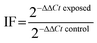Open Access Article
Open Access ArticleOrigin, exposure routes and xenobiotics impart nanoplastics with toxic effects on freshwater bivalves†
Adeline
Arini
a,
Sandra
Muller
a,
Véronique
Coma
b,
Etienne
Grau
b,
Olivier
Sandre
 *b and
Magalie
Baudrimont
*b and
Magalie
Baudrimont
 *a
*a
aUniv. Bordeaux, CNRS, Bordeaux INP, EPOC, UMR 5805, F-33600 Pessac, France. E-mail: magalie.baudrimont@u-bordeaux.fr
bUniv. Bordeaux, CNRS, Bordeaux INP, LCPO, UMR 5629, F-33600 Pessac, France. E-mail: olivier.sandre@u-bordeaux.fr
First published on 15th March 2023
Abstract
Various environmentally aged plastic wastes were collected from the environment and crushed to the submicronic scale to get a mix of nanoplastics (NPs) of different natures – mostly polyolefins (PE, PP), polyesters (PET), polyvinylics (PS and PVC) and undefined shapes (denoted NP-L, mean hydrodynamic diameter of 285 nm). We aimed to test the toxic effects of NPs of environmental relevance on freshwater bivalves and compare results to commonly used styrenic NP-PS (206 nm). Corbicula fluminea was exposed to four different conditions with NPs (0.008 to 10 μg L−1), for 21 days and kept under depuration conditions for 21 additional days: 1) waterborne exposure to NP-L (from the Leyre River), 2) diet-borne exposure to NP-L, 3) synergic waterborne exposure to NP-L and AlCl3 salt (1 mg L−1), and 4) waterborne exposure to NP-PS. Enzyme activities and gene expressions were assessed and behavioural tests were performed. Trophic and synergic exposure to Al3+ ions triggered more gene modulations than direct exposure to NP-L (namely on 12s, atg12, gal, segpx, p53 and ache). NP-PS were also more harmful than NP-L, but only at high concentrations (10 μg L−1). The effects of each treatment lasted until 7 days of depuration and no more gene inductions were observed after 21 days in clean water. Very few effects were shown on phenol-oxidase (PO), and glutathione S-transferase (GST). However, the inhibition of acetylcholinesterase (AchE) was concomitant with an increase of the filtration activity of bivalves exposed to NP-L (trophic route) and NP-PS, suggesting neurotoxic effects. By disturbing the ventilatory activity, NPs could have direct effects on xenobiotic accumulation and excretion capacities. The results point out how the structure, aging, exposure routes and additional xenobiotics can interact with adverse outcomes of NPs in bivalves. These findings underline the importance of considering naturally aged environmental NPs in ecotoxicological studies rather than synthetic latexes, i.e. crosslinked nanospheres prepared from virgin polymers. This manuscript presents the first data of toxic effects on freshwater organisms exposed to nanoplastics coming from natural sources. Whereas the majority of papers have dealt with non-environmentally representative plastics (mainly commercially available polystyrene latexes) to evaluate nanoplastic effects on organisms, this study develops methods to prepare model nanoplastics from plastic wastes collected from rivers, and to assess their real adverse effects on aquatic organisms. Significant differences were found between the inflammatory effects triggered by nanoplastics coming from natural sources and polystyrene nanobeads. This work suggests that the data published so far in the literature may underestimate the toxicity of nanoplastics spread in the environment on the aquatic organisms at the bottom of the food chain, which might consequently impact halieutic resources in the long term.
Environmental significanceWithin the size continuum of plastic debris, nanoplastics (NPs) are potentially the most worrying due to their small size and difficulties of being detected and quantified in complex bio-organic matrices. Therefore it is crucial to assess the environmental fate and behavior of NPs in terms of bioavailability and toxicity towards aquatic organisms according to their origin, morphology, oxidation state, and the presence of additional xenobiotics such as metals. In the present study, we test different exposure routes on Corbicula fluminea, types of NPs (environmentally aged vs. virgin polymers) and biological readouts, such as filtration behaviour, gene expression and enzymatic activities, to get an integrative view of NPs' toxic effects on these freshwater bivalves at environmentally realistic concentrations. |
Introduction
The increasing production of plastics has resulted in millions of tons of plastic wastes spread in the environment. Large plastic debris can break down into micro- and nanometric particles. Several definitions exist to define nanoparticles of plastics, commonly called “nanoplastics” (NPs). They correspond to plastic particles whose size ranges between 1 and 1000 nm, resulting mainly from the degradation of larger plastic particles and having a colloidal behaviour in the environment.1–3 In addition to the degradation of plastic debris, another important source of NPs arises from textile fibres rejected by washing machines and the inability of wastewater treatment plants to efficiently filter all these particles before releasing water into the aquatic environment.4Characterizing these particles at the nanometric scale and at very low concentrations in the environment requires solving several analytical issues. One of the biggest challenges lies in the lack of methods for the quantification of NPs, most of which are at their infancy, making it almost impossible to obtain real concentrations of NPs in the environment.5–7 It is thus difficult even now to know the sources of emission, and the fate and responsiveness of NPs.5 Therefore, ecotoxicology studies carried out on NP toxicity coming from the environment are still very scarce.
Owing to their nanoscale size, NPs' properties greatly differentiate them from the rest of plastic wastes.6 The hydrophobicity of NPs added to their important surface/volume ratio facilitates their interactions with system components such as organic matter, microorganisms, dissolved metals and organic pollutants,7 as well as the adsorption of hydrophobic organic and inorganic compounds present in the environment.8–10 As a consequence, NPs can act as vectors of contamination in the environment and even in aquatic organisms, which ingested or absorbed them, since they can cross biological barriers (cells, tissues, etc.).11
Several studies have reported their toxic effects, in particular, on growth, locomotion, and energetic metabolism, associated with the production of reactive oxygen species (ROS) that can lead to death.12–16 These important interactions with aquatic life suggest that NPs could impact the functioning of ecosystems. However, to date, more than 95% (source Scopus, publications from 2017–2022) of studies refer to only one type of commercial NP: polystyrene (PS) in the form of commercial latexes, which are spherical and chemically crosslinked particles, without taking into account the variability of the plastic polymers produced and released into the environment.1,17–20
Moreover, most of the studies in ecotoxicology conducted to date have been performed at unrealistic doses of NP exposure, in the range of mg L−1.1 In addition, NPs are not only potentially toxic to aquatic life, but they can also act as carriers of chemicals, which may modify their behaviour and their reactivity and induce the release of contaminants farther away from the original sources. Coupling NPs with other types of contaminants is a very innovative approach since no data exist so far about the potential synergistic effects of NPs with associated contaminants in freshwater species. It is also worth noting that depuration phases are rarely included in experimental studies and very scarce data exist about the recovery capacities of organisms.21
Our study aimed to use plastic wastes collected by volunteers from the Leyre River (South-West of France), because it is of great ecological, touristic, and economic importance. The intense nautical activities in summer can be sources of various plastic wastes in the Leyre, potentially carrying adsorbed contaminants. Different collected plastic wastes were mixed and crushed to the nanometric scale before being used under laboratory conditions to mimic environmental NPs.
The objectives of this study were to assess the toxicity of these “environmental NP” suspensions and compare them with nanoparticles made from virgin polystyrene (NP-PS) commonly used in the literature. We tested different contamination routes (water- and diet-borne exposures) on a freshwater bivalve, Corbicula fluminea. We used environmentally realistic concentrations (0.008 to 10 μg L−1),22,23 with or without synergetic contamination by aluminium cations (Al3+, abbreviated Al) naturally found in the Leyre river from the leaching of aluminosilicate minerals. Different outcomes were tested, from molecular to individual levels, to get an integrative overview of the NPs' toxicity. The exposure was followed by a depuration phase to explore the recovery capacities of bivalves after the different exposure treatments.
This study should bring the first outcomes about the toxicity of environmentally realistic NPs on a freshwater species, and help understand to which extent NPs' toxicity may depend on the exposure route and the potential interference with associated contaminants. It also investigates for the first time the recovery capacities of bivalves after different NP exposures, at the gene and enzymatic levels.
Methods
Nanoplastic preparation and characterization
Plastic macro-wastes were collected at different sites on the banks of the Leyre River (South-West of France) in July 2020, by the citizen association “La Pagaie Sauvage” (https://lapagaiesauvage.org). After alkaline treatment (KOH 3 M for 48 h at 20 °C) to remove any adsorbed biological organic matter, a random mix of plastic wastes was crushed into small pieces in a mill grinder (IKA-A10) filled with liquid nitrogen to make the plastics more brittle. The millimetric powder was then crushed in liquid nitrogen (centrifugal grinder Retsch ZM 200) and sifted using a final 80 μm fine sieve. The fine powder was resuspended in MilliQ water and sonicated for 10 min with an ultrasound bath. The suspension was filtered with glass-fibre syringe pre-filters (Nalgene, pore size of 1 μm) to obtain a nanoscale suspension called NP-L.Polystyrene pellets were purchased from Sigma Aldrich (CAS 9003-53-6, Mw = 200 kg mol−1, Đ = Mw/Mn = 2.2 according to size exclusion chromatography, see Fig. S1†). PS pellets were dissolved in tetrahydrofuran solvent (THF purchased from VWR, CAS 109-99-9) (10 mg PS per mL THF) for 10 min under agitation. Then, MilliQ water (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 volume ratio) was added under stirring to perform the “solvent shift” or “nanoprecipitation” process of PS chains into nanoparticles, and left under agitation for 2 h at 50 °C, according to the standard procedure as in Schubert et al.24 The nanoprecipitated PS suspension was sonicated for 10 min in an ultrasound bath and filtered with 1 μm glass-fibre syringe pre-filters to obtain a nanoscale suspension called NP-PS. The suspension was left open under a hood for one week before use, to enable total evaporation of the THF solvent. Analysis by GC-MS was performed on the filtrate of an aliquot of the NP-PS suspension, indicating no detectable residual presence of THF inside the sample (data not shown).
1 volume ratio) was added under stirring to perform the “solvent shift” or “nanoprecipitation” process of PS chains into nanoparticles, and left under agitation for 2 h at 50 °C, according to the standard procedure as in Schubert et al.24 The nanoprecipitated PS suspension was sonicated for 10 min in an ultrasound bath and filtered with 1 μm glass-fibre syringe pre-filters to obtain a nanoscale suspension called NP-PS. The suspension was left open under a hood for one week before use, to enable total evaporation of the THF solvent. Analysis by GC-MS was performed on the filtrate of an aliquot of the NP-PS suspension, indicating no detectable residual presence of THF inside the sample (data not shown).
