Deploying a molecular copper catalyst for efficient degradation of commercial and industrial dyes under practical conditions†
Abstract
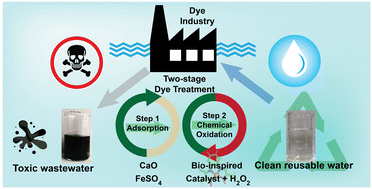
Water pollution due to the discharge of inadequately treated contaminated water from industry creates an ecological imbalance posing several health hazards leading to the depletion of aquatic flora and fauna. Therefore, an immediate solution pertaining to stringent action and awareness is warranted to preserve natural water bodies from industrial effluent. Here, we report a two-step method for wastewater treatment, wherein the first step deploys an adsorption-based dye-treatment method (coagulation and flocculation). In contrast, the second step enforces chemical oxidation induced via a combination of a bio-inspired molecular copper complex and H2O2. This catalytic unit efficiently degrades a versatile array of toxic industrial dyes, with varying molecular templates, present in aqueous solution at room temperature. The dye treatment process was primarily monitored via optical spectroscopy as decolouration of the solution indicated oxidative degradation of the dye molecules. A complementary gas chromatography experiment established that CO2 gas is produced as the major product of this process. Detailed mechanistic studies revealed that the chemical process proceeds via the formation of a Cu(III)-hydroxo intermediate, which actively destroys the aromatic backbone of the dyes without following traditional radical-based Fenton's chemistry. This two-step process was active over a broad pH range (pH 3–11) of aqueous solutions, while it exhibited excellent efficiency even during the degradation of actual industrial dyes in their original form without any pre-treatment. The capability of this dye-treatment process was successfully tested at 100 liter scale with industrial dyes, showcasing the high technology readiness level (TRL) of this process.
- This article is part of the themed collection: Outstanding Papers 2023 – Environmental Science: Water Research & Technology


 Please wait while we load your content...
Please wait while we load your content...