Structure and energetics of hydrogen bonding networks in dilute HOD/H2O solutions confined in silica nanopores†
Abstract
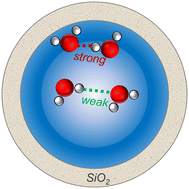
Hydrogen-bonding (H-bonding) networks in liquid H2O define its reactivity and physico-chemical properties. In nanopores, H-bonding is not easily predictable due to the competing effects on water structure from the opposing surfaces. Here we explore H-bonding under nanoconfinement in silica nanopores. We use isotopically dilute deuterated water to exclude intermolecular oscillator coupling effects, and utilize temperature-controlled Raman spectroscopy, water sorption measurements, and molecular dynamics simulations to decipher H-bonding structures and energetics. We found two opposite trends for H-bonding under nanoconfinement: in the interfacial binding layers at the silica surfaces nanoconfinement increases H-bond strength, while in the body of the nanopore H-bond strength is decreased in comparison to bulk water. The existence of two populations of water in nanopores could lead to the contrasting reactivity trends under nanoconfinement for the chemical species residing near pore surfaces versus those residing in the middle of the pore. In the near-interfacial regions, strong H-bonding environments affect reactions involving charge transfer and solvation; alternatively, in the middle of the pore, weakened H-bonding interactions could decrease de-solvation costs and shift equilibrium constants. The observed H-bond weakening in SiO2 nanopore body may also indicate a decrease in the H2O liquid–vapor phase transition temperature.
- This article is part of the themed collection: Nanomaterial applications in water


 Please wait while we load your content...
Please wait while we load your content...