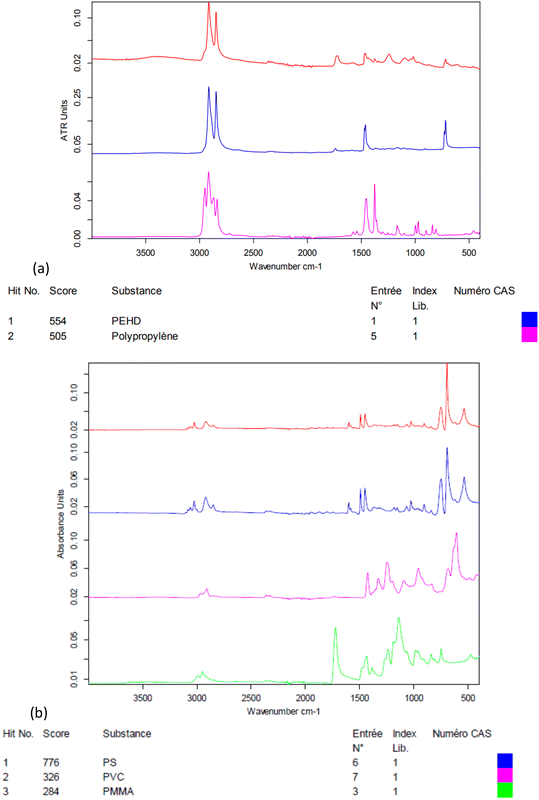
The electrokinetic zeta-potential of particles was measured by phase analysis light scattering zetametry (PALS, Malvern NanoZS) to determine the surface charge of NPs. The hydrodynamic size of the particles was assessed by dynamic light scattering (DLS, Vasco™ Flex instrument from Cordouan technologies, Pessac, France), working at a backscattering angle of 165° and using both the cumulant fitting method and the Pade–Laplace multimodal analysis (Fig. S2†). NP suspensions were imaged by transmission electron microscopy (TEM) with samarium acetate staining with a Hitachi H7650 electron microscope operated at 80 kV. The weight concentrations of NPs in the suspensions (dry extracts) were measured by thermogravimetry analysis (TGA, Q50, TA Instruments, Fig. S4†). The polymers present in NP suspensions were analysed by Fourier-transform infrared spectroscopy (FTIR, Bruker Vertex 70, Fig. 1).
Corbicula fluminea collection and acclimation
Around 300 Asiatic clams Corbicula fluminea were collected in a small tributary of the Isle River in Montpon-Ménestérol, considered as clean. Clams were kept in clean water for 2 weeks for acclimation, until used for experiments. Clams were fed with the green algae Scenedesmus subspicatus (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per individual per L) every other day.
000 cells per individual per L) every other day.
Experimental design
Glass vessels were filled with 1 L of dechlorinated tap water, at 15 °C, and oxygenated with aeration pumps two days before the start of the experiment. Light was provided by artificial neon lamps (photoperiod 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12). The day before the start of the experiment, 8 clams were introduced in each experimental unit to get used to the experimental conditions.
12). The day before the start of the experiment, 8 clams were introduced in each experimental unit to get used to the experimental conditions.
The day when the experiment started, contaminants were spiked directly into tanks intended for waterborne exposures. Three concentrations of NP-L and NP-PS were used: 0.008, 1, and 10 μg L−1. The concentrations were chosen according to published data of microplastic concentrations in deep sea and oceanic gyres,22,23 since no data exist so far for NP concentrations in the environment. Clams were exposed to NP-L with or without an additional contaminant: aluminium salt (AlCl3), at 1 mg L−1.
Clams dedicated to diet-borne exposures were fed with algae solutions that had been contaminated with 0.008, 1 or 10 μg L−1 NP-L under agitation, 48 h before the start of exposure. The day of the start of the experiment, contaminated algae were introduced into the experimental units (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per individual per L).
000 cells per individual per L).
Clams dedicated to waterborne exposures were fed with Scenedesmus subspicatus (70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per individual per L) 2 h before the exposure started, to avoid contamination by the trophic route once NPs were spiked into the experimental units.
000 cells per individual per L) 2 h before the exposure started, to avoid contamination by the trophic route once NPs were spiked into the experimental units.
There were four experimental replicates per condition, according to the following denominations:
– Controls
– Waterborne exposure to NPs from the Leyre River: NP-L
– Waterborne exposure to PS: NP-PS
– Synergic waterborne exposure to NPs and Al: Al + NP-L
– Waterborne exposure to Al: Al
– Diet-borne exposure to Scenedesmus subspicatus contaminated with NP-L: diet NP-L
The temperature, Al concentrations and water levels were checked daily to be adjusted, if necessary. Under the waterborne conditions, clams were fed every other day, 2 hours before the water was changed. The dechlorinated water was renewed every other day in each tank, and a contaminant was added after water renewal (either spiked contaminants or contaminated algae) to be kept as close as possible to nominal concentrations.
No mortality was observed during the experiment. Clams were sacrificed after 7 (T7) and 21 days (T21) of exposure to NPs and/or Al, and after 7 (D7) and 21 days (D21) of depuration. The gills and visceral mass were dissected. Organs from two individuals were pooled to get enough mass, and split for different analyses: Al bioaccumulation, enzyme activities and gene expression. The gills and visceral mass dedicated to transcriptomic and enzymatic tests were stored at −80 °C before analysis. Bioaccumulation and enzyme activities were analysed on samples from T7, T21 and D7 (since no significant differences were shown after 7 days of depuration), whereas transcriptomic analyses were continued until D21.
Aluminium bioaccumulation
Samples collected for Al bioaccumulation were dried at 40 °C for 48 h before mineralization (1 mL of nitric acid at 100 °C for 3 hours). A volume of 6 mL of ultrapure water was added to digestates and samples were stored at 4 °C before analysis. Aluminium was analysed by atomic absorption spectrometry (AAS, 240Z AA graphite furnace spectrometer, Agilent Technologies). The validity of the aluminium titration method was periodically checked with an internal laboratory certified reference material of the Al element.Enzyme assays
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 15 min to retrieve the supernatant used for assays. Protein concentrations were measured using the Bradford assay. Briefly, 5 μL of each homogenized sample were added to 250 μL of Coomassie blue reagent. The samples were incubated for 10 minutes at room temperature before the absorbance was read on a microplate spectrometer (BioTek EPOCH) at 620 nm. A standard curve was built using BSA (bovine serum albumin) dilutions from 0.1 to 1000 μg mL−1. The slope of the standard curve was used to calculate the protein concentration of samples. A sample concentration of 300 μg mL−1 was used for each enzymatic assay (4 analytical replicates per sample).
000 rpm for 15 min to retrieve the supernatant used for assays. Protein concentrations were measured using the Bradford assay. Briefly, 5 μL of each homogenized sample were added to 250 μL of Coomassie blue reagent. The samples were incubated for 10 minutes at room temperature before the absorbance was read on a microplate spectrometer (BioTek EPOCH) at 620 nm. A standard curve was built using BSA (bovine serum albumin) dilutions from 0.1 to 1000 μg mL−1. The slope of the standard curve was used to calculate the protein concentration of samples. A sample concentration of 300 μg mL−1 was used for each enzymatic assay (4 analytical replicates per sample).
| PO specific activity (U mg−1 protein) = (absorbance per min × dilution factor)/total protein concentration |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) of reduced L-glutathione (GSH, 2.225 mM in PB) and 1-chloro-2,4-dinitrobenzene (CDNB, 38 mM in ethanol) was prepared.
1) of reduced L-glutathione (GSH, 2.225 mM in PB) and 1-chloro-2,4-dinitrobenzene (CDNB, 38 mM in ethanol) was prepared.
180 μL of the GSH/CDNB mixture were added to each well right before reading the absorbance every 60 seconds, for 10 minutes at 340 nm. 20 μL of Tris buffer were added instead of samples, as a negative control. The specific GST activity (nmol min−1 per mg of protein) was calculated according to the following formula:
| GST = (ΔOD × reaction volume/(ε × L))/protein concentration |
| AchE = (ΔOD × (reaction volume/ε × L))/protein concentration |
Transcriptomic analyses
Real-time PCR was performed to assess the differential gene expression in gills and visceral mass of clams. An RNA isolation system kit (SV total, Promega) was used to extract the total mRNA, according to the manufacturer's instructions. The mRNA concentration was measured in a Take3 plate with a microplate spectrometer (BioTek EPOCH). The RNA purity was checked by comparing the ratio between values measured at 260 and 280 nm and ensured when the ratio was over 2. The RNA samples were diluted to a concentration of 100 ng μL−1. They were used for reverse transcription (RT), using a RT system kit (GoScript, Promega). RT was performed in an Eppendorf Mastercycler™ to synthesize copy DNAs. Samples were kept at −20 °C until their use for qPCR amplifications.Quantitative polymerase chain reaction (qPCR) was performed in a LightCycler 480 (Roche) using a GoTaq® qPCR Master Mix kit (Promega) in 384-well plates. A mix of primer pair was prepared for each gene (5 μM, Table 1). 1 μL of the pair mix, 12.5 μL of the qPCR Master Mix mixed with 6.5 μL of RNA free water, and 5 μL of cDNA were added to each well. The qPCR program started by heating the samples at 95 °C for 10 min and then ran 40 cycles as follows: 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 30 s. The samples were gradually heated from 60 to 95 °C to get a dissociation curve and check for amplification specificity. Two reference genes were used to normalize gene expression: β-actin and rpl7 (according to the GeNorm method). The differential gene expression was calculated using the 2−ΔΔCt method described by Livak and Schmittgen.26 The gene expression was expressed as induction factors compared to controls, according to the following formula:
Nineteen genes involved in endocytosis, detoxification, respiratory chain, DNA repair, oxidative stress, apoptosis, neurotransmission and reproduction were assessed (Table 1).
