SOB can use reduced sulfur compounds, such as sulfide, as electron donors to transform nitrite/nitrate into N2 (eqn (3) and (4)).
| 3HS− + 8NO2− + 5H+ → 3SO42− + 4N2 + 4H2O | (3) |
| 5HS− + 8NO3− + 3H+ → 5SO42− + 4N2 + 4H2O | (4) |
The use of membrane bioreactors (MBR)s has been considered by several authors as an interesting approach to treat effluents from mainstream UASB systems,8,10,20,21 for two main reasons: i) the high quality of the effluent (free of suspended solids) and ii) to guarantee the complete retention of slow-growth denitrifiers such as anammox, N-damo bacteria, and N-damo archaea, microbes of great interest considering the low concentration of COD present in the effluent of UASB systems to denitrify.
Silva-Teira et al.8 investigated the use of a pre-anoxic/aerobic MBR system (56 L) to minimize the impact of the effluent from a UASB (120 L) treating synthetic domestic sewage at room temperature. The post-treatment system was composed of a first mechanically stirred anoxic compartment with biofilm carriers and a second aerobic membrane filtration compartment. In the latter, ammonium was oxidized, and suspended biomass/effluent separation was carried out. A recirculation from the aerobic to the anoxic compartment was needed to provide an electron acceptor for nitrogen removal. Nitrate was the main electron acceptor available, and nitrite was barely present. Methane removal efficiencies of 80% and nitrogen removals between 10 and 15 mg N LFeed−1 were achieved in the anoxic compartment of the MBR.
Using the same technology and similar operating conditions, although with the presence of nitrite and nitrate in the anoxic compartment, Alvarino et al.10 achieved much higher nitrogen removals in the anoxic compartment, 35 mg TN LFeed−1. The authors suggest that a higher nitrite presence in the anoxic compartment promoted the growth of anammox. Nevertheless, methane removal efficiencies or rates were similar to Silva-Teira et al.8
In the present study, the impact of dissolved sulfide on the performance of a hybrid membrane bioreactor (MBR) treating the effluent of a mainstream UASB is evaluated. A pilot-scale UASB-MBR integrated system (176 L) fed with low-strength synthetic sewage and continuously operated at ambient temperature was employed. This study introduces a technological enhancement to the hybrid MBR when compared to our prior prototype.8,10 This improvement entails the inclusion of an extra aerobic chamber utilizing suspended biofilm carriers (Biochip). Its primary purpose is to facilitate denitrification processes, as demonstrated by Allegue et al.9 Two main operating periods were distinguished based on the absence or presence of dissolved sulfide in the UASB effluent. In the first period, no dissolved sulfide was present in the UASB effluent, while in the second period, dissolved sulfide was introduced by adding sulfate to the UASB feeding substrate. The two periods were compared, with a focus on the elimination of organic matter, dissolved methane, nitrogen, and organic micropollutants (OMP)s in the MBR post-treatment system. Additionally, microbial communities were investigated.
2. Methods
2.1 Reactor set-up
In this study, a pilot-scale UASB-MBR integrated system of 176 L was operated (Fig. 1). The system consisted of a methanogenic UASB reactor (120 L) coupled in series to a hybrid MBR post-treatment system (56 L) with three different compartments: i) a first anoxic (36 L); ii) a second aerobic (8 L), and a third aerobic filtration unit (12 L) with a submerged hollow-fiber membrane. The anoxic compartment was mixed mechanically, while the aerobic and filtration compartments were mixed pneumatically using aeration. The organic matter in the UASB is transformed into biogas under anaerobic conditions, while the simultaneous removal of methane and nitrogen occurs in the anoxic compartment. In the MBR, the aerobic compartment facilitates the conversion of ammonium into oxidized nitrogen species (NOx), while the filtration membrane compartment guarantees biomass retention.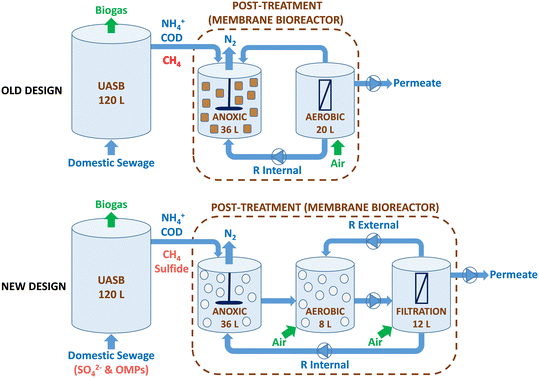 | ||
| Fig. 1 The diagram presents a comparison of the previous (top) and new (bottom) designs of the UASB-MBR. The new design is the one used in the present study, whereas the old design was used in previous studies.8,10 | ||
The system utilized two different recirculation methods, including an internal recirculation from the filtration membrane to the anoxic compartment with a recycling ratio (R) of 3.5 and an external recirculation from the filtration membrane to the aerobic compartment (R = 1). The internal recycling ratio of 3.5 was consistently applied to optimize the removal of both methane and nitrogen, as demonstrated in a study by Silva-Teira et al.8 in a similar UASB-MBR. A ZW-10 Zenon ultrafiltration membrane module, featuring a pore size of 0.04 μm and a total surface area of 0.9 m2, was placed in the filtration compartment. The membrane operates in cycles of 7 minutes, with a 0.5 min relaxation period.
