Degradation of organic contaminants by peroxymonosulfate activated with zeolitic imidazolate framework-based catalysts: performances, mechanisms and stability
Chao
Guo
a,
Min
Cheng
 *a,
Gaoxia
Zhang
*b,
Weiping
Xiong
a,
Chengyun
Zhou
*a,
Gaoxia
Zhang
*b,
Weiping
Xiong
a,
Chengyun
Zhou
 a,
Biao
Song
a,
Biao
Song
 a,
Li
Du
a,
Ling
Li
a,
Chensi
Tang
a,
Guangfu
Wang
a and
Hongda
Liu
a
a,
Li
Du
a,
Ling
Li
a,
Chensi
Tang
a,
Guangfu
Wang
a and
Hongda
Liu
a
aCollege of Environmental Science and Engineering, and, Key Laboratory of Environmental Biology and Pollution Control of Ministry of Education, Hunan University, Changsha 410082, China. E-mail: chengmin@hnu.edu.cn
bCarbon Neutrality Research Institute of Power China Jiangxi Electric Power Construction Co., Ltd., Nanchang 330001, China. E-mail: zhanggaoxia@hnu.edu.cn
First published on 3rd April 2023
Abstract
For the past few years, sulfate radical-based advanced oxidation processes (SR-AOPs) have been developed rapidly due to their unique advantages for the degradation of organic contaminants. Zeolitic imidazolate framework (ZIF)-based materials have been considered to be potential catalysts to activate peroxymonosulfate (PMS) for the generation of SO4˙−. ZIFs are constructed from tetrahedrally coordinated transition metal (M) ions (Zn, Co cation) linked by organic imidazole (Im) units and possess similar structures to conventional aluminosilicate zeolites, where M occupies the position of silicon and the role of oxygen is substituted by Im. ZIFs have high thermal stability and chemical robustness and can be used in many common solvents. Besides, ZIFs have tailorable pore sizes and structures for modification into ideal composite catalysts. Therefore, it is necessary to systematically examine the recent advances of PMS activation on ZIF-based catalysts for the removal of organic contaminants. This review discussed the recent progress on ZIF-based catalysts for PMS activation. Firstly, the applications of ZIF-based catalysts for the removal of contaminants are reviewed. Secondly, the different mechanisms (including radical pathways and non-radical pathways) of PMS activation by pristine ZIFs, ZIF composites and ZIF derivatives are elucidated respectively. Particular emphasis is given to the influence of structure on catalytic activity. Finally, some challenges existing in ZIF/PMS systems are pointed out and the possible research directions are proposed. This review aims to provide novel insight for the application of ZIF-based materials in PMS activation and propose a deeper understanding of ZIF-based materials.
 Top (from left to right): Weiping Xiong; Chengyun Zhou; Biao Song; Li Du. Bottom (from left to right): Ling Li; Guangfu Wang; Chensi Tang; Hongda Liu |
Environmental significanceDue to the fact that a large number of emerging organic contaminants harm the environment, this review mainly discussed the recent progress on ZIF-based catalysts in PMS activation for the removal of organic contaminants. However, the metal leaching of ZIF based materials could damage the ecosystem and threaten human health. We summarized some methods to solve this problem and broaden the horizon for the development of novel ZIF-based materials. |
1. Introduction
With the development of modern industry, a large number of emerging organic contaminants are discharged into the water environment through various ways.1,2 Most organic contaminants have stable structure, high carcinogenicity, teratogenicity and mutagenicity, which significantly damage the ecosystem and severely threaten safety and health.3,4 Therefore, it is very significant to deeply investigate effective methods to degrade organic contaminants.Various technologies have been developed to solve the problems of water pollution, including adsorption,5 photocatalytic degradation,6–10 biological treatment,11 enzymatic degradation12,13 and advanced oxidation processes (AOPs).14–19 Among them, AOPs are effective means to oxidize macromolecular organic contaminants into harmless or low-toxicity mineralized products by generating highly reactive oxygen species (ROS), such as sulfate (SO4˙−) and hydroxyl (˙OH) radicals. Theoretically, SO4˙− can effectively and rapidly degrade most organic contaminants in water, such as organic dyes, antibiotics, pesticides, and endocrine disruptors.20 In recent years, SO4˙− based AOPs (SR-AOPs) have been widely studied for their high standard reduction potential (2.5–3.1 V), wide pH application range (2–8), long half-life period (30–40 μs), and high selectivity and efficiency of the reaction.21–24