| Function | Gene | Name | Specific primers (5′–3′) |
|---|---|---|---|
| a Forward primer. b Reverse primer. | |||
| Reference | β-actin | Beta-actin | GATGGATGGTCCAGACTCGTa |
| GGTCTGGATCGGTGGTTCTAb | |||
| rpl7 | P18124 | CACCATCGTTGAAGTGGTTGa | |
| CTTCAAACAGGCTGCCAACTb | |||
| Endocytosis | cltl | Clathrin light chain 1 | GCAAACATCACACAGGTTGCa |
| CGTGCGTAGTTGGACACATTb | |||
| cav | Caveolin | GTCCAGCACCATACGTGGTTa | |
| AACTGTTGACCACCCTCTGTGb | |||
| Detoxification | mt | Metallothionein | CGGCTATCTCCCGCGAa |
| AGCTTTTACCAGAACCAAACAGTb | |||
| Mxr | Multixenobiotic resistance | ATTCTTCTGCTGGACGAGGCa | |
| ACATGTTCTCTACCCGCCTGTGb | |||
| Respiratory chain | cox1 | Cytochrome C oxidase I | CCTGTTTGGAGAAAGGGTCAa |
| CCGTGGCATTCCACTTATTCb | |||
| 12s | Mitochondrial 12S rRNA | AGCATTACTATGTTACGACTTACCTCAa | |
| AGTTCAGGTAGACGTGTAGGGb | |||
| Immunity | atg12 | Autophagy related 12 | CCTCTCAACTGCCCATTTCTa |
| GTACACCAACGAGTCCTTTGCb | |||
| gal | Galanin | TACTGACTTCCCGCTCTTCGa | |
| CACTTTGATGTGCGCTTCCb | |||
| Oxidative stress | sod1 | Superoxide dismutase [Cu–Zn] | CCAGCAGCCAGACCAGTTATa |
| AGGGAGACGCTAATGTGTCGb | |||
| sod2 | Superoxide dismutase [Mn] | CCAGGCTAATGGCAGACTTCa | |
| GTAGGCATGCTCCCAAACATb | |||
| cat | Catalase | CACCAGGTGTCCTTCCTGTTa | |
| CTCAGCATTCACCAGCTTGAb | |||
| se-gpx | Glutathione peroxidase | TGAGCCTCAGTTCTCGTTGAa | |
| Selenium dependent | ACTCGTTGTCGTCGCTAGGTb | ||
| gst | Glutathione S-transferase | GCCAGGCTACCGTATCTa | |
| TGTCCACCTTTAGGGCCTCb | |||
| Apoptosis | gadd45 | Growth arrest and DNA damage | GGAGCAGGTGATGCTGTGTAa |
| CCAGCAGTGTGCCTCAATAAb | |||
| p53 | Tumor protein 53 | TCCTGCCACAGTCACAAATGa | |
| GTCGAGATTTTCCCTCCTTAGCb | |||
| bax | BCL2 associated X | AAAGGGGAGGATGGGAGATa | |
| GCTATAACTGCCCCTGCTGTb | |||
| Neurotransmission | ache | Acetylcholinesterase | CTTGCTCTGGATCCTGCTCCa |
| TGCAAAAACCGGGACTCCAAb | |||
| Reproduction | err2 | Estrogen receptor 2 | CAGCTAATGGGGACTGCGAAa |
| CACCTTCCCTGAGCATTCCAAb | |||
| vit | Vitellogenin | CCCTAAAACATCCTGGCCGAa | |
| CCCAGTTGCCCCTTTCAAGAb | |||
Data treatment
Significant differences between treatments were tested using non-parametric Kruskal–Wallis tests, with post hoc exact permutation tests for inter-group comparison after sequential Bonferroni correction (XL-Stat software version 2013.5.09, 1995e2013 Addinsoft). A statistical parameter p < 0.05 was considered significant for all statistical tests. Multivariate analyses of the results were conducted using Prism 8.0.2 (GraphPad Software).Results
Nanoplastic suspension characterization
As seen in Fig. 1, the crushed environmental nanoplastic sample NP-L contains mostly the classical polyolefins PE and PP. The band near 1700 cm−1 indicates the presence also of ester bonds, which might either arise from other common polymers (e.g. PMMA or PET) or be ascribed to polyolefin oxidation into carboxylic acid functions generated by outdoor weathering,27 in accordance with the negative zeta potential of −32 mV (Table 2). The zeta potential measured for NP-PS that is even more negative than that of the environmental NP-L might be explained by the adsorption of bicarbonate anions onto hydrophobic surfaces, as shown by Yan et al.28 In practice, thanks to their negative surface charges, both suspensions of NP-L and NP-PS remained in a dispersed state for several months, as evidenced by a kinetic study by DLS (Fig. S2†). Right after their preparation, the NP-L suspension exhibits a single peak at 334 nm hydrodynamic diameter using the Pade–Laplace multimodal relaxation fitting of the DLS auto-correlogram acquired from backscattering (165° scattering angle), whereas NP-PS shows two peaks at 155 nm and 406 nm, with respective weighting factors to the scattered intensity of 57% and 39%. After 3 months, both suspensions still have mean hydrodynamic sizes of around 300 nm with polydispersity indices PDI = 0.27–0.28. The slight aggregation at longer time (8 months) could simply be suppressed by sonicating the stock suspensions for 10 min before their usage to contaminate the algae and the clams.| Stock solution concentration (mg L−1) | Z-Average diameter (nm)/PDI | Zeta potential (mV) | Polymer composition | |
|---|---|---|---|---|
| NP-L | 147 | 285/0.25 | −32 ± 6 | Polypropylene and polyethylene mostly |
| NP-PS | 420 | 206/0.17 | −44 ± 6 | Polystyrene |
Aluminium bioaccumulation
Al concentrations were measured in dry weights (DWs) of the gills and visceral mass of bivalves exposed to both NP-L and Al, or Al alone (Fig. 2). Surprisingly, no significant bioaccumulation of Al could be observed in the gills, compared to the controls, except for the 1 μg L−1 conditions at T7 (203.4 ± 14.8 μg g−1 DW). Overall, the Al concentrations were around 10 times lower in the visceral mass than in the gills. The Al bioaccumulation significantly increased in the visceral mass of bivalves exposed to Al alone, at each sampling time, as well as under the Al + NP-L conditions at 10 μg L−1 and T21 (10.6 ± 1.6 μg g−1 DW). After 7 days of depuration, there are still significant Al deposits in the visceral mass of bivalves exposed to Al + NP-L at 1 μg L−1 and to Al salt alone.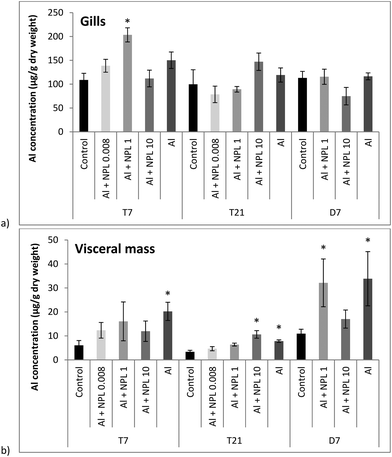 | ||
| Fig. 2 Aluminium concentrations in gills (a) and in visceral mass (b) of the clams exposed to Al + NP-L or Al alone (mean ± SE, n = 4). *: significantly different from controls (p < 0.005). | ||
Transcriptomic analyses
Globally, the gene expressions were more modulated in the visceral mass than in the gills (38.8 and 23.7% of genes modulated under the 10 μg L−1 conditions, respectively), even for the direct exposure to NP-L (Tables 3 and 4). They followed a dose–response trend towards the three concentrations tested. Gene modulations were observed at concentrations as low as 0.008 μg L−1 in both gills and visceral mass, after direct or dietary exposures. Gene modulations followed the same pattern between the water- and diet-borne exposures. However, genes were more upregulated in the gills of clams exposed via the trophic route than the direct route (21 and 16%, respectively, for the 10 μg L−1 conditions after 21 days), whereas the direct exposure triggered more gene modulation in the visceral mass than the trophic route (42 and 26% of genes, respectively, for the 10 μg L−1 conditions after 21 days). Several genes were still upregulated after 7 days of depuration in the visceral mass and gills of clams exposed through the direct and trophic routes. At 1 μg L−1, the waterborne exposure to NP-L modulated more genes than that to NP-PS in gills (10.5 and 5.3% of genes up-regulated at T7, respectively), whereas NP-PS triggered more gene up-regulations at 10 μg L−1 in gills than NP-L (10.5 and 5.3% of genes up-regulated at T7, respectively). The opposite trend was observed in the visceral mass. The effects of Al were stronger than those of Al + NP-L under all conditions and times tested, except in the gills at 10 μg L−1 for T7 and T21. Similarly, the effects of the waterborne exposure were stronger than those of the synergic exposure with Al (Al + NP-L) under all conditions tested, except in the gills at 1 μg L−1 for T7 and T21. The effects of Al + NP-L lasted longer than those of Al and NP-L during the depuration phase.| Concentration | clhc | cav | mt | mxr | 12s | cox1 | atg12 | Gal | sod1 | sod2 | cat | se-gpx | gst | gadd45 | p53 | bax | ache | err2 | vit | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T7 | NP-L | 0.008 | 3.42 | 1.60 | 3.71 | ||||||||||||||||
| 1 | 3.61 | 2.56 | |||||||||||||||||||
| 10 | 5.21 | ||||||||||||||||||||
| Diet NP-L | 0.008 | 0.05 | 2.51 | 0.28 | 0.44 | ||||||||||||||||
| 1 | 0.26 | 3.46 | 0.51 | ||||||||||||||||||
| 10 | 1.63 | 0.39 | 5.08 | 0.52 | |||||||||||||||||
| Al + NP-L | 0.008 | 0.31 | 3.10 | 0.47 | 0.42 | 9.00 | |||||||||||||||
| 1 | 0.49 | 2.78 | 0.42 | ||||||||||||||||||