The UASB-MBR system combines three distinct redox environments (anaerobic, anoxic, and aerobic) and three types of biomass (granular in the UASB, adhered to carriers, and suspended biomass in the MBR). Fresh and active biomass from a similar pilot plant configuration was used as inoculum for both compartments of the system (UASB and MBR); therefore, no stabilization time was required.10 In addition to suspended biomass, the growth of adhered biomass in the anoxic and aerobic compartments was encouraged by adding biofilm carriers called Biochip. The Biochips have an external surface area of 2174 m2 m−3 and a volume per carrier particle of 4.15 × 10−4 L, allowing for a higher nitrification capacity per unit volume as the high surface area facilitates oxygen transfer. Allegue et al.9 observed improved methane and nitrogen removal due to the accumulation of methane oxidizers and anammox bacteria adhered to the Biochips in the aerobic compartment. Biochips were also added to the anoxic compartment to promote the growth of anaerobic microbes, such as N-damo, that might be sensitive to the oxygen in the internal recirculation stream. The apparent volume of Biochips in the anoxic and aerobic compartments was 17% and 20%, respectively.
In this study, modifications were made to the design of the UASB-MBR system compared to the previous prototype,8,10 as shown in Fig. 1. Firstly, the previous aerobic compartment, which housed the submerged membrane module, was split into two separate compartments: the membrane filtration compartment and the aerobic compartment. The addition of an aerobic compartment allows for the incorporation of Biochip carriers, which have been shown to improve methane and nitrogen removal in the MBR.9 The objective of employing Biochip carriers in the aerobic compartment is to facilitate the proliferation of denitrifiers and methane oxidizers within the biofilm. Additionally, Biochip were preferred over Levapor carriers for utilization in the anoxic compartment of the MBR. Earlier studies using Levapor demonstrated that the microbial communities in the suspended biomass and adhered biomass were similar, implying that Levapor does not encourage the formation of biofilms with distinct attributes.10
The UASB was fed with low-strength synthetic wastewater composed of a concentrated medium (containing skimmed milk, NaHCO3, NH4Cl, trace elements, and OMPs) diluted with tap water, and with the following final composition: NaHCO3 (200 mg L−1); NH4Cl (9.3 mg N L−1); trace elements concentrations and composition as indicated by Silva-Teira et al.;8 10 μg L−1 of 15 different organic micropollutants (OMPs), including endocrine disruptors (bisphenol BPA, estrone E1, β-estradiol E2, α-ethinylestradiol EE2), anti-inflammatories (ibuprofen IBP, naproxen NPX, diclofenac DCF), a disinfectant (triclosan TCS), antibiotics (sulfamethoxazole SMX, trimethoprim TMP, erythromycin ERY, roxithromycin ROX), and neuro drugs (diazepam DZP, carbamazepine CBZ, fluoxetine FLX). Collapsible silicone bags were used to house the concentrated medium, and refrigeration was employed to ensure its preservation.
The study was divided into two main operating periods, based on the absence (days 0–70) or presence (days 71–154) of sulfate in the feed, referred to as Periods I and II, respectively. During Period II, 50 mg SO42−–S L−1 was added to the feed as Na2SO4.
2.2 Analytical methods
Standard methods22 were used to measure total (CODT) and soluble chemical oxygen demand (CODS), nitrogen species (nitrite, nitrate, and ammonium), mixed-liquor total (MLTSS), and volatile suspended solids (MLVSS), pH, and temperature. The methane dissolved in the liquid phase was determined taking samples of approximately 300 mL in watertight 500 mL Pyrex flasks. Exsolved methane to gas phase was analyzed by Gas Chromatography (GC), as described by Silva-Teira et al.8 A volumetric gas-flow meter MGC-10 (Ritter®) was used to measure the biogas production.The biogas composition was analyzed using a gas chromatograph 5890 Series II (Hewlett-Packard) with a thermal conductivity detector and a column of Porapack Q80/100 2 m × 1/8′′ (SUPELCO). The column and detector temperature were set at 34 °C and 110 °C, respectively, using helium as the carrier gas. Total sulfide species (including H2S, HS−, S2−) in the liquid phase were measured using a spectrophotometric sulfide test method (Spectroquant®) according to APHA 4500. An 861 Advanced Compact Ion Chromatograph (IC) with a Metrosep C3-250 column, and an 838 Advanced Sample Processor, were used to measure sulfate ions. Intermediary sulfur compounds (S0, S2O32−, and SO32−) were not analyzed.