In SR-AOPs, ROS can be generated by the activation of peroxymonosulfate (PMS) and peroxodisulfate (PDS). Numerous investigations have shown that PMS is easier to activate than PDS, which is largely attributed to their different physicochemical properties: (a) the standard reduction potential of PMS is 1.82 VNHE while that of PDS is 2.08 VNHE; (b) PMS has an asymmetric structure while PDS has a symmetric structure; and (c) the O–O bond length of PDS (1.497 Å) is longer than that of PMS (1.453 Å).25 Although PMS is a thermodynamically strong oxidant, its direct reaction with most contaminants is too slow and therefore activation is required. So far, there have been many methods to generate SO4˙− by activating PMS, such as direct use of energy (e.g., heat, ultraviolet, ultrasonic, etc.) and catalysis through different transition metals, metal oxides and non-metallic materials in alkaline media. Among them, it has been proven that the use of catalysts could be a more efficient and cost-effective strategy.16 Numerous studies have suggested that metal-based catalysts have high efficiency in activating PMS, but their problems including secondary pollution, metal ion toxicity and mass dissolution of metal ions hinder their further application.26 Besides, the activation of PMS using metal-free carbon materials does not suffer from the above problems, but their catalytic performance is still unsatisfactory. Therefore, it is of great significance to develop novel catalysts with low cost, excellent activity and environmental friendliness.
Metal–organic frameworks (MOFs), which are constructed from organic linkers and metal centers, have been widely investigated in various applications, including, but not limited to, separation,27 chemical sensing,28 catalysis,29 gas storage,30 biomedicine,31 and energy storage and conversion.32 Compared with traditional porous materials such as zeolite, biochar and graphene, MOFs have proven to be superior catalysts due to their tunable pore size, large surface area and porosity, and unsaturated metal sites.33,34 As a class of MOF materials, zeolitic imidazolate frameworks (ZIFs) are constructed from tetrahedrally coordinated transition metal (M) ions (Zn, Co cation) linked by organic imidazole (Im) units and possess similar structures to conventional aluminosilicate zeolites, where M occupies the position of silicon and the role of oxygen is substituted by Im.35 ZIFs have rich structural topologies and high porosity, as well as easily tailorable pore sizes and structures, which facilitate their modification and bring some new characteristics. Compared with other MOF materials such as the Material of Institute Lavoisier (MIL) series, the University of Oslo (UiO) series, and the Hong Kong University of Science and Technology (HKUST) series, ZIF materials have the advantages of permanent porosity, high thermal stability, and outstanding chemical stability. For example, ZIF-8 remains structurally stable at temperatures above 500 °C and maintains its crystallinity and porosity after use in many common solvents.35,36 Besides, the high surface area of ZIF-67 makes it rich in active sites, which is favorable for the reaction. Due to the tight coordination of the constructed imidazole linkers with Zn2+ or Co2+, ZIFs can be prepared rapidly at room temperature without the use of toxic solvents.37 These advantages make ZIF-based materials potential catalysts for PMS activation. Specially, ZIFs, represented by ZIF-67 and ZIF-8 and their derivatives, have been gradually applied in PMS activation.
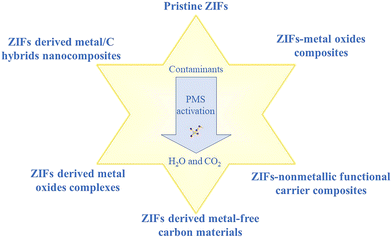
In the past few years, ZIF-based materials have made rapid progress in PMS activation. More and more research studies on this topic suggest that ZIF-based catalysts, including pristine ZIFs, ZIF composites and their derivatives, will play an important role in the removal of contaminants by PMS activation.38,39 However, the role of ZIF-based catalysts in PMS activation and the associated mechanisms have not been elaborated so far. Therefore, a review of ZIF-based catalysts in PMS activation is highly expected. Accordingly, this review mainly introduces the activation of PMS based on ZIF-based catalysts, including pristine ZIFs, ZIF composites and their derivatives (Fig. 1). Most importantly, in order to highlight the recent progress in the removal of contaminants by PMS activation of ZIF-based materials, some representative examples are analyzed in this review. Particular emphasis is given to the influence of structure on catalytic activity and to the mechanisms of catalysis. Finally, the existing problems of ZIF-based catalysts for PMS activation are pointed out, and some recommendations are proposed.