| 10 | 0.47 | 2.60 | |||||||||||||||||||
| NP-PS | 1 | 3.50 | |||||||||||||||||||
| 10 | 3.68 | 12.99 | |||||||||||||||||||
| Al | 0.04 | 12.41 | 0.06 | 8.42 | 2.47 | 874.24 | |||||||||||||||
| T21 | NP-L | 0.008 | 1.82 | 2.35 | |||||||||||||||||
| 1 | 2.94 | 0.49 | 1.52 | 0.36 | 0.44 | 0.26 | |||||||||||||||
| 10 | 0.27 | 0.22 | 0.46 | 0.38 | 0.32 | 0.32 | 1.66 | 0.22 | 0.14 | 0.32 | 0.12 | 0.19 | 0.14 | ||||||||
| Diet NP-L | 0.008 | 2.85 | 0.40 | 1.85 | 3.22 | 0.34 | 2.21 | 0.27 | 0.29 | 0.31 | 0.37 | ||||||||||
| 1 | 3.06 | 0.44 | 2.06 | 3.42 | 0.45 | 2.38 | 0.32 | 0.42 | 0.49 | ||||||||||||
| 10 | 3.48 | 3.08 | 5.30 | 2.66 | 0.45 | ||||||||||||||||
| Al + NP-L | 0.008 | 0.28 | 3.10 | 0.34 | 0.52 | 0.50 | 0.16 | 0.35 | 0.15 | 0.24 | 0.18 | 0.22 | |||||||||
| 1 | 0.16 | 0.29 | 1.51 | 0.37 | 0.29 | 0.36 | 0.11 | 0.16 | 0.08 | 0.22 | 0.13 | ||||||||||
| 10 | 1.57 | 0.41 | 4.53 | 3.08 | 0.47 | 0.26 | 0.47 | 0.50 | 0.06 | ||||||||||||
| NP-PS | 1 | 0.31 | 0.47 | 0.51 | 0.25 | 0.43 | 0.17 | 0.42 | 0.19 | ||||||||||||
| 10 | 1.77 | 2.33 | 0.50 | 0.51 | |||||||||||||||||
| Al | 9.44 | ||||||||||||||||||||
| D7 | NP-L | 1 | 0.51 | 0.06 | |||||||||||||||||
| 10 | 0.15 | ||||||||||||||||||||
| Diet NP-L | 1 | 0.47 | 0.47 | 1.67 | 0.10 | ||||||||||||||||
| 10 | 2.02 | 2.02 | |||||||||||||||||||
| Al + NP-L | 1 | 2.29 | 1.69 | 1.58 | 1.84 | ||||||||||||||||
| 10 | 0.36 | 1.92 | 2.22 | 0.49 | 1.84 | 2.05 | 1.93 | 2.58 | 1.84 | ||||||||||||
| NP-PS | 1 | 1.67 | 1.93 | 1.53 | 1.64 | 0.53 | 1.79 | ||||||||||||||
| 10 | 1.52 | 1.85 | 1.77 | 1.78 | 0.50 | ||||||||||||||||
| Al | 0 | 9.89 | 9.12 | ||||||||||||||||||
| D21 | NP-L | 1 | |||||||||||||||||||
| 10 | 0.51 | 1.61 | |||||||||||||||||||
| Diet NP-L | 1 | 0.51 | 0.49 | 0.51 | 0.18 | 0.45 | 0.15 | 0.44 | 0.35 |
| Concentration | clhc | cav | mt | mxr | 12s | cox1 | atg12 | gal | sod1 | sod2 | cat | se-gpx | gst | gadd45 | p53 | bax | ache | err2 | vit | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T7 | NP-L | 0.008 | 0.24 | 0.49 | 1.88 | 1.89 | |||||||||||||||
| 1 | 0.23 | 2.15 | |||||||||||||||||||
| 10 | 0.04 | ||||||||||||||||||||
| Diet NP-L | 0.008 | 1.55 | 0.21 | 2.18 | 1.55 | 2.21 | |||||||||||||||
| 1 | 1.55 | 0.41 | 2.17 | ||||||||||||||||||
| 10 | 0.21 | 1.64 | 1.73 | 1.78 | 0.28 | 2.18 | 1.94 | 3.34 | |||||||||||||
| Al + NP-L | 0.008 | 0.14 | 0.12 | 0.43 | 0.22 | 0.22 | 0.39 | 0.03 | 0.12 | 0.27 | 0.06 | 0.36 | 0.43 | 0.25 | 0.10 | ||||||
| 1 | 0.05 | 0.04 | 0.45 | 0.73 | 0.13 | 0.29 | 0.16 | 0.37 | 0.23 | 0.08 | 0.15 | 0.05 | 0.12 | 0.15 | 0.18 | 0.07 | |||||
| 10 | 0.05 | 26.65 | 0.08 | 0.34 | 0.12 | 0.21 | 0.26 | 0.02 | 0.07 | 0.26 | 0.27 | 0.06 | 0.16 | 0.29 | 0.30 | 0.07 | |||||
| NP-PS | 1 | 0.12 | 1.78 | ||||||||||||||||||
| 10 | 0.17 | 1.60 | |||||||||||||||||||
| Al | 48.05 | 0.26 | 0.15 | 3.02 | 27.78 | 0.12 | 1.90 | 0.00 | |||||||||||||
| T21 | NP-L | 0.008 | 7.44 | 43.15 | 0.10 | 0.00 | 0.00 | 0.00 | |||||||||||||
| 1 | 13.48 | 160.68 | 0.37 | 0.00 | 0.00 | 1.85 | |||||||||||||||
| 10 | 25.42 | 542.20 | 4.04 | 8.79 | 2.11 | 13.64 | 3.92 | 0.00 | 0.00 | 4.11 | |||||||||||
| Diet NP-L | 0.008 | 0.33 | 19.59 | 158.92 | 0.27 | 0.33 | 0.33 | 0.17 | 0.36 | ||||||||||||
| 1 | 11.69 | 668.66 | 0.30 | 3.33 | 2.78 | 1.76 | 2.74 | 1.94 | |||||||||||||
| 10 | 17.48 | 578.24 | 0.15 | 2.09 | 0.47 | 2.36 | 1.77 | ||||||||||||||
| Al + NP-L | 0.008 | 0.18 | 15.50 | 0.08 | 193.86 | 0.05 | 0.23 | 0.33 | 0.32 | 0.34 | |||||||||||
| 1 | 0.06 | 20.01 | 0.21 | 184.11 | 0.28 | 0.10 | 0.16 | 0.13 | 0.12 | 0.14 | 0.39 | 0.10 | |||||||||
| 10 | 0.08 | 18.01 | 0.07 | 207.70 | 0.06 | 0.15 | 0.31 | 0.18 | 0.18 | 0.28 | |||||||||||
| NP-PS | 1 | 18.37 | 0.22 | 729.98 | 0.50 | 1.81 | 2.11 | 0.28 | |||||||||||||
| 10 | 0.10 | 14.45 | 0.08 | 185.59 | 0.13 | 0.26 | 0.20 | 0.05 | 0.04 | 0.22 | 0.05 | 0.04 | 0.05 | 0.08 | 0.11 | 0.02 | |||||
| Al | 2.79 | 4.53 | 2.36 | 2.61 | |||||||||||||||||
| D7 | NP-L | 1 | 1.96 | 20.13 | 4.74 | 1.50 | 1.58 | ||||||||||||||
| 10 | 0.50 | 5.83 | 32.62 | 0.23 | 7.38 | 2.14 | 0.10 | 2.01 | |||||||||||||
| Diet NP-L | 1 | 18.91 | 4.94 | 2.06 | |||||||||||||||||
| 10 | 2.31 | 0.07 | 3.14 | 3.14 | 2.30 | ||||||||||||||||
| Al + NP-L | 1 | 0.34 | 39.41 | 31.14 | 4.69 | 1.88 | 0.34 | ||||||||||||||
| 10 | 46.44 | 22.73 | 5.35 | 2.67 | 1.58 | ||||||||||||||||
| NP-PS | 1 | 1.63 | 0.02 | 7.15 | 11.12 | 2.28 | 3.30 | 0.11 | 1.93 | 19.91 | 14.20 | 5.53 | 0.47 | 2.05 | 3.44 | 123.46 | |||||
| 10 | 1.37 | 0.03 | 6.01 | 8.06 | 2.06 | 2.85 | 0.08 | 2.39 | 6.19 | 9.13 | 5.60 | 0.38 | 2.97 | 72.00 | |||||||
| Al | 0 | 2.79 | 4.53 | 2.36 | 2.61 | ||||||||||||||||
| D21 | NP-L | 1 | 0.45 | 0.44 | 0.47 | ||||||||||||||||
| 10 | 0.44 | 0.32 | 0.47 | 0.52 | 0.26 | 0.49 | 0.37 | 0.39 | 0.51 | ||||||||||||
| Diet NP-L | 1 | 0.43 | 0.16 | 0.35 | 0.15 | 0.22 |
No induction of the detoxification gene mt was observed under any of the tested conditions, including Al, whereas it led to an increase of the mxr expression at T7 (12.41). The mxr gene was repressed under the NP-L and Al + NP-L conditions at T21 and over-expressed under the NP-PS conditions (1.77 for 10 μg L−1). It was up-regulated after 7 days of depuration under the Al + NP-L and diet NP-L conditions and down-regulated after 21 days under the NP-L conditions.
The 12s gene was repressed for the three concentrations of Al + NP-L (0.31–0.47) and diet NP-L (0.05–0.39) after 7 days. It was also repressed under all conditions except Al after 21 days of contamination. No gene modulation was observed during the depuration phase. In the opposite trend, the cox1 expression increased under all conditions tested at T7 (2.51 to 5.21), except Al, and under the Al + NP-L conditions at T21 (1.51 for 1 μg L−1). The over-expression lasted until 21 days of depuration under the NP-PS conditions.
A low immunity response was triggered during the exposure phase. Only the 3 concentrations tested under the diet NP-L conditions led to an increase of the atg12 gene (1.85 to 3.08). However, some gene inductions were observed during the depuration phase, namely atg12 under the Al + NP-L conditions (2.22 for 10 μg L−1, at D7), atg12 and gal under the NP-PS (1.93 and 1.53 for 1 μg L−1 at D7) and NP-L (1.61 for 10 μg L−1 at D21) conditions.
Few effects were observed in response against oxidative stress after 7 days of contamination. Only the Al conditions led to an up-regulation of the cat gene (8.42), whereas se-gpx was repressed under the Al + NP-L and diet NP-L conditions. After 21 days of contamination, sod1, sod2 and se-gpx were repressed under several conditions, namely NP-L, Al + NP-L, NP-PS and diet NP-L (only for se-gpx), whereas cat and gst were up-regulated under many conditions. During the first 7 days of the depuration phase, gst was still up-regulated under all conditions except NP-L.
The genes gadd45, p53 and bax were repressed after 21 days of contamination under all conditions tested except Al. During the depuration phase, concomitant with the induction of gst, the exposure to Al + NP-L led to an up-regulation of gadd45 (1.58 for 1 μg L−1) and bax (1.84–1.93 for 1 and 10 μg L−1, respectively). The NP-PS exposure also led to an increase of gadd45 expression at D7 (1.64–1.78 for 1 and 10 μg L−1, respectively), while p53 was repressed (0.53–0.50 for 1 and 10 μg L−1, respectively).
The neurotransmission gene of AchE was repressed after 7 days under the Al + NP-L and diet NP-L conditions (0.42 and 0.44 for 0.008 μg L−1, respectively), while it was over-expressed under the Al conditions (2.47). The gene ache was repressed after 21 days of exposure under the conditions Al + NP-L (0.50 for 10 μg L−1), NP-L (0.12 for 10 μg L−1), and diet NP-L (0.37 for 0.008 μg L−1). However, its expression was up-regulated during the 7 first days of depuration under the Al + NP-L, diet NP-L and NP-PS conditions (2.58, 1.49 and 1.79, respectively).