To prepare for OMPs analysis, the samples were prefiltered (using AP3004705, Millipore), preconcentrated by solid-phase extraction, and then preserved at 4 °C. The antibiotics, neuro drugs, and hormones concentrations were determined using liquid chromatography coupled to mass spectrometry (LC-MS-MS), whereas gas chromatography coupled to mass spectrometry (GC-MS) was utilized to measure the concentrations of anti-inflammatories, TCS, and BPA. OMPs samplings were carried out during Period I (days 57, 64, and 70) and Period II (141, 145, 147, and 149). Alvarino et al.23 provided a detailed description of the OMPs analysis. The microbial communities were characterized through 16S rRNA gene amplicon sequencing on days 70 (Period I) and 154 (Period II). The DNA extraction and bioinformatic analysis were conducted as indicated by Arias et al.24
3. Results and discussion
The reactor was operated for 154 d with temperatures ranging between 18 and 22 °C, and with a consistent feed flow of 138 ± 15 L d−1. The hydraulic retention times applied in the UASB and MBR post-treatment system were 21 ± 2 h and 12 ± 1 h, respectively. Internal and external recycling ratios (R) of 3.5 and 1, respectively, were applied during the study. The pH in the UASB and the anoxic compartment remained constant at 7.5 ± 0.5 and 7.6 ± 0.3, respectively. The pH of the aerobic compartment during Period II, 8.0 ± 0.1, was higher than that observed in Period I, 7.7 ± 0.1, probably due to a higher denitrifying activity. The MLVSS concentration measured in the anoxic compartment were 5.8 ± 0.4 and 4.8 ± 0.3 g MLVSS L−1 for Periods I and II, respectively.3.1 COD removal
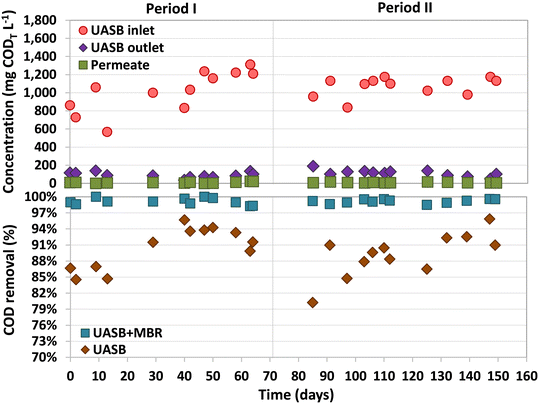
The average CODT and CODS of the inflow were 1046 ± 176 mg L−1 and 906 ± 180 mg L−1, respectively (Fig. 2). COD removal efficiencies above 98.2% were observed in the UASB-MBR system throughout the entire study, with most of the removal happening in the UASB, 90 ± 4% (Fig. 2). Analysis of the COD balances showed that 73% of the organic matter fed to the UASB was converted into CH4. The biogas produced had an average composition of 76 ± 4% CH4, 15 ± 3% CO2, and 8 ± 3% N2. During Period II, a trace amount of H2S (0.5%) was detected in the biogas, which was produced by SRB.The CODT and CODS concentrations in the UASB effluent were 103 ± 35 and 47 ± 23 mg L−1 during the entire study, respectively. These values exclusively account for the COD contribution derived from organic matter present in the UASB effluent, leaving out the COD contributions of dissolved methane and sulfide in this measurement. The fraction of organic matter not removed in the UASB reactor can be used as an electron donor to denitrify in the pre-anoxic compartment of the MBR. The MBR permeate contained only 11.9 ± 9.3 mg COD L−1 (Period I) and 7.5 ± 3.7 mg COD L−1 (Period II), with concentrations never exceeding 23 mg COD L−1 during the entire operation. It should be considered that the CODT and CODS values in the permeate are the same as no suspended solids can go through the ultrafiltration membrane. There was no noticeable difference in the permeate COD levels between Periods I and II, indicating that the presence of sulfide did not have an impact on the COD removal potential of the MBR.
3.2 Dissolved methane removal
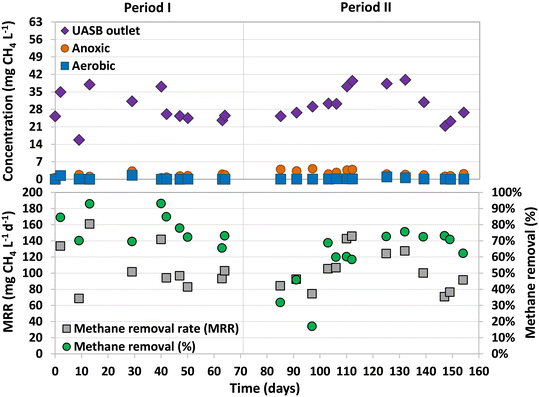
The dissolved methane concentration in the effluent of the UASB system was 24.4 ± 1.7 mg L−1 (Period I) and 28.4 ± 7.4 mg L−1 (Period II), making up 11.8 ± 1.1% and 13.9 ± 3.0% of total methane produced, respectively (Fig. 3). During Period I (days 42–70), an average dissolved methane removal efficiency of 74.8 ± 7.2% was achieved in the anoxic compartment. However, with the addition of sulfate into the feeding substrate, the removal efficiency considerably decreased in the early stages of Period II (days 70–97) to values ranging between 17% and 46%. The activity of methane oxidizers was found to be adversely affected by the initial presence of sulfide dissolved in the anoxic compartment. Over time, as the sulfide was completely oxidized by sulfur-oxidizing bacteria, the methane removal efficiencies recovered, reaching high and stable values from day 103 until the end of the study, 70.9 ± 5.1% (similar to Period I). The absence of dissolved sulfide in the anoxic compartment of the MBR from day 112 onwards was confirmed, but earlier dissolved sulfide concentrations were not measured.Similar methane removal rates (MRR)s were observed in the anoxic compartment for both periods (Fig. 3), 94.0 ± 7.3 (Period I) and 93.3 ± 23.4 mg CH4 L−1 d−1 (Period II). The dissolved methane that was not removed in the anoxic compartment could have either been released into the atmosphere through aeration or oxidized by aerobic methanotrophs in the subsequent aerobic and/or filtration compartments. During most of the operation, the concentration of dissolved methane in the aerobic compartment was either not detected or insignificant (Fig. 3).
These results are similar to the ones achieved in previous studies that used the old design of the UASB-MBR system (Fig. 1) and similar experimental conditions.8,10 Using the old design, Silva-Teria et al.8 accomplished a maximum MRR of 195 ± 17 mg CH4 L−1 d−1, doubling the values observed in the present study. The same experimental conditions were used, but higher feed flows were applied (355 L d−1). They found that the MRR was dependent on the feed flow rate, showing the best results with the highest feed flows. However, the methane removal efficiency was not influenced by the feed flow rate, but by the recirculation ratio between the aerobic and anoxic compartments. The lower feed flow rate used in the present study could explain the lower MRRs obtained.