2. Application in organic contaminant treatment
2.1 Pristine ZIFs for PMS activation
Transition metal Co has been widely used to activate PMS and has shown the most effective activation ability.40 Therefore, as a representative of Co-based pristine ZIFs, ZIF-67 has been used as a catalyst to activate PMS. In 2015, Lin et al. reported the pioneering work of using pristine ZIFs as novel catalysts to degrade Rhodamine B (RhB) by PMS activation (Fig. 2a).41 Experimental results suggested that ZIF-67 exhibited superior catalytic performance to Co3O4 nanoparticles (NPs) because of its higher surface area and porosity. In addition, they found that the degradation efficiency remained over 90% after being reused three times, displaying the excellent chemical stability of the catalyst. During the degradation of RhB, Co acted as the active site and reacted with PMS to generate SO4˙− (eqn (1) and (2)). The generated SO4˙− had strong oxidizing property and could eventually degrade RhB into CO2 and H2O.| Co2+@ZIF-67 + HSO5− → Co3+@ZIF-67 + SO4˙− + OH− | (1) |
| Co3+@ZIF-67 + HSO5− → Co2+@ZIF-67 + SO5˙− + H+ | (2) |
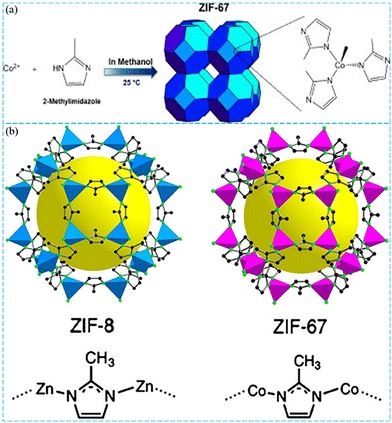 | ||
| Fig. 2 (a) Scheme of the synthesis of zeolitic imidazole framework (ZIF)-67. Reproduced with permission.41 Copyright 2015, Elsevier. (b) Crystal structure of ZIF-8 and ZIF-67. Reproduced with permission.9 Copyright 2021, Elsevier. | ||
Considering that ZIFs possessed different structural topologies and the types of solvents affect the structures and properties of ZIFs in the synthesis process, Li et al. synthesized Co-based MOFs (Co3(BTC)2·12H2O (Co–BTC)) as catalysts to degrade dibutyl phthalate (DBP) by PMS activation.150 Co–BTC(A) and Co–BTC(B) were prepared through using different solvents, leading to different catalytic activities in the reaction. The Co–BTC(A)/PMS system exhibited higher performance for the removal of DBP than the Co–BTC(B)/PMS system, and this consequence was related to the particle size and specific surface area of the catalysts. In addition to this, considering that the catalytic activity is connected with the structure of the catalyst, Cong et al. prepared ZIF-9 and ZIF-12 with different structural topologies to degrade RhB via PMS activation.42 ZIF-9 had a sodalite (SOD) topology with a cubic spatial structure, while ZIF-12 had a rhombic (RHO) topology with a rhombic dodecahedron spatial structure. The results indicated that the performance of the catalyst was closely related to the particle size and structure topology. At the beginning of the catalytic process, ZIF-9 showed better catalytic efficiency than ZIF-12 because its nanoscale made it come into closer contact with PMS. However, with increasing time, ZIF-12 exhibited higher degradation performance because its RHO topology gave it a larger micropore volume and larger surface area.