The reproduction pathways were also affected after 7 days of contamination, through the direct route with the upregulation of the err2 (1.39–1.60) and vit genes (2.56–3.71). All conditions led to an up-regulation of vit after 7 days of exposure, except the diet NP-L conditions. However, err2 was repressed after 21 days of exposure under all conditions except Al. The NP-L and Al + NP-L conditions also led to a down-regulation of the vit gene. During the depuration phase, the Al + NP-L and Al conditions triggered an over-expression of err2 and vit (1.84 and 9.12, respectively, for 10 μg L−1), whereas the water- and diet-borne exposures led to a down-regulation of vit, which lasted until D21 for the diet NP-L conditions.
The mt gene was strongly upregulated, except for the Al conditions, after 21 days of contamination (from a 7.44 to 25.42-fold change for the NP-L conditions, for instance). The upregulation lasted until 7 days of depuration, and then mt expression was repressed throughout the rest of the experiment, under most of the conditions tested. Only NP-PS led to an upregulation of mxr after 7 days of depuration.
The mitochondrial metabolism was affected with a strong increase of the 12s gene expression after 21 days of contamination, under all conditions tested. The induction reached 542.20 and 578.24-fold changes under the NP-L and diet-borne NP-L conditions at 10 μg L−1. The over-expression was observed until 7 days of depuration, except for the Al conditions, and was then repressed. The cox1 gene was down-regulated after 21 days of exposure through the trophic route and to NP-PS.
Immunity was also strongly induced, through the atg12 gene. It was upregulated after diet NP-L exposure from T7 to D7. The NP-L exposure also led to an increase of atg12 expression (3.33 for 10 μg L−1). The atg12 gene upregulation was observed until 7 days of depuration under all conditions tested, except Al, and was then repressed. The gal gene was strongly induced after 7 days of exposure to Al (27.78) and 21 days to NP-PS (1.81 for 1 μg L−1) but not for the other conditions.
The exposure to Al + NP-L led to a strong down-regulation of all genes involved against oxidative stress after 7 days and of cat and se-gpx after 21 days. The exposure to diet NP-L led to a down-regulation of cat and an increase of se-gpx expression (2.18 for both 0.008 and 10 μg L−1) after 7 days. No effects were observed for the NP-L and NP-PS conditions after 7 days of exposure.
After 21 days of exposure, the cat and se-gpx expressions were still repressed under the Al + NP-L conditions, whereas the Al conditions up-regulated the cat and gst expressions (1.52 and 4.83, respectively). An induction of sod1, sod2, cat and gst was observed for the highest concentration tested for the NP-L conditions (8.79, 2.11, 13.64, 3.92, respectively). Diet NP-L exposure also led to the upregulation of sod1, sod2 and cat, whereas the NP-PS exposure decreased the expression of sod2, cat, se-gpx and gst. During the depuration phase, the gst gene was induced after 7 days, under all conditions tested but Al + NP-L. NP-PS exposure also triggered the upregulation of sod2, cat, and se-gpx after 7 days of depuration.
The gadd45 gene involved in DNA repair was downregulated after 7 and 21 days of exposure under several conditions, especially Al + NP-L. A low apoptotic response was observed, with the downregulation of p53 and bax after 7 days of exposure to Al + NP-L and under most of the conditions tested, after 21 days. The diet NP-L conditions only led to the over-expression of p53 (1.94 and 2.36 for 10 μg L−1 after 7 and 21 days, respectively), while bax was upregulated after 7 days of exposure to Al and NP-L (1.90 and 1.88, respectively, for 0.008 μg L−1). No clear trend was observed towards the apoptotic response during the depuration phase.
Neurotransmission was assessed through the ache expression, which was upregulated after 7 days of exposure through the direct and trophic routes, as well as NP-PS, whereas it was downregulated under the Al + NP-L conditions. After 21 days, the ache expression was repressed under all conditions, except Al. During the depuration phase, its expression was again over-expressed under the Al + NP-L and NP-PS conditions.
The reproduction pathway was also affected by several conditions, especially after 21 days. Both direct and trophic routes triggered an increase in err2 expression (1.85–4.11 and 1.94–1.77 for direct and trophic conditions, respectively, for the 1 and 10 μg L−1 conditions, respectively). Its expression was decreased under the Al + NP-L conditions after 7 and 21 days, as well as the vit expression. The expression of err2 and vit was decreased under the NP-PS conditions after 21 days and increased after 7 days of depuration (123.46 and 72 for vit at 1 and 10 μg L−1, respectively).
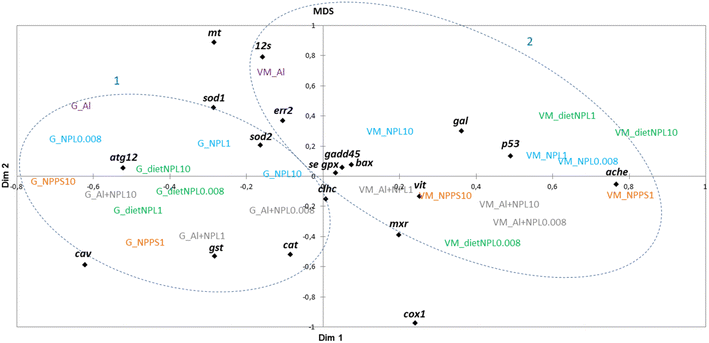
The results obtained in the visceral mass and gills of clams after 21 days of exposure were plotted according to a multidimensional scaling (MDS) analysis, made from a similarity matrix (Spearman correlation, Fig. 3). The analysis shows a clear discrepancy between the results from gills (1) and visceral mass (2). The gills respond mainly by endocytosis and oxidative stress, whereas the visceral mass is rather linked to processes involved in detoxification, mitochondrial metabolism, oxidative stress and apoptosis. The MDS analysis also shows that all tested conditions triggered many gene modulations involved in a variety of biological processes, without forming distinguished groups.
Filtration test
A filtration test was performed after 21 days of experiment (Fig. 4). The exposure to NP-L led to a significant decrease of the filtration rate after 60 min under the 1 μg L−1 conditions (129![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 ± 27
900 ± 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 cells per mL per individual, compared to control reaching 336
600 cells per mL per individual, compared to control reaching 336![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 ± 75
800 ± 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cells per mL per individual). The bivalves exposed to diet NP-L showed an opposite behaviour, with a significant increase of their filtration rate after 30 and 45 min, for the 1 and 10 μg L−1 concentrations (345
200 cells per mL per individual). The bivalves exposed to diet NP-L showed an opposite behaviour, with a significant increase of their filtration rate after 30 and 45 min, for the 1 and 10 μg L−1 concentrations (345![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 47
000 ± 47![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 and 328
100 and 328![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 ± 24
600 ± 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cells per mL per individual, respectively after 45 min, compared to control reaching 195
200 cells per mL per individual, respectively after 45 min, compared to control reaching 195![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ± 35
500 ± 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 cells per mL per individual). The same observation was obtained under the NP-PS conditions, with increasing filtration rates in clams exposed to 1 and 10 μg L−1, but only in the early stages of the test (5 to 20 min). The filtration rates averaged 162
200 cells per mL per individual). The same observation was obtained under the NP-PS conditions, with increasing filtration rates in clams exposed to 1 and 10 μg L−1, but only in the early stages of the test (5 to 20 min). The filtration rates averaged 162![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 36
000 ± 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 248
000 and 248![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 88
000 ± 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL per individual for 1 and 10 μg L−1 after 20 min, while the control did not go higher than 104
000 cells per mL per individual for 1 and 10 μg L−1 after 20 min, while the control did not go higher than 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ± 48
000 ± 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 cells per mL per individual. No significant results were obtained for the Al + NP-L conditions.
500 cells per mL per individual. No significant results were obtained for the Al + NP-L conditions.