During this study, it was possible that some of the dissolved methane was removed through AMO-D (eqn (1) and (2)) as dissolved oxygen was externally recirculated from the membrane filtration to the anoxic compartment. Assuming that all the recirculated oxygen was solely used in AMO-D, and considering eqn (1), methane removals up to 19.5 and 27 mg CH4 L−1 d−1 could be expected. This accounts for 21% and 29% of the total methane removal achieved during Periods I and II, respectively. The remaining removal of methane may be attributed to other methane oxidation processes, such as N-damo or, as suggested by Kalyuzhnaya et al.,25 to aerobic methane oxidizers that convert methane into fermentation products under oxygen-limited conditions.
3.3 Total nitrogen removal
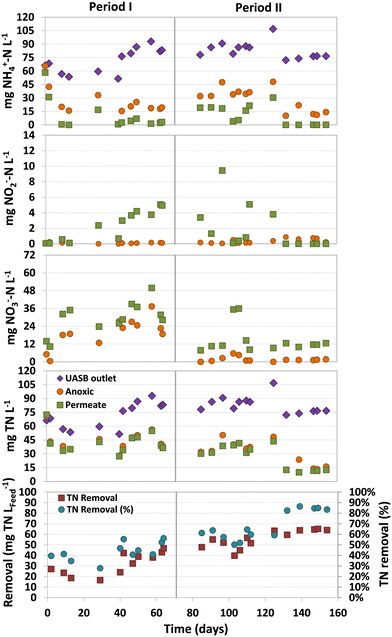
One of the challenges in treating domestic sewage in methanogenic bioreactors is the high levels of total nitrogen (TN) present in their effluents, mostly as ammonium. The limited availability of easily biodegradable organic matter exacerbates this issue, making it imperative to find alternative electron acceptors to improve denitrification. In this sense, the presence of either dissolved methane or sulphur compounds in the wastewater may be an alternative to obtain effluents with lower TN. However, the hydrogen sulfide produced in the UASB stage may also inhibit the activity of microbes involved in eliminating both nitrogen and dissolved methane.An average ammonium concentration of 71 ± 19 (Period I) and 84 ± 10 (Period II) mg NH4+–N L−1 was measured in the UASB (Fig. 4). Although low concentrations of ammonium were observed in the permeate by the end of Period I, complete aerobic ammonium oxidation was only achieved by the end of Period II. High ammonium concentrations were detected in the permeate at the beginning of Period II (days 85–125), up to 30 mg NH4+–N L−1. This accumulation could be attributed to the presence of dissolved sulfide in the UASB effluent, which are known to inhibit nitrifiers at low concentrations.18
Nitrate was the major ammonium oxidation product, and significant concentration variations were observed in both periods (Fig. 4). During Period I, higher concentrations of nitrate were found in both the anoxic compartment and permeate, with concentrations of 23.1 ± 6.7 and 33.2 ± 7.6 mg NO3−–N L−1, respectively. However, at the beginning of Period II, the nitrate concentration decreased significantly, especially in the anoxic compartment, with concentrations of only 1.9 ± 1.7 mg NO3−–N L−1. This remarkable lower nitrate concentration could be caused by the higher nitrate denitrification rate of SOB26 due to the presence of sulfide in the effluent of the anaerobic compartment. The observed nitrate in the permeate was usually below 15 mg NO3−–N L−1, which was lower than the concentration observed in Period I. The TN removals in the MBR during Period I (days 42–70) and Period II (days 132–154) were 40.2 ± 5.0 and 63.4 ± 2.2 mg TN LFeed−1, respectively (Fig. 4).
The increased TN removals in Period II can be attributed to the presence of dissolved sulfide in the UASB effluent (14.2 ± 4.0 mg S L−1), which could be used by SOB to denitrify. This implies that, apart from residual organic matter and dissolved methane, dissolved sulfide served as an electron donor for denitrification, as demonstrated by the considerable reduction in nitrate concentration compared to Period I. The observation suggests that the presence of dissolved sulfide boosted the denitrification capacity of the system, rather than inhibiting it.
It should be highlighted that nitrogen removals were not conducted only in the anoxic compartment of the MBR, but also in the aerobic compartment. This is demonstrated by the fact that the average TN concentration in the permeate was lower than that measured in the anoxic compartment (Fig. 4). The differences in TN concentrations between these two compartments were consistently detected. Through mass balances, it was estimated that 21 ± 14% (Period I) and 18 ± 6% (Period II) of the TN removal in the MBR was conducted in the aerobic compartment. This removal was likely achieved in the biofilm located in the aerobic compartment, as reported by other authors using a similar system.9 Most of the nitrogen was removed in the anoxic compartment, where nitrogen removal rates of 134.1 ± 28.8 (Period I) and 188.5 ± 10.2 mg N L−1 d−1 (Period II) were accomplished.
The TN concentration removed in this study, even during Period I without the SOB contribution (40.2 ± 5.0 mg TN LFeed−1), was much higher than that in previous studies with the old design of the UASB-MBR system, with 15 and 20 mg TN L−1.8,21 The enhanced denitrification potential observed with the new design was attributed to the presence of biofilm carriers in the aerobic compartment, where different denitrifying microorganisms can thrive.