In general, the active metal center of ZIFs is the main site for their catalytic activity. Compared to monometallic ZIFs, bimetallic ZIFs have been proven to have superior catalytic performance. Therefore, doping of one or more metal centers in pristine ZIFs is receiving increasing attention, as this combination can enhance their special activity. Yao's group synthesized ZIF-8, ZIF-67 and Co/ZIF-8 with ideal particle size and morphology as heterogeneous catalysts for PMS activation.43 The crystal structures of ZIF-67 and ZIF-8 are shown in Fig. 2b. The results suggested that Co/ZIF-8 displayed enhanced stability and catalytic activity compared to ZIF-67 and ZIF-8, which was attributed to the fact that the presence of bimetals in Co/ZIF-8 could significantly improve the chemical stability and provide more active sites. Besides, Zareba et al. obtained Co/ZIF-8 with adjustable particle sizes via room temperature synthesis using different ratios of Co direct doping.44 When the doping concentration of Co was increased from 60% to 100%, the particle size increased from 60 nm to more than 500 nm. Similarly, in Yao's report, the particle size was gradually tunable from 30 nm to over 300 nm at different levels of Co doping from 0% to 100%. Besides, Zn/Co MOFs prepared by Fang's group and ZIF-11(Zn/Co) prepared by Ling's group both exhibited excellent catalytic activity for water treatment.45,46 Gu et al. prepared core–shell bimetallic MIL-101/ZIF-67x with superior catalytic performance for the degradation of 2-chlorophenol (2-cp).47 The past research studies on the activation of PMS for degradation of organic contaminants by pristine ZIFs are summarized in Table 1.
| Catalysts | Contaminants | Optimal experiment terms | Removal efficiency | Reusability | Ref. |
|---|---|---|---|---|---|
| ZIF-67 | Rhodamine B (RhB) | RhB = 50 mg L−1, catalyst = 10 mg L−1, PMS = 50 mg L−1, T = 20 °C | 100% (60 min) | The recycling efficiency was slightly decreased after 3 cycles | 41 |
| Fe3O4@ZIF-67 | Tetrabromobisphenol A (TBBPA) | TBBPA = 40 mg L−1, catalyst = 0.1 g L−1, PMS = 0.1 g L−1, | 100% (3 min) | Catalytic performances without significant loss after 5 recycles | 50 |
| Fe3O4-PVP@ZIF-67 | Bisphenol F (BPF) | BPF = 20 mg L−1, catalyst = 150 mg L−1, PMS = 0.3 mM | 99.8% (60 min) | Slight loss of the catalytic activity after 4 recycles | 54 |
| Fe3O4@Zn/Co-ZIFs | Carbamazepine (CBZ) | CBZ = 5 mg L−1, catalyst = 25 mg L−1, PMS = 0.4 mM, pH = 6.8 | 100% (30 min) | Slight loss of the catalytic activity after 4 recycles | 59 |
| ZIF-67/PAN | Acid yellow-17 (AY) | AY = 500 mg L−1, catalyst = 233 mg L−1, PMS = 500 mg L−1, T = 20 °C | 95.1% (10 min) | Catalytic performances almost unchanged after 5 recycles | 63 |
| ZIF-9@GEL, ZIF-12@GEL | p-Nitrophenol (PNP) | ZIF-9@GEL = 0.61 mg L−1, ZIF-12@GEL = 0.79 mg L−1, pH = 6, T = 25 °C | 90% (60 min) | Kept excellent degradation performance after 3 cycles | 73 |
| CoFe2O4/ZIF-8 | Methylene blue (MB) | AY = 20 mg L−1, catalyst = 50 mg L−1, PMS = 300 mg L−1, T = 20 °C | 97.9% (60 min) | The degradation efficiency has not been greatly reduced after four catalytic cycles | 48 |
| Mn3O4/ZIF-8 | Rhodamine B (RhB) | RhB = 10 mg L−1, catalyst = 300 mg L−1, PMS = 300 mg L−1, T = 23 °C | 98% (60 min) | The catalytic activity remained high after five runs | 49 |
| ZIF@resin | Rhodamine B (RhB) | RhB = 10 mg L−1, catalyst = 50 mg L−1, PMS = 50 mg L−1, T = 30 °C | 100% (30 min) | It remained very effective for activation over four cycles | 75 |
| Ag/ZIF-67@GO | Phenol | Phenol = 20 mg L−1, catalyst = 50 mg L−1, PMS = 300 mg L−1, T = 25 °C | 100% (30 min) | The activity of the recovered nanocomposite did not change after four repetitive catalytic cycles | 153 |
| Ag/AgCl@ZIF-8 modified g-C3N4 | Levofloxacin (LVFX) | Phenol = 20 mg L−1, catalyst = 50 mg L−1, PMS = 300 mg L−1, T = 25 °C | 87.3% (60 min) | The degradation efficiency was slightly decreased after 4 times of degradation | 154 |