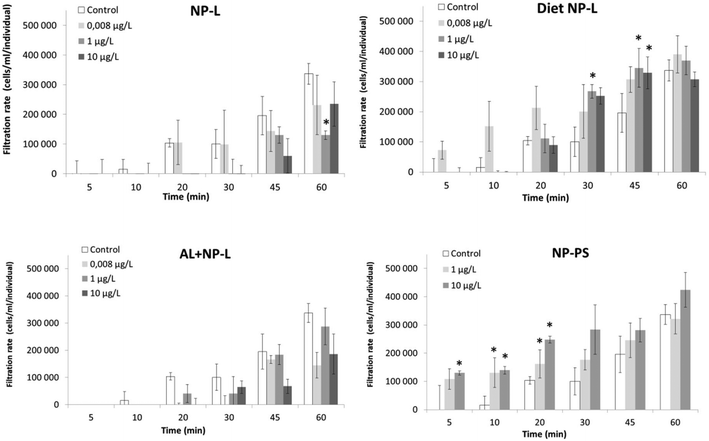 | ||
| Fig. 4 Filtration rates of clams exposed to the different conditions (mean ± SD, n = 4). *: significantly different from controls (Student's test p < 0.005). | ||
Phenol oxidase activity
The phenol oxidase (PO) activity was measured after 7 and 21 days of exposure and after 7 days of depuration (Fig. 5). Very few results were different from controls. After 7 days of exposure to NP-PS, the activity was significantly higher than that in controls, reaching 0.68 ± 0.04 and 0.32 ± 0.08 U per mg of proteins, respectively. No other significant result was observed after 7 and 21 days of exposure. After 7 days of depuration, the clams under the diet NP-L conditions at 10 μg L−1 showed an activity significantly higher than those in controls (0.83 ± 0.08 and 0.36 ± 0.01 U per mg of proteins, respectively), but not under the other conditions.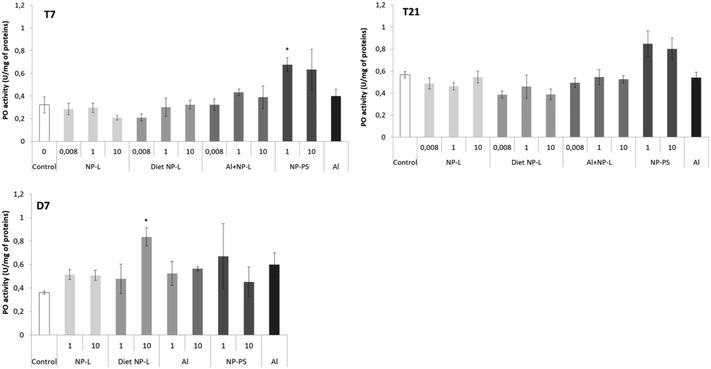 | ||
| Fig. 5 Phenol oxidase activity in gills of clams exposed to the different conditions (mean ± SD, n = 4). *: significantly different from controls (Student's test coefficient p < 0.005). | ||
GST activity
No difference in the GST activity was observed after 7 days of contamination (Fig. 6). After 21 days, the GST activity was significantly lower under the Al + NP-L conditions at 1 μg L−1 compared to controls (1.60 ± 0.26 and 2.97 ± 0.26 nmol min−1 per mg of proteins, respectively). After 7 days of depuration, the activity was also significantly decreased under the NP-L and diet NP-L conditions, at 1 μg L−1, compared to controls (2.07 ± 0.25, 1.78 ± 0.26 and 2.80 ± 0.14 nmol min−1 per mg of proteins, respectively).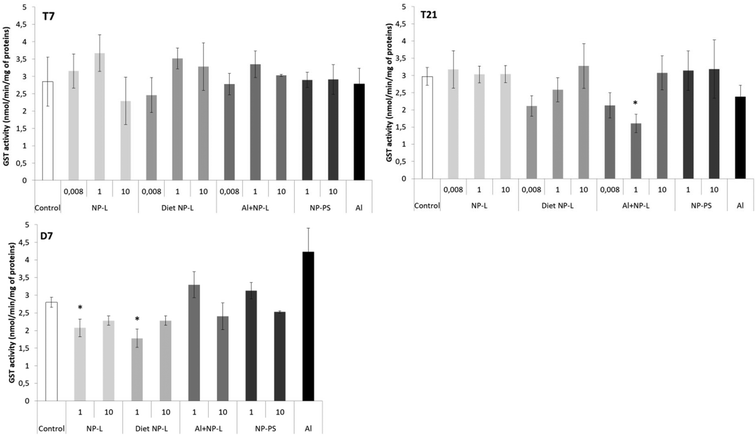 | ||
| Fig. 6 GST activity in the visceral mass of clams exposed to the different conditions (mean ± SD, n = 4). *: significantly different from controls (p < 0.005). | ||
AchE activity
After 7 days, the activity of AchE was significantly higher in bivalves exposed to Al + NP-L at 0.008 and 1 μg L−1 (3.04 ± 0.37, 2.16 ± 0.02 nmol min−1 per mg of proteins, respectively) and to NP-PS at 1 μg L−1 and Al (2.18 ± 0.25 and 2.18 ± 0.38 nmol min−1 per mg of proteins, respectively) than controls (1.40 ± 0.09 nmol min−1 per mg of proteins) (Fig. 7). After 21 days, the activity of AchE significantly decreased under the diet NP-L conditions (0.46 ± 0.06 and 0.80 ± 0.13 nmol min−1 per mg of proteins for 0.008 and 1 μg L−1 under diet NP-L, respectively) compared to controls (2.34 ± 0.49 nmol min−1 per mg of proteins), as well as under the Al + NP-L conditions at 0.008 μg L−1, in contrast to the increase at 1 μg L−1. Similarly, the activity decreased after 7 days of depuration under the diet NP-L conditions for the 1 and 10 μg L−1 concentrations and increased under the NP-PS conditions at 1 μg L−1.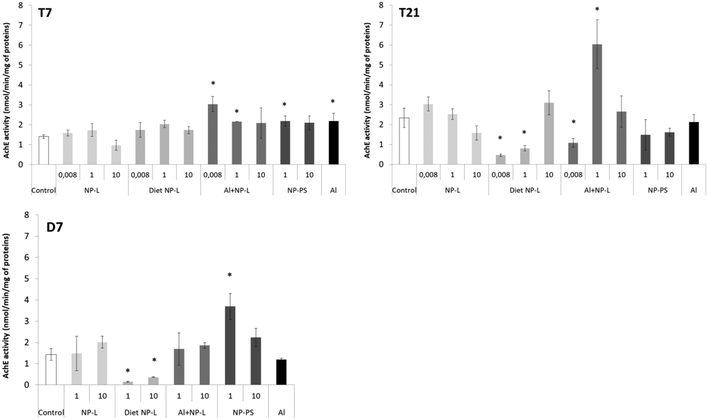 | ||
| Fig. 7 AchE activity in visceral mass of clams exposed to the different conditions (mean ± SD, n = 4). *: significantly different from controls (p < 0.005). | ||
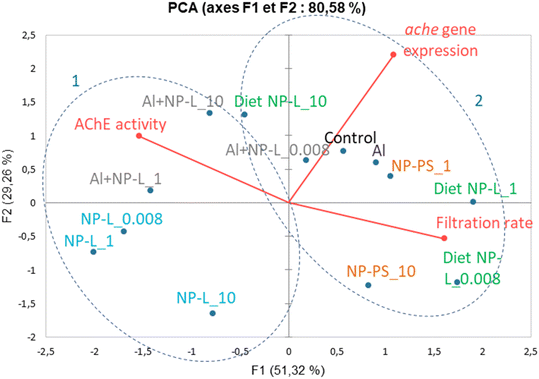
A principal component analysis (PCA, Spearman correlation) was realized from the results of AchE activity, filtration rates and ache gene expression measured in the visceral mass of clams after 21 days of exposure to the different conditions. Vectors from the PCA show that the AchE activity is diametrically opposed to the filtration rate. The gene expression rate is correlated neither to the enzyme activity nor to the filtration rate. The ache gene was repressed under all conditions tested whether its enzyme activity was inhibited or not. Two groups can be circled from the PCA results: 1) NP-L and Al + NP-L treatments and 2) NP-PS and diet NP-L treatments. The NP-PS and diet NP-L treatments led to an increased filtration rate possibly due to the inhibition of AchE enzyme activity. Conversely, the NP-L and AL + NP-L conditions resulted in an increase of AchE activity and a decrease of the filtration rate of clams, as ascribed to the loss of Ach in neurotransmission pathways (Fig. 8).
Discussion
Nanoplastic characterization
The NPs crushed from the Leyre River (NP-L) were mainly composed of PP and PE. However, so far, NPs used in the literature for ecotoxicological studies are overwhelmingly PS. This observation motivated our choice to use NP-PS to compare our data with NP-L. Our objective was to point out how studies commonly based on the toxicity of NP-PS may underestimate the effects of realistic NP mixtures which are actually composed of different polymers (PP, PE for instance).Aluminium bioaccumulation
No significant bioaccumulation of Al could be observed in gills of bivalves exposed to either Al or Al + NP-L, except for one condition: Al + NP-L at 1 μg L−1 and 7 days. In a previous work by Rosseland et al.,29 it was demonstrated that Al can provoke osmoregulatory failure in gills and lead to the precipitation of Al. This phenomenon was ascribed to the acidification of mucus secretion that prevented Al from binding to gill cells.30Surprisingly, Al concentrations were lower in the visceral mass compared to those in gills in controls. However, significant increases were observed in the bivalves exposed to Al alone or punctually under the Al + NP-L conditions. This suggests that NPs may affect the metal accumulation. This could result from a lower bioavailability of Al in the presence of NPs, potentially due to Al adsorption onto the NPs' surface. It has been suggested that NPs can be directly rejected through pseudo-faeces or mucus secretion,31,32 and that the bioavailability of a metalloid such as arsenic was lowered in the presence of NPs in mangrove oysters, due to its adsorption onto the surface of NPs.16 On the assumption that some of the Al are bound to NPs, it could explain, at least partly, the low accumulation of Al in gills and visceral mass under the conditions with NPs.
Transcriptomic analyses
Our study aimed to compare gene expression after water- and diet-borne exposures to NPs, with NP-L or NP-PS commonly used in the literature, as well as synergic effects with a metal: aluminium. Two organs were investigated to see the differences between exposure routes: gills and visceral mass.Moreover, the cell permeability of gills, in direct contact with the surrounding medium, can act as a protective barrier against xenobiotics. This could reduce pollutant accumulation through the direct route.33 We observed through the waterborne exposure a strong production of mucus coming out from the clams' valves which could also act as a protective mechanism of gills towards contamination by trapping NPs and rejecting them into the surrounding medium. This phenomenon has also been reported on oysters exposed to NPs.32,34
After 21 days of exposure, the gills mainly responded to the surrounding contamination through the endocytosis pathway, with the up-regulation of the caveolin gene under all conditions tested, except NP-PS. The endocytosis was concomitant with the decrease in the 12s expression under the NP-L conditions and with the up-regulation of gst and cat. This could underline a disturbance of the energetic balance leading to oxidative stress in clams after NP-L entry. Similar trends were observed in previous studies on the Pacific oyster I. alatus exposed through the direct route to NPs, with the up-regulation of the cav, 12s and cat genes,34 whereas this was not observed after dietary exposure.16 Surprisingly, no response was observed towards apoptosis in gills, with the global inhibition of gadd45, p53 and bax under all conditions tested (except Al). This suggests that responses against oxidative stress in gills could have efficiently counteracted the effects of NPs and did not end up in apoptosis.
The results in visceral mass were more pronounced than in gills after 21 days of exposure. We observed a very strong induction of the mt (except under the Al conditions) and 12s genes under all conditions tested, while cox1 was repressed under the diet NP-L and NP-PS conditions. The results suggest that metallothioneins, well known for their key role in metal detoxification,35 could also be involved in the case of NP exposure, probably through the oxidative stress generated by their high surface reactivity. They could have also reacted to metals or additives released in the tissues after absorption of NPs in the cells to sequester or excrete them. The fact that Al did not trigger an up-regulation of mt was already described in the literature in freshwater molluscs such as Dreissena polymorpha.36 But considering the very low bioaccumulation observed in tissues, the weak concentrations of Al may also have not been sufficient to induce a response towards the mt gene, even if this metal generated oxidative stress (cat induction). This confirms our hypothesis that the mt gene can actually be modulated by NPs themselves, and not only in the case of combined exposure with metals. To the best of our knowledge, this is the first evidence of mt being involved in the response against NPs in bivalves. Unlike in gills, we observed in the visceral mass a very strong increase of 12s expression under all conditions tested. Such up-regulations have been reported in other studies on the oyster I. alatus after direct exposure to environmental NPs for one week.32,34 This may show a trial to increase the mitochondria number that could be at least partly related to the increase in energy demand to excrete NPs. Again, Al alone led to much lower inductions than Al + NP-L, showing that gene responses are mainly driven by NPs themselves.