To estimate the nitrogen that could be denitrified using the organic matter, dissolved methane, and dissolved sulfide measured in the effluent of the UASB, mass balances were conducted. Conventional heterotrophic denitrification requires approximately 4.98 or 2.98 g COD g−1 N, using nitrate or nitrite as an electron acceptor, respectively. By using stoichiometric analyses and considering that all the organic matter present in the UASB effluent was used to denitrify, up to 15.3 (Period I) and 14.1 (Period II) mg NO3−–N Lfeed−1, could be removed in the MBR system. This suggests that the organic matter content in the UASB effluent was not high enough to explain the observed total nitrogen removals. The methane dissolved in the UASB effluent could also have been used for denitrification in the MBR. In AMO-D processes using methanol as an intermediary electron donor, 0.95 g CH4 g−1 NO3−–N is theoretically required. By using stoichiometric reactions and if all the oxygen consumed in the anoxic compartment was used only for AMO-D, 3.5 (Period I) and 5.8 mg NO3−–N L−1 (Period II) could have been removed.
Moreover, the dissolved sulfide present in the UASB effluent (14.2 mg L−1) could have also played a significant role in the denitrification activity (eqn (3) and (4)). SOB was found to have fully oxidized the sulfide between days 112 and 154, as it was not detected in the anoxic and aerobic compartments. Based on eqn (4), it was estimated that 9.9 mg NO3−–N L−1 could have been denitrified in Period 2 by SOB. Considering nitrite as the electron donor (eqn (3)), this value could increase up to 16.5 mg NO2−–N L−1. The combined impact of conventional heterotrophic denitrification, AMO-D, and sulfide oxidation processes could result in a total estimated denitrification of 18.8 and 29.8 mg TN L−1 for Periods I and II, respectively. However, this capacity was still lower than the actual TN removal observed in Period I (40.2 mg TN L−1) and Period II (63.4 mg TN L−1).
Using the old UASB-MBR system prototype, Silva-Teira et al.8 achieved TN removals (10–15 mg N LFeed−1) significantly lower compared to this study. Using the same technology as Silva-Teira et al.,8 but with presence of nitrite and nitrate in the anoxic compartment, Alvarino et al.10 achieved higher nitrogen removals of 35 mg TN LFeed−1, but still lower than with the new UASB-MBR prototype. This highlights the importance of the extra aerobic compartment with suspended biofilm carriers added to the MBR.
In another study, Pelaz et al.7 proposed the use of a fixed film bioreactor filled with corrugated PVC rings to treat the effluent from an anaerobic membrane bioreactor containing methane, sulfide, low concentrations of organic matter, and nitrogen. That research proved the feasibility of using all these compounds as electron donors to remove around 73 mg TN L−1 at 18 °C and 2 h of HRT, a value which is higher than in the present study. However, the quality of their effluent, in terms of COD, was lower (58.6 mg COD L−1), which might be explained by the absence of an ultrafiltration membrane in the post-treatment system.
3.4 Organic micropollutants removal
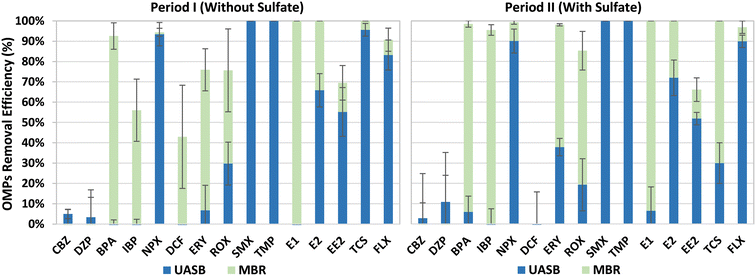
The OMPs removal efficiencies were calculated by using mass balances in both, the UASB and the MBR of the integrated system. Overall, high OMPs removal efficiencies were observed in the UASB-MBR system during the entire experimentation (Fig. 5), with no major differences between Periods I and II. This suggests that sulfate addition did not affect the OMPs removal efficiencies. NPX, SMX, TMP, and FLX were mainly removed in the UASB system with anaerobic conditions, while most of the BPA, IBP, and E1 removals were observed in the MBR post-treatment system. The removal of ERY, ROX, E2, EE2, and TCS was observed in the UASB and the MBR, indicating the key role of the MBR to obtain higher biotransformation efficiencies of most of the OMPs and particularly of those which were not affected by the UASB. CBZ, DZP, and DCF were barely removed during this study, indicating the recalcitrant nature of these OMPs against biological degradation. Similar OMPs removal efficiencies were observed in our previous study using the old UASB-MBR prototype (Fig. 1),10 and other studies using another type of integrated technologies combining different redox conditions.27 These results prove the robustness of this system to remove most of the OMPs.SMX, TMP, and NPX are easily biodegradable in anaerobic conditions.27 This explains why SMX and TMP antibiotics were entirely removed and why NPX, an anti-inflammatory drug, experienced significant biotransformation (>90%) during Periods I and II. FLX, a lipophilic compound, was also highly removed in the UASB reactor with anaerobic conditions, with efficiencies above 83% during both periods. The high removals observed can be explained by the fast sorption capacity of this neuro drug onto the granular sludge.28 Similar removals could be expected for another lipophilic micropollutant such as TCS, however, despite the high efficiencies attained during Period I (95.6%), an average value of only 30% was achieved for this disinfectant during Period II. The reason for this lower degradation observed in the last period was not elucidated. The remaining fraction of FLX and TCS present in the UASB effluent was almost or fully degraded in the subsequent MBR post-treatment system.
IBP and BPA were mainly removed in the MBR system with alternating anoxic and aerobic conditions. Most of the IBP removal was conducted most likely in the aerobic compartment since this anti-inflammatory is known to be easily biodegradable under aerobic conditions as reported by Suarez et al.29 The higher removals observed during Period II could be explained by the higher nitrification activities observed in the aerobic compartment of the MBR, compared to Period I. The BPA biodegradation could have also occurred under aerobic conditions through nitrification processes as reported by other authors.30,31 E1 and E2 were fully removed during both periods. E1 was mostly removed in the MBR, while E2 was removed jointly between the UASB and the MBR. The removal of BPA, a well-known endocrine disruptor, took place in the MBR, showing its recalcitrant nature under anaerobic conditions.