2.2 ZIF composites for PMS activation
The unique physical structure and chemical properties of ZIFs provide a broad platform for the study and design of ZIFs in PMS activation, making them a hot research topic for water pollution treatment. However, some inevitable disadvantages of ZIFs limit their development in practical applications. For example, the active sites in pristine ZIFs are limited which result in unsatisfactory catalytic performance. The inevitable ion leaching during the application of pristine ZIFs may bring the risk of secondary contamination.41 Besides, in practical applications for wastewater treatment, ZIF nanoparticles are difficult to separate from the reaction solution for recycling due to their highly dispersive nature. Therefore, with the demand for functional and high-performance materials increasing, it is necessary to modify pristine ZIFs to expand their applications. At present, the controlled integration of ZIFs and functional materials can lead to the formation of novel multifunctional composites, which have attracted much attention by showing superior performance over individual components through the collective behavior of each functional unit. For example, by compositing ZIFs with magnetic Fe3O4 or loading ZIFs on some suitable support materials, the resulting composite materials can solve the recycling problem of ZIFs while maintaining their nanoscale reactivity. Generally, ZIF composites include ZIF-metal oxide composites and ZIF-nonmetallic functional carrier composites as catalysts for PMS activation (Table 1).In addition to this supporting, ZIFs and magnetic metal oxides can form unique core–shell structures with abundant active sites to enhance catalytic performance and ameliorate recovery. Recently, core–shell structure catalysts with Fe3O4 as the core and ZIF as the shell have been widely studied in water treatment. Chen's group prepared magnetic Fe3O4@ZIF-67 composites to degrade tetrabromobisphenol A (TBBPA).50 Using Fe3O4@ZIF-67 as the catalyst, complete removal of TBBPA was reached in only 3 min. By contrast, Fe3O4 only degraded 7.1% (in 30 min), while ZIF-67 degraded TBBPA completely in 30 min.41 The whole catalytic mechanism of Fe3O4@ZIF-67 is proposed in Fig. 3. PMS could be activated in the N-doped domains of Fe3O4@ZIF-67 by a non-radical process to produce 1O2.51 Meanwhile, SO4˙− could be generated through Co2+ species in Fe3O4@ZIF-67 activating PMS, and the SO4˙− and the Co2+ were respectively converted to ˙OH and Co3+.52,53 Moreover, the interactions between ZIF-67 and Fe3O4 through Fe–N binding showed an interesting synergistic catalytic structural mode. The results indicated that the Fe2+ in Fe3O4 could donate electrons to enhance the recycling of Co2+ to Co3+, thus ensuring a strong catalytic performance for PMS activation.
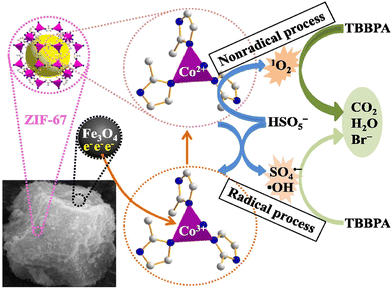 | ||
| Fig. 3 Synergistic catalytic mechanism of PMS activation by Fe3O4@ZIF-67 for the degradation of TBBPA. Reproduced with permission.50 Copyright 2021, Elsevier. | ||
Poly vinyl pyrrolidone (PVP), an amphiphilic non-ionic polymer, is widely used for the synthesis of catalysts due to its advantages. For example, Cui et al. firstly prepared the Fe3O4–PVP@ZIF-67 nanocomposite for the degradation of bisphenol F (BPF).54 In this work, PVP was used not only to regulate the size and morphology of the nanoparticles,55 but also to optimize the crystal structure of ZIF-67.56 PVP acted as a template to form different shapes by controlling the growth rate of different molecules and ions in contact.57,58 Besides, PVP also held abundant pores that was beneficial for catalysis. Compared with the ZIF-67/PMS and Co3O4/PMS systems, Fe3O4-PVP@ZIF-67 exhibited the highest degradation performance, decomposing 99.8% of BPF within 60 min.