Increased expressions were also observed for genes involved in immunity, oxidative stress, and apoptosis in visceral mass (atg12, sod1, sod2 and cat). This led to an apoptotic response under the NP-L diet-borne conditions only, with the up-regulation of p53, as shown for the pacific oyster I. alatus.34
Genes involved in reproduction were also tested and the results showed an up-regulation of err2 in the visceral mass of clams exposed through the direct and trophic routes (1 and 10 μg L−1), but no effects were observed for the vitellogenin gene. This underlines the first evidence of reproductive gene impairments after exposure to NPs at such low concentrations. Reproductive adverse effects have already been described in Pacific oysters exposed to 50 nm NP-PS but at higher concentrations (0.1, 1, 10 and 25 mg L−1). The authors showed that NPs could alter fertilization success, lead to larval teratologies, and arrest growth development.37
After 7 days of exposure, very few effects were observed under the waterborne conditions, both in the gills and visceral mass. The strongest gene modulations were shown after the diet-borne exposure in the visceral mass, with the up-regulation of gene expressions among all functions tested (namely 12s, atg12, gal, se-gpx, p53 and ache) and in a dose–response manner. This suggests that NPs may trigger more effects on bivalves via the diet borne route than the waterborne one. Another study comparing water- and diet-borne exposures to nanoparticles of gold in Corbicula fluminea showed that the effects on gene expressions were much lower after the diet-borne exposure.38 The authors suggested that this was probably due to valve closures occurring in response to water contamination. This phenomenon has been documented for metals in Corbicula fluminea,39 but due to the lack of analytical methods available to quantify NPs in tissues, it is difficult to actually attribute toxicity to NP bioaccumulation. However, our results from the filtration test showed that the ventilatory activity of Corbicula fluminea decreased after the waterborne exposure, while it increased after the diet-borne exposure. This suggests a stronger NP accumulation under the diet NP-L conditions, coinciding with the up-regulation of most genes tested under these conditions.
Gene expressions in gills followed the same trends after 21 days of exposure to either the water or the trophic route. The main differences were observed in the visceral mass. Both conditions led to an increase of gene expressions of mt, 12s and atg12. However, responses differed for genes involved in oxidative stress response and apoptosis. The direct exposure triggered a response against oxidative stress but not towards apoptosis, whereas we observed the opposite pattern for the trophic route in visceral mass.
After 21 days of contamination in the visceral mass, the effects of Al alone led to much lower inductions of mt and 12s, than those after the Al + NP-L exposure, showing that the responses are mainly driven by NP exposures. Moreover, the effects of Al + NP-L lasted longer in the depuration phase than the single Al or NP-L exposures.
We also observed delayed effects of NP-PS and Al + NP-L with the up-regulation of genes involved in the detoxification, DNA repair, oxidative stress and apoptosis occurring in the gills and visceral mass during the depuration phase. This suggests that the clams are recovering from the exposure and able to implement gene responses against the remaining stress. After 21 days of depuration, most of the tested genes were repressed in the gills and visceral mass. A study on Pecten maximus showed that 68% of the initial burden of 250 nm 14C-radiolabeled NP-PS had been purged after 3 days of depuration.44 This suggests fast depuration capacities in bivalves, which is in agreement with our results on gene up-regulations, lasting no longer than 7 days after the end of exposure.
Immune and anti-oxidative responses through PO and GST activities
The transcriptomic results showed that NPs triggered oxidative stress and immune responses. However, very few immune and anti-oxidative responses were observed at the enzymatic level, regarding PO and GST activities. Some studies have shown the inhibition of GST and PO activities in Corbicula fluminea in response to model NP-PS exposure for 96 h,18 but the authors used higher concentrations than those in the present study, ranging from 0.1 to 5 mg L−1. It is possible that the concentrations used in this study were not sufficient to induce such an enzymatic response.Neurotoxicity through AchE activity and filtration behaviour
AchE inhibition following NP exposure has been reported in several species. For instance, a study on zebrafish reported a decrease in AchE activity after a 72 h exposure to 1 mg L−1 50 nm model NP-PS.15 Another study on M. galloprovincialis also resulted in a significantly decreased cholinesterase activity after 96 h exposure to model NP-PS (0.05 up to 50 mg L−1).46 It has been suggested that modification of AchE activity could result from ROS production, namely H2O2, as it could promote disturbances in the cholinergic system by altering the AchE structure.47 In our study, the AchE activity was significantly decreased after 21 days of diet-borne exposure, coinciding with the strongest induction of genes involved against oxidative stress. However, no such relationship could be observed for other conditions.
A study on Corbicula fluminea exposed to environmental NPs (1–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μg L−1) did not show any effect on the filtration rates of clams after 48 h of direct exposure.31 However, the authors showed an increase in pseudo-faeces production, suggesting an effect on excretion. Our results report the first evidence linking AchE altered activity (gene repression and enzyme inhibition) to actual behavioural impairments in bivalves exposed to NPs.
000 μg L−1) did not show any effect on the filtration rates of clams after 48 h of direct exposure.31 However, the authors showed an increase in pseudo-faeces production, suggesting an effect on excretion. Our results report the first evidence linking AchE altered activity (gene repression and enzyme inhibition) to actual behavioural impairments in bivalves exposed to NPs.
Conclusion
This study aimed at testing different exposure routes, types of NPs and biological readouts to obtain an integrative view of NPs' toxicity on freshwater bivalves. Results have shown that NPs in the presence of Al affected the metal bioaccumulation, probably due to its adsorption onto the NPs' surface leading to a lower bioavailability of this element. Moreover, gene expression modulation was observed at concentrations as low as 0.008 μg L−1 in both gills and visceral mass, after either direct or dietary exposures, with responses more deeply modulated in the visceral mass than in the gills. The exposure routes also seem to be a crucial criterion to investigate NPs' toxicity. After the trophic route of exposure and in the presence of Al, indeed synergistic effects were observed on gene expression, which was more disrupted than that after direct exposure to NP-L. The inhibition of AchE, concomitant with an increase of the filtration activity of bivalves exposed to NP-L (trophic route) and NP-PS controls, suggested also neurotoxic effects. The effects were observed even after 7 days of depuration in clean water, but after 21 days, they returned to basal levels of expression. In terms of the origin of NPs, discrepancies were observed in gene expression. In the visceral mass, most of the genes from clams exposed to NP-PS were repressed, while they were all upregulated under the NP-L conditions, for the different concentrations tested. This lack of gene upregulation during NP-PS exposure underlines that the origin, morphology, ageing, oxidation state, and additional xenobiotics can interact with internalization and adverse pathways in clams. These findings show the importance of considering naturally aged environmental NPs in ecotoxicological studies rather than synthetic NP-PS latexes, like commercial crosslinked PS nanospheres used in most NP studies published to date or submicronic particles prepared by nanoprecipitation from virgin polymer chains used as controls in this work. Following these findings, further studies may give greater consideration to more environmentally relevant NPs, to get closer to field conditions experienced by aquatic organisms.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We want to thank the LabEx COTE funding for supporting the PLASCOTE project (ANR 10-LABX-0045) and the University of Bordeaux for funding through its “inter-department research call” in 2021. We also thank the molecular biology platform of the EPOC laboratory for giving us access to the transcriptomic and enzymatic assays and PCR equipment, Amélie Vax-Weber for the search of possible THF traces in the NP-PS medium by GC-MS and Diana Kazaryan for obtaining the TEM images of NP-L and NP-PS samples at the Bordeaux Imaging Centre electron microscopy facility (https://www.bic.u-bordeaux.fr/).References
- T. M. Nolte, N. B. Hartmann, J. M. Kleijn, J. Garnæs, D. Van De Meent, A. J. Hendriks and A. Baun, The toxicity of plastic nanoparticles to green algae as influenced by surface modification, medium hardness and cellular adsorption, Aquat. Toxicol., 2017, 183, 11–20 CrossRef CAS PubMed.
- N. B. Hartmann, T. Nolte, M. A. Sørensen, P. R. Jensen and A. Baun, Aquatic ecotoxicity testing of nanoplastics lessons learned from nanoecotoxicology, Sound/Visual production (digital), DTU Environment, 2015, https://backend.orbit.dtu.dk/ws/portalfiles/portal/106390042/ASLO_Hartmann_et_al_nanoplastics_Final_clean.pdf Search PubMed.
- J. Gigault, A. Ter Halle, M. Baudrimont, P.-Y. Pascal, F. Gauffre, T.-L. Phi, H. El Hadri, B. Grassl and S. Reynaud, Current opinion: what is a nanoplastic?, Environ. Pollut., 2018, 235, 1030–1034 CrossRef CAS PubMed.
- M. A. Browne, P. Crump, S. J. Niven, E. Teuten, A. Tonkin, T. Galloway and R. Thompson, Accumulation of microplastic on shorelines woldwide: sources and sinks, Environ. Sci. Technol., 2011, 45, 9175–9179 CrossRef CAS PubMed.
- D. Allen, S. Allen, S. Abbasi, A. Baker, M. Bergmann, J. Brahney, T. Butler, R. A. Duce, S. Eckhardt and N. Evangeliou, Microplastics and nanoplastics in the marine-atmosphere environment, Nat. Rev. Earth Environ., 2022, 3, 393–405 CrossRef CAS.
- A. A. Koelmans, E. Besseling and W. J. Shim, Nanoplastics in the aquatic environment, Critical review, Marine anthropogenic litter, 2015, pp. 325–340 Search PubMed.
- H. Bouwmeester, P. C. Hollman and R. J. Peters, Potential health impact of environmentally released micro-and nanoplastics in the human food production chain: experiences from nanotoxicology, Environ. Sci. Technol., 2015, 49, 8932–8947 CrossRef CAS PubMed.
- C. M. Rochman, C. Manzano, B. T. Hentschel, S. L. M. Simonich and E. Hoh, Polystyrene plastic: a source and sink for polycyclic aromatic hydrocarbons in the marine environment, Environ. Sci. Technol., 2013, 47, 13976–13984 CrossRef CAS PubMed.
- I. Velzeboer, C. Kwadijk and A. Koelmans, Strong sorption of PCBs to nanoplastics, microplastics, carbon nanotubes, and fullerenes, Environ. Sci. Technol., 2014, 48, 4869–4876 CrossRef CAS PubMed.
- A. L. Andrady, Microplastics in the marine environment, Mar. Pollut. Bull., 2011, 62, 1596–1605 CrossRef CAS PubMed.
- L. Manfra, A. Rotini, E. Bergami, G. Grassi, C. Faleri and I. Corsi, Comparative ecotoxicity of polystyrene nanoparticles in natural seawater and reconstituted seawater using the rotifer Brachionus plicatilis, Ecotoxicol. Environ. Saf., 2017, 145, 557–563 CrossRef CAS PubMed.
- T. Kögel, Ø. Bjorøy, B. Toto, A. M. Bienfait and M. Sanden, Micro-and nanoplastic toxicity on aquatic life: Determining factors, Sci. Total Environ., 2020, 709, 136050 CrossRef PubMed.
- Z. Liu, M. Cai, D. Wu, P. Yu, Y. Jiao, Q. Jiang and Y. Zhao, Effects of nanoplastics at predicted environmental concentration on Daphnia pulex after exposure through multiple generations, Environ. Pollut., 2020, 256, 113506 CrossRef CAS PubMed.