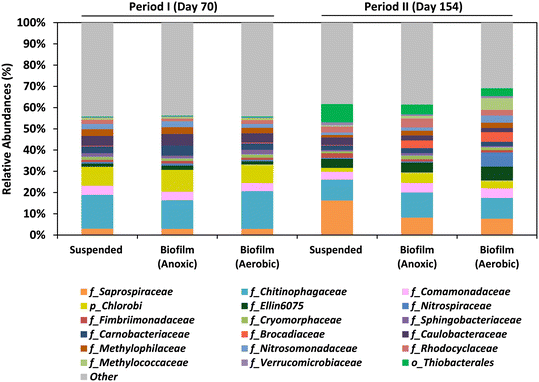
3.5 Microbial communities in the MBR
The study focused on both the suspended biomass in the MBR and the biofilm communities that adhered to the biochip carriers in the anoxic and aerobic compartments by the end of Period I (day 70) and Period II (day 154). The dominant bacteria found in the MBR system during both periods were members of the Chitinophagaceae and Saprospiraceae families, as well as the Chlorobi phylum (Fig. 6; Table S1†).Saprospiraceae are aerobic heterotrophs considered saprophytic microorganisms. The role of Chitinophagaceae and Chlorobi in the MBR remains unclear. The presence of the family Comamonadaceae (including heterotrophic denitrifiers), was confirmed in all the samples. The presence of the Comamonadaceae family (which includes heterotrophic denitrifiers) was confirmed in all samples and it is suggested that they utilized various organic compounds as electron donors for denitrification in the MBR.
Aerobic methanotrophs, mainly of the Methyloccoccaceae and Methylophilaceae families, were also found in all samples, with abundances ranging from 1.8 to 8.1%. The remarkable abundance of these bacteria in the adhered biomass of the aerobic compartment during both periods was detected, with abundances of 3.6% and 8.1% in Periods I and II, respectively. This fact confirms that dissolved methane was eliminated in both compartments of the MBR. The presence of N-damo bacteria which was detected in an analog system by FISH analysis8 was not detected in the present study for any of the samples.
The presence of anammox bacteria (Brocadiaceae), which can oxidize ammonium by reducing nitrite in anoxic conditions,32 was low in Period I (<0.4%) but higher values were observed in the adhered biomass in the anoxic (3.7%) and aerobic compartments (4.6%) during Period II. SOB, belonging to the Thiobacterales order, were abundant in all samples analyzed in Period II, with abundances ranging from 3.6 to 8.5%. This indicates that sulfide oxidation and denitrification processes were conducted in both compartments of the MBR. The SOB proliferation during Period II is explained by the presence of sulfide in the UASB effluent.
Anaerobic environments can be generated in the inner layers due to the existence of distinct dissolved oxygen concentration gradients within the biofilm carriers. This is a noteworthy feature of biofilm systems that could explain the observed nitrogen removal in the aerobic compartment. The co-occurrence of nitrification–denitrification processes in the Biochip underscores the importance of the supplementary aerobic compartment, which was added to improve methane and nitrogen removals compared to earlier studies.8,10
4. Conclusions
The presence of dissolved sulfide in the UASB effluent did not impact the COD and OMPs removals attained in the hybrid MBR post-treatment system. Initially, sulfide had a negative effect on the methane removals in the anoxic compartment. Once sulfide was fully oxidized into sulfate, the efficiencies were restored with values similar to those in Period I, without sulfide (>70%).The sulfide addition significantly improved the nitrogen removals achieved in the MBR, leading to 63.4 mg TN Lfeed−1 (Period II), compared to the 40 mg TN Lfeed−1 observed during Period I without sulfide. The higher removals in Period II can be attributed to the presence of SOB. Microbial analysis and mass balances indicate that not all the nitrogen removal occurred in the anoxic compartment (79%), but also in the aerobic one (up to 21%). Microbial analysis suggests that nitrogen removal in the MBR could have been achieved through a combination of AMO-D, heterotrophic denitrification, sulfide oxidation, and anammox processes, among others.
The TN removals in the MBR, even during Period I (without sulfide addition), doubled the values achieved in previous studies with a similar UASB-MBR system under similar operating conditions. The improvement observed with the new prototype is attributed to the addition of an extra aerobic compartment in the MBR, which enables the growth of denitrifying microbes in the inner layers of the suspended biofilm carriers within anoxic environments. This technological advance allowed remarkable denitrification activities in the MBR, especially when considering the low concentrations of COD found in the UASB effluent.
Abbreviations
| AMO-D | Aerobic methane oxidation coupled to denitrification |
| CODS | Soluble chemical oxygen demand |
| CODT | Total chemical oxygen demand |
| HRT | Hydraulic retention time |
| MBR | Membrane bioreactor |
| MLTSS | Mixed liquor total suspended solids |
| MLVSS | Mixed liquor volatile suspended solids |
| MRR | Methane removal rate |
| N-damo | Nitrate/nitrite-dependent anaerobic methane oxidation |
| N-damo archaea | Nitrate-dependent anaerobic methane-oxidizing archaea |
| N-damo bacteria | Nitrite-dependent anaerobic methane-oxidizing bacteria |
| OMP | Organic micropollutants |
| R | Recycling ratio |
| SOB | Sulfur-oxidizing bacteria |
| SRB | Sulfate-reducing bacteria |
| TN | Total nitrogen |
| UASB | Up-flow anaerobic sludge blanket |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the Ministry of Science, Innovation and Universities of Spain through the Antares project (PID2019-110346RB-C21). Tomás Allegue extends his appreciation to the Ministry of Economy and Competitiveness (Spain) for granting him a research scholarship (BES-2014-069114). The authors are part of the Galician Competitive Research Groups (GRC) ED431C-2021/37.References
- C. A. L. Chernicharo, Post-treatment options for the anaerobic treatment of domestic wastewater, Rev. Environ. Sci. Bio/Technol., 2006, 5, 73–92, DOI:10.1007/s11157-005-5683-5.