The problems of metal element leaching and active component loss in Fe3O4-based composite catalysts greatly reduce the recyclability. Wu et al. designed an effective method to solve these problems by placing and confining Fe3O4 nanoparticles into hollow cavities of Zn/Co-ZIFs.59 As shown in Fig. 4, Fe3O4 nanoparticles were pretreated with polyvinylpyrrolidone (PVP), and then ZIF shells were grown by a solvothermal method. Fe3O4 and ZIF-67 could be wrapped by the shell structure of ZIF-8, which could help confine and protect the leaching of metal ions. They found that the leaching concentration of Co ions in the Fe3O4@Zn/Co-ZIFs/PMS system was much lower than that of the ZIF-67/PMS system and Fe3O4@Co-ZIFs/PMS system under the same experimental conditions. In addition, the prepared Fe3O4@Zn/Co-ZIF composite showed superior catalytic degradation performance (near 100% removal in 30 min) for the removal of carbamazepine (CBZ). In addition, Wu et al. prepared CuFe2O4@ZIF-67 for the activation of PMS, and the Cu2+/Cu+ redox cycles in this process further improved the activation ability.60
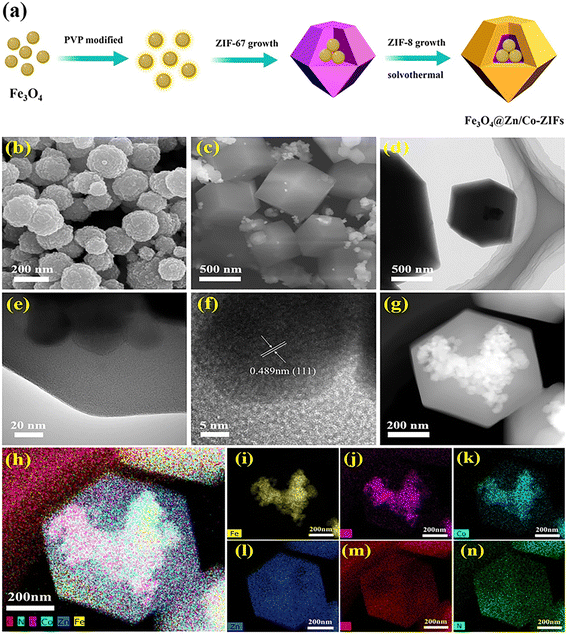 | ||
| Fig. 4 (a) Schematic illustration of the preparation process of Fe3O4@Zn/Co-ZIFs. SEM images of (b) Fe3O4 and (c) Fe3O4@Zn/Co-ZIFs, (d and e) TEM, (f) HRTEM, and (g) HAADF-STEM images and (h–n) element mapping of Fe3O4@Zn/Co-ZIFs. Reproduced with permission from ref. 59. Copyright 2020, Elsevier. | ||
The electrospinning technique is widely used to immobilize nanoparticles in or on fibers, which overcomes the disadvantage of poor catalyst recovery.64–67 Wang et al. firstly prepared ZIF-67/polyacrylonitrile (PAN) composite nanofibers with one-dimensional (1D) nanostructure via electrospinning.63 The ZIF-67/PAN composite nanofibers exhibited excellent degradation efficiency toward acid yellow (AY) (nearly 95.1% removal efficiency in 10 min) and retained high catalytic stability (over 98%) after 5 cycles. The electrospun PAN nanofibers with a high surface area and flexible properties not only provided an efficient contact between the composite catalysts and the contaminants, but also would be beneficial to separate and recycle the catalysts.68 Moreover, the same research group reported that ZIF-67/PAN fibers could be deposited on a glass sand funnel to form a catalytic membrane, which exhibited high efficiency to activate PMS for the removal of organic contaminants.69 This flexible and reusable catalytic membrane demonstrated great promise for practical industrial applications.