- W. S. Lee, H.-J. Cho, E. Kim, Y. H. Huh, H.-J. Kim, B. Kim, T. Kang, J.-S. Lee and J. Jeong, Bioaccumulation of polystyrene nanoplastics and their effect on the toxicity of Au ions in zebrafish embryos, Nanoscale, 2019, 11, 3173–3185 RSC.
- Q. Chen, D. Yin, Y. Jia, S. Schiwy, J. Legradi, S. Yang and H. Hollert, Enhanced uptake of BPA in the presence of nanoplastics can lead to neurotoxic effects in adult zebrafish, Sci. Total Environ., 2017, 609, 1312–1321 CrossRef CAS PubMed.
- M. Lebordais, Z. Venel, J. Gigault, V. S. Langlois and M. Baudrimont, Molecular Impacts of Dietary Exposure to Nanoplastics Combined or Not with Arsenic in the Caribbean Mangrove Oysters (Isognomon alatus), Nanomaterials, 2021, 11, 1151 CrossRef CAS PubMed.
- E. Bergami, S. Pugnalini, M. Vannuccini, L. Manfra, C. Faleri, F. Savorelli, K. Dawson and I. Corsi, Long-term toxicity of surface-charged polystyrene nanoplastics to marine planktonic species Dunaliella tertiolecta and Artemia franciscana, Aquat. Toxicol., 2017, 189, 159–169 CrossRef CAS PubMed.
- Z. Li, C. Feng, Y. Wu and X. Guo, Impacts of nanoplastics on bivalve: Fluorescence tracing of organ accumulation, oxidative stress and damage, J. Hazard. Mater., 2020, 392, 122418 CrossRef CAS PubMed.
- M. Prüst, J. Meijer and R. H. Westerink, The plastic brain: neurotoxicity of micro-and nanoplastics, Part. Fibre Toxicol., 2020, 17, 1–16 CrossRef PubMed.
- W. Fan, P. Yang, Y. Qiao, M. Su and G. Zhang, Polystyrene nanoplastics decrease molting and induce oxidative stress in adult Macrobrachium nipponense, Fish Shellfish Immunol., 2022, 122, 419–425 CrossRef CAS PubMed.
- H. Cai, E. G. Xu, F. Du, R. Li, J. Liu and H. Shi, Analysis of environmental nanoplastics: Progress and challenges, Chem. Eng. J., 2021, 410, 128208 CrossRef CAS.
- J.-P. W. Desforges, M. Galbraith, N. Dangerfield and P. S. Ross, Widespread distribution of microplastics in subsurface seawater in the NE Pacific Ocean, Mar. Pollut. Bull., 2014, 79, 94–99 CrossRef CAS PubMed.
- M. C. Goldstein, A. J. Titmus and M. Ford, Scales of spatial heterogeneity of plastic marine debris in the northeast Pacific Ocean, PLoS One, 2013, 8, e80020 CrossRef PubMed.
- S. Schubert, J. T. Delaney Jr and U. S. Schubert, Nanoprecipitation and nanoformulation of polymers: from history to powerful possibilities beyond poly (lactic acid), Soft Matter, 2011, 7, 1581–1588 RSC.
- C. Le Bris, G. Richard, C. Paillard, C. Lambert, C. Seguineau, O. Gauthier, F. Pernet and F. Guérard, Immune responses of phenoloxidase and superoxide dismutase in the manila clam Venerupis philippinarum challenged with Vibrio tapetis–Part I: Spatio-temporal evolution of enzymes' activities post-infection, Fish Shellfish Immunol., 2015, 42, 16–24 CrossRef CAS PubMed.
- K. J. Livak and T. D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method, Methods, 2001, 25, 402–408 CrossRef CAS PubMed.
- Y. Lv, Y. Huang, J. Yang, M. Kong, H. Yang, J. Zhao and G. Li, Outdoor and accelerated laboratory weathering of polypropylene: A comparison and correlation study, Polym. Degrad. Stab., 2015, 112, 145–159 CrossRef CAS.
- X. Yan, M. Delgado, J. Aubry, O. Gribelin, A. Stocco, F. Boisson-Da Cruz, J. Bernard and F. Ganachaud, Central role of bicarbonate anions in charging water/hydrophobic interfaces, J. Phys. Chem. Lett., 2018, 9, 96–103 CrossRef CAS PubMed.
- B. O. Rosseland, I. A. Blakar, A. Bulger, F. Kroglund, A. Kvellstad, E. Lydersen, D. H. Oughton, B. Salbu, M. Staurnes and R. Vogt, The mixing zone between limed and acidic river waters: complex aluminium chemistry and extreme toxicity for salmonids, Environ. Pollut., 1992, 78, 3–8 CrossRef CAS PubMed.
- M. Berntssen, F. Kroglund, B. O. Rosseland and S. E. W. Bonga, Responses of skin mucous cells to aluminium exposure at low pH in Atlantic salmon (Salmo salar) smolts, Can. J. Fish. Aquat. Sci., 1997, 54, 1039–1045 CAS.
- M. Baudrimont, A. Arini, C. Guégan, Z. Venel, J. Gigault, B. Pedrono, J. Prunier, L. Maurice, A. Ter Halle and A. Feurtet-Mazel, Ecotoxicity of polyethylene nanoplastics from the North Atlantic oceanic gyre on freshwater and marine organisms (microalgae and filter-feeding bivalves), Environ. Sci. Pollut. Res., 2020, 27, 3746–3755 CrossRef CAS PubMed.
- A. Arini, J. Gigault, Z. Venel, A. Bertucci and M. Baudrimont, The underestimated toxic effects of nanoplastics coming from marine sources: A demonstration on oysters (Isognomon alatus), Chemosphere, 2022, 295, 133824 CrossRef CAS PubMed.
- R. Zhou, G. Lu, Z. Yan, R. Jiang, Y. Sun and P. Zhang, Effects of polystyrene nanoplastics on the bioaccumulation, distribution and parental transfer of ethylhexyl salicylate, Environ. Sci.: Nano, 2022, 9, 1025–1036 RSC.
- A. Arini, Z. Venel, H. Tabuteau, J. Gigault and M. Baudrimont, Early molecular responses of mangrove oysters to nanoplastics using a microfluidic device to mimic environmental exposure, J. Hazard. Mater., 2022, 436, 129283 CrossRef CAS PubMed.
- M. Baudrimont, J. Metivaud, R. Maury-Brachet, F. Ribeyre and A. Boudou, Bioaccumulation and metallothionein response in the Asiatic clam (Corbicula fluminea) after experimental exposure to cadmium and inorganic mercury, Environ. Toxicol. Chem., 1997, 16, 2096–2105 CrossRef CAS.
- M. M. Desouky, Metallothionein is up-regulated in molluscan responses to cadmium, but not aluminum, exposure, J. Basic Appl. Zool., 2012, 65, 139–143 CrossRef CAS.
- K. Tallec, A. Huvet, C. Di Poi, C. González-Fernández, C. Lambert, B. Petton, N. Le Goïc, M. Berchel, P. Soudant and I. Paul-Pont, Nanoplastics impaired oyster free living stages, gametes and embryos, Environ. Pollut., 2018, 242, 1226–1235 CrossRef CAS PubMed.
- A. Arini, F. Pierron, S. Mornet and M. Baudrimont, Bioaccumulation dynamics and gene regulation in a freshwater bivalve after aqueous and dietary exposures to gold nanoparticles and ionic gold, Environ. Sci. Pollut. Res., 2020, 27, 3637–3650 CrossRef CAS PubMed.
- D. Tran, P. Ciret, A. Ciutat, G. Durrieu and J. C. Massabuau, Estimation of potential and limits of bivalve closure response to detect contaminants: application to cadmium, Environ. Toxicol. Chem., 2003, 22, 914–920 CrossRef CAS PubMed.
- F. Blancho, M. Davranche, F. Fumagalli, G. Ceccone and J. Gigault, A reliable procedure to obtain environmentally relevant nanoplastic proxies, Environ. Sci.: Nano, 2021, 8, 3211–3219 RSC.
- P. Ruenraroengsak and T. D. Tetley, Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: robust response of alveolar type 1 epithelial cells, Part. Fibre Toxicol., 2015, 12, 1–20 CrossRef CAS PubMed.
- D. B. Zorov, M. Juhaszova and S. J. Sollott, Mitochondrial ROS-induced ROS release: an update and review, Biochim. Biophys. Acta, Bioenerg., 2006, 1757, 509–517 CrossRef CAS PubMed.
- C. E. Purman, P. L. Collins, S. I. Porter, A. Saini, H. Gupta, B. P. Sleckman and E. M. Oltz, Regional gene repression by DNA double-strand breaks in G1 phase cells, Mol. Cell. Biol., 2019, 39(24), e00181-19 CrossRef PubMed.
- M. Al-Sid-Cheikh, S. J. Rowland, K. Stevenson, C. Rouleau, T. B. Henry and R. C. Thompson, Uptake, whole-body distribution, and depuration of nanoplastics by the scallop Pecten maximus at environmentally realistic concentrations, Environ. Sci. Technol., 2018, 52, 14480–14486 CrossRef CAS PubMed.
- Z. Venel, H. Tabuteau, A. Pradel, P.-Y. Pascal, B. Grassl, H. El Hadri, M. Baudrimont and J. Gigault, Environmental Fate Modeling of Nanoplastics in a Salinity Gradient Using a Lab-on-a-Chip: Where Does the Nanoscale Fraction of Plastic Debris Accumulate?, Environ. Sci. Technol., 2021, 55, 3001–3008 CrossRef CAS PubMed.
- I. Brandts, M. Teles, A. Gonçalves, A. Barreto, L. Franco-Martinez, A. Tvarijonaviciute, M. Martins, A. Soares, L. Tort and M. Oliveira, Effects of nanoplastics on Mytilus galloprovincialis after individual and combined exposure with carbamazepine, Sci. Total Environ., 2018, 643, 775–784 CrossRef CAS PubMed.
- A. Garcimartín, M. E. López-Oliva, M. P. González, F. J. Sánchez-Muniz and J. Benedí, Hydrogen peroxide modifies both activity and isoforms of acetylcholinesterase in human neuroblastoma SH-SY5Y cells, Redox Biol., 2017, 12, 719–726 CrossRef PubMed.
- R. S. Costa, P. Oliveira and L. Guilhermino, in Advances in Geoethics and Groundwater Management: Theory and Practice for a Sustainable Development, Springer, 2021, pp. 279–282 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3en00022b |
| This journal is © The Royal Society of Chemistry 2023 |