- C. A. L. Chernicharo, J. B. Lier, A. Noyola and T. B. Ribeiro, Anaerobic sewage treatment: state of the art, constraints and challenges, Rev. Environ. Sci. Bio/Technol., 2015, 14, 649–679, DOI:10.1007/s11157-015-9377-3.
- B. C. Crone, J. L. Garland, G. A. Sorial and L. M. Vane, Significance of dissolved methane in effluents of anaerobically treated low strength wastewater and potential for recovery as an energy product: A review, Water Res., 2016, 104, 520–531, DOI:10.1016/j.watres.2016.08.019.
- G. Myhre, D. Shindell, F. Bréon, W. Collins, J. Fuglestvedt, J. Huang, D. Koch, J. Lamarque, D. Lee, B. Mendoza and Others, Anthropogenic and natural radiative forcing, 2013, pp. 658–740, DOI:10.1017/CBO9781107415324.018.
- E. Foresti, M. Zaiat and M. Vallero, Anaerobic processes as the core technology for sustainable domestic wastewater treatment: Consolidated applications, new trends, perspectives, and challenges, Rev. Environ. Sci. Bio/Technol., 2006, 5, 3–19, DOI:10.1007/s11157-005-4630-9.
- D. Wang, Y. Wang, Y. Liu, H. H. Ngo, Y. Lian, J. Zhao, F. Chen, Q. Yang, G. Zeng and X. Li, Is denitrifying anaerobic methane oxidation-centered technologies a solution for the sustainable operation of wastewater treatment Plants?, Bioresour. Technol., 2017, 234, 456–465, DOI:10.1016/j.biortech.2017.02.059.
- L. Pelaz, A. Gómez, G. Garralón, A. Letona and M. Fdz-Polanco, Denitrification of the anaerobic membrane bioreactor (AnMBR) effluent with alternative electron donors in domestic wastewater treatment, Bioresour. Technol., 2017, 243, 1173–1179, DOI:10.1016/j.biortech.2017.06.168.
- A. Silva-Teira, A. Sánchez, D. Buntner, L. Rodríguez-Hernández and J. M. Garrido, Removal of dissolved methane and nitrogen from anaerobically treated effluents at low temperature by MBR post-treatment, Chem. Eng. J., 2017, 326, 970–979, DOI:10.1016/j.cej.2017.06.047.
- T. Allegue, M. N. Carballo-Costa, N. Fernández-González and J. M. Garrido, Simultaneous nitrogen and dissolved methane removal from an upflow anaerobic sludge blanket reactor effluent using an integrated fixed-film activated sludge system, J. Environ. Manage., 2020, 263, 110395, DOI:10.1016/j.jenvman.2020.110395.
- T. Alvarino, T. Allegue, N. Fernandez-Gonzalez, S. Suarez, J. M. Lema, J. M. Garrido and F. Omil, Minimization of dissolved methane, nitrogen and organic micropollutants emissions of effluents from a methanogenic reactor by using a preanoxic MBR post-treatment system, Sci. Total Environ., 2019, 671, 165–174, DOI:10.1016/j.scitotenv.2019.03.169.
- T. Allegue, A. Arias, N. Fernandez-Gonzalez, F. Omil and J. M. Garrido, Enrichment of nitrite-dependent anaerobic methane oxidizing bacteria in a membrane bioreactor, Chem. Eng. J., 2018, 347, 721–730, DOI:10.1016/j.cej.2018.04.134.
- O. Modin, K. Fukushi and K. Yamamoto, Denitrification with methane as external carbon source, Water Res., 2007, 41, 2726–2738, DOI:10.1016/j.watres.2007.02.053.
- M. F. Haroon, S. Hu, Y. Shi, M. Imelfort, J. Keller, P. Hugenholtz and Z. Yuan, Anaerobic oxidation of methane coupled to nitrate reduction in a novel archaeal lineage, Nature, 2013, 2–8, DOI:10.1038/nature12375.
- K. F. Ettwig, M. K. Butler, D. Le Paslier, E. Pelletier, S. Mangenot, M. M. M. Kuypers, F. Schreiber, B. E. Dutilh, J. Zedelius, D. de Beer, J. Gloerich, H. J. C. T. Wessels, T. van Alen, F. Luesken, M. L. Wu, K. T. van de Pas-Schoonen, H. J. M. Op den Camp, E. M. Janssen-Megens, K.-J. Francoijs, H. Stunnenberg, J. Weissenbach, M. S. M. Jetten and M. Strous, Nitrite-driven anaerobic methane oxidation by oxygenic bacteria, Nature, 2010, 464, 543–548, DOI:10.1038/nature08883.
- J. Delgado Vela, L. B. Stadler, K. J. Martin, L. Raskin, C. B. Bott and N. G. Love, Prospects for biological nitrogen removal from anaerobic effluents during mainstream wastewater treatment, Environ. Sci. Technol. Lett., 2015, 2, 234–244, DOI:10.1021/acs.estlett.5b00191.