Cellulose aerogels hold great promise as a support carrier due to their advantages of large specific surface area, low cost and narrow pore size distribution.70 In addition, the superior physical and chemical stability of cellulose aerogels makes it easy to separate and recycle the catalyst from the reaction system.71,72 Ren et al. successfully obtained ZIFs (ZIF-9 and ZIF-12)@cellulose aerogels to degrade p-nitrophenol (PNP) by PMS activation.73 The results showed that all the prepared ZIFs@cellulose aerogels (GELs) exhibited efficient degradation of PNP with up to 90% degradation rate after 1 h. It was worth mentioning that the degradation rate of ZIF-9@GEL was higher than that of ZIF-12@GEL, which is due to the higher loading and smaller pore size of ZIF-9@GEL,74 resulting in a shorter transfer distance for PMS to Co ions.42 Similarly, zeolite beads, ion-exchange resins and nickel foam have been proven to be good carrier materials for PMS activation.75–77
In recent years, the application of ZIF-based heterostructure composites with excellent photocatalytic performance in PMS activation has attracted extensive attention owing to the synergistic effect of their metal centers and organic linkages.78 The heterojunction constructed by the combination of graphitic carbon nitride (g-C3N4) and ZIFs can improve the photocatalytic performance, but the activation ability and the stability for activating PMS are limited. Therefore, Luo et al. prepared ZIF-67/MOF-74(Ni)/g-C3N4 and ZIF-67/MIL-100(Fe)/g-C3N4 composite catalysts by adding MOF-74(Ni) and MIL-100(Fe) respectively to activate PMS for the degradation of venlafaxine (VEN).79 The results indicated that compared with the unmodified composite catalysts, the catalytic performance and the stability of the modified ones were greatly improved. However, the reduction of the surface area and active sites of ZIF-based composite functional materials is limited because the additive layer encapsulates the ZIF nanoparticles. Thus, on the premise of maintaining the advantages of the pristine ZIFs, it is still a challenge to construct ZIF-based macro-building materials.
2.3 ZIF derivatives for PMS activation
The above description demonstrates that pristine ZIFs and ZIF composites exhibit excellent performance to activate PMS for the removal of organic contaminants. More interestingly, they have been widely used as ideal self-sacrificing templates to prepare various nanomaterials by pyrolysis under controlled atmospheres, including carbon materials, metal oxides, and metal/C nanohybrid materials. The metal/C hybrid nanocomposites can be produced by the pyrolysis of ZIFs under an inert atmosphere (N2, Ar). Carbon materials can be generated by further acid etching to remove metal. In addition, metal oxides can be produced by direct thermal treatment of ZIFs in air. The derivatives obtained through pyrolysis can inherit the advantages of high porosity and large specific surface area of ZIF materials, and at the same time enhance some functions such as adsorption properties, water stability, chemical stability and thermal stability.119 Besides, N-doped carbon can be synthesized by carbonizing the N contained in ZIFs, which is an effective method to improve the electrical conductivity of carbon materials.116 Herein, ZIF derivatives are highlighted as emerging catalysts with good performance in PMS activation.In another work, a similar situation was observed where N-doped carbon nanotube frameworks (NCNTFs) were synthesized by pyrolysis and acid-washing of ZIF-67 (Fig. 5a).85 The NCNTFs had many nanotubes on the surface, which endowed them with rich mesopores and were beneficial to transfer and diffuse the reactive material. The results indicated that the NCNTFs obtained by pyrolysis at 800 °C possessed high N content and good graphitization degree, which displayed superior catalytic performance to degrade BPA. The possible mechanism is shown in Fig. 5b; PMS was easily absorbed on the surface of carbon nanotubes and interacted closely with active N-doped carbon networks. The catalyst would act as an electron transfer intermediate to enhance the electron transfer from BPA to PMS. Then the O–O bond of PMS was broken and various ROS were produced to degrade BPA. During the reaction process, the non-radical pathway (1O2) and the radicals (SO4˙−, ˙OH and O2˙−) were both involved in the removal of BPA.
