Emerging investigator series: microplastic-based leachate formation under UV irradiation: the extent, characteristics, and mechanisms†
Abstract
Microplastics in the aquatic system are among the many inevitable consequences of plastic pollution, which has cascading environmental and public health impacts. Our study aimed at analyzing surface interactions and leachate production of six microplastics under ultraviolet (UV) irradiation. Leachate production was analyzed for the dissolved organic content (DOC), UV254, and fluorescence through excitation emission (EEM) to determine the kinetics and mechanisms involved in the release of organic matter by UV irradiation. The results suggested there was a clear trend of organic matter being released from the surface of the six microplastics caused by UV irradiation based on DOC, UV254 absorbance, and EEM intensity increasing with time. Polystyrene had the greatest and fastest increase in DOC concentrations, followed by the resin coated polystyrene. Experiments conducted at different temperatures indicated the endothermic nature of these leaching mechanisms. The differences in leachate formation for different polymers were attributed to their chemical makeup and their potency to interact with UV. The aged microplastic samples were analyzed by Fourier-transform infrared spectroscopy (FT-IR), Raman, and X-ray photoelectron spectroscopy (XPS), to determine the surface changes with respect to leachate formation. Results indicated that all microplastics had increasing carbonyl indices when aged by UV with polystyrene being the greatest. These findings affirm that the leachate formation is an interfacial interaction and could be a significant source of organic compound influx to natural waters due to the extremely abundant occurrence of microplastics and their large surface areas.
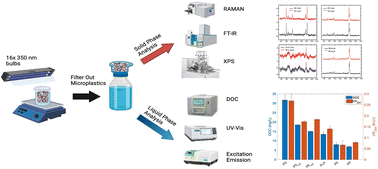
- This article is part of the themed collections: Outstanding Papers 2023 – Environmental Science: Water Research & Technology and Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...