- J. E. Sánchez-Ramírez, A. Seco, J. Ferrer, A. Bouzas and F. García-Usach, Treatment of a submerged anaerobic membrane bioreactor (SAnMBR) effluent by an activated sludge system: The role of sulphide and thiosulphate in the process, J. Environ. Manage., 2015, 147, 213–218, DOI:10.1016/j.jenvman.2014.04.043.
- S. A. Saad, L. Welles, C. M. Lopez-Vazquez, M. C. M. van Loosdrecht and D. Brdjanovic, Sulfide effects on the anaerobic metabolism of polyphosphate-accumulating organisms, Chem. Eng. J., 2017, 326, 68–77, DOI:10.1016/j.cej.2017.05.074.
- D. I. Bejarano Ortiz, F. Thalasso, F. D. M. Cuervo López and A. C. Texier, Inhibitory effect of sulfide on the nitrifying respiratory process, J. Chem. Technol. Biotechnol., 2013, 88, 1344–1349, DOI:10.1002/jctb.3982.
- Y. Pan, L. Ye and Z. Yuan, Effect of H2S on N2O reduction and accumulation during denitrification by methanol utilizing denitrifiers, Environ. Sci. Technol., 2013, 47, 8408–8415, DOI:10.1021/es401632r.
- D. Buntner, A. Sánchez and J. M. Garrido, Feasibility of combined UASB and MBR system in dairy wastewater treatment at ambient temperatures, Chem. Eng. J., 2013, 230, 475–481, DOI:10.1016/j.cej.2013.06.043.
- A. Sánchez, L. Rodríguez-Hernández, D. Buntner, A. L. Esteban-García, I. Tejero and J. M. Garrido, Denitrification coupled with methane oxidation in a membrane bioreactor after methanogenic pre-treatment of wastewater, J. Chem. Technol. Biotechnol., 2016, 91, 2950–2958, DOI:10.1002/jctb.4913.
- American Public Health Association, Standard Methods for the Examination of Water and Wastewater, ed. E. W. Rice, R. B. Baird, A. D. Eaton and L. S. Clesceri, American Public Health Association, American Water Works Association, Water Environment Federation, 22nd edn, 2012 Search PubMed.
- T. Alvarino, S. Suarez, E. Katsou, J. Vazquez-Padin, J. M. Lema and F. Omil, Removal of PPCPs from the sludge supernatant in a one stage nitritation/anammox process, Water Res., 2015, 68, 701–709, DOI:10.1016/j.watres.2014.10.055.
- A. Arias, T. Allegue, A. Darwich, M. Fachal-Suárez, M. Martínez-Quintela, F. Omil and J. M. Garrido, Operating strategies to optimize a membrane bioreactor enriched in nitrite-dependent anaerobic methane-oxidizing bacteria, Chem. Eng. J., 2022, 450 DOI:10.1016/j.cej.2022.138289.
- M. G. Kalyuzhnaya, S. Yang, O. N. Rozova, N. E. Smalley, J. Clubb, A. Lamb, G. A. N. Gowda, D. Raftery, Y. Fu, F. Bringel, S. Vuilleumier, D. A. C. Beck, Y. A. Trotsenko, V. N. Khmelenina and M. E. Lidstrom, Highly efficient methane biocatalysis revealed in a methanotrophic bacterium, Nat. Commun., 2013, 4, 1–7, DOI:10.1038/ncomms3785.
- J. Reyes-Avila, E. Razo-Flores and J. Gomez, Simultaneous biological removal of nitrogen, carbon and sulfur by denitrification, Water Res., 2004, 38, 3313–3321, DOI:10.1016/j.watres.2004.04.035.
- T. Alvarino, S. Suárez, M. Garrido, J. M. Lema and F. Omil, A UASB reactor coupled to a hybrid aerobic MBR as innovative plant configuration to enhance the removal of organic micropollutants, Chemosphere, 2016, 144, 452–458, DOI:10.1016/j.chemosphere.2015.09.016.
- W. Xue, C. Wu, K. Xiao, X. Huang, H. Zhou, H. Tsuno and H. Tanaka, Elimination and fate of selected micro-organic pollutants in a full-scale anaerobic/anoxic/aerobic process combined with membrane bioreactor for municipal wastewater reclamation, Water Res., 2010, 44, 5999–6010, DOI:10.1016/j.watres.2010.07.052.
- S. Suarez, J. M. Lema and F. Omil, Removal of Pharmaceutical and Personal Care Products (PPCPs) under nitrifying and denitrifying conditions, Water Res., 2010, 44, 3214–3224, DOI:10.1016/j.watres.2010.02.040.
- P. Guerra, M. Kim, S. Teslic, M. Alaee and S. A. Smyth, Bisphenol-A removal in various wastewater treatment processes: Operational conditions, mass balance, and optimization, J. Environ. Manage., 2015, 152, 192–200, DOI:10.1016/j.jenvman.2015.01.044.
- S. Yang, F. I. Hai, W. E. Price, J. McDonald, S. J. Khan and L. D. Nghiem, Occurrence of trace organic contaminants in wastewater sludge and their removals by anaerobic digestion, Bioresour. Technol., 2016, 210, 153–159, DOI:10.1016/j.biortech.2015.12.080.
- M. Strous, J. G. Kuenen and M. S. M. Jetten, Key Physiology of Anaerobic Ammonium Oxidation Key Physiology of Anaerobic Ammonium Oxidation, Appl. Environ. Microbiol., 1999, 65, 3, DOI:10.1128/AEM.65.7.3248-3250.1999.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ew00404j |
| This journal is © The Royal Society of Chemistry 2023 |