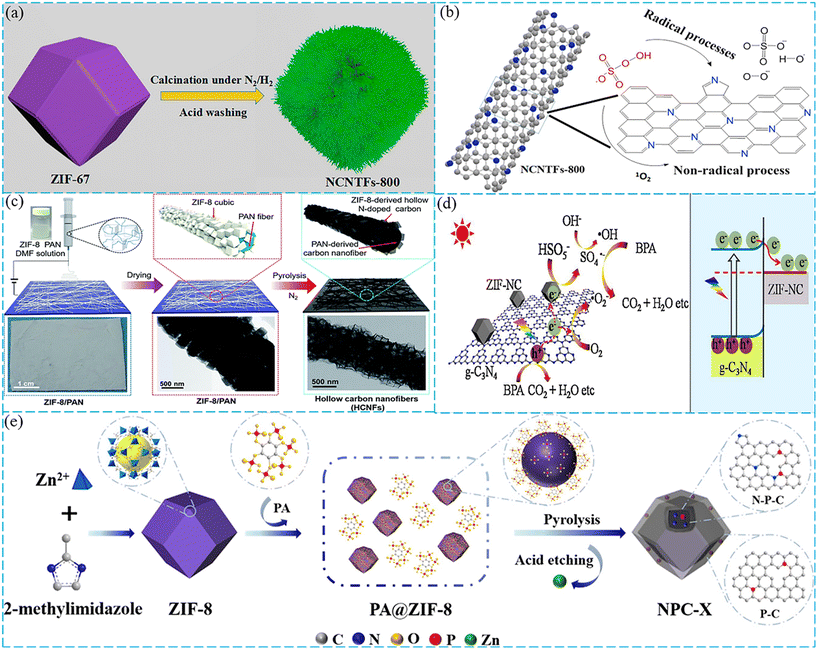 | ||
| Fig. 5 (a) Preparation of NCNTFs-800 using ZIF-67 as the precursor. (b) The radical and non-radical processes in PMS activation by NCNTFs-800. Reproduced with permission.85 Copyright 2018, Elsevier. (c) The schematic of the synthesis of MOF-based hollow carbon nanofibers (HCNFs). Reproduced with permission.86 Copyright 2019, The Royal Society of Chemistry. (d) Proposed photocatalytic mechanism of ZIF-NC/g-C3N4. Reproduced with permission.93 Copyright 2018, Elsevier. (e) Illustration of synthesis of NPC-X from ZIF-8 and PA. Reproduced with permission.90 Copyright 2021, Elsevier. | ||
Among the various structures of porous carbon materials, hollow structures have received much attention because they can provide more active sites. Wang et al. prepared one-dimensional (1D) hollow carbon nanofibers (HCNFs) with highly porous structures through the pyrolysis of ZIF-8/PAN composite nanofibers (Fig. 5c).86 During the carbonization process, the PAN layers caused the ZIF-8 to shrink from the inside out, resulting in a hollow structure, which acted as a “highway” to transfer electrons quickly. In addition, the hollow nanoparticles derived from ZIF-8 formed a unique nanostructure of HCNF through a network of fibers connected to each other, which accelerated the electron transport for the reaction. Therefore, up to 80% tetracycline (TC) removal was achieved within 20 min with HCNFs used as catalysts. In another report, Li et al. prepared N-doped three-dimensional (3D) hierarchically porous carbon by pyrolysis of core–shell composites composed of polystyrene (PS) cores and ZIF-8 shells.87 During high-temperature pyrolysis, Zn would be evaporated and the ZIF-8 shell could be pyrolyzed into a porous carbon material, while the PS cores decomposed to form hollow carbon materials due to the poor thermal stability. The results indicated that the large surface area and pore sizes of the catalyst increased the adsorption and catalytic activity of phenol by PMS activation.
Adding other nonmetallic atoms such as S, B or P is an effective method to further adjust the microstructure and catalytic activity of carbon materials.88,89 However, choosing P or S to dope porous carbon materials is more intractable than N, because the atomic radii of P (1.30 Å) and S (1.04 Å) are much larger than that of C (0.86 Å), while the radius of N (0.80 Å) is smaller. In recent years, Li et al. synthesized N–P co-doped porous carbons (NPC) with a core–shell structure by pyrolysis and acid etching of ZIF-8@phytic acid (PA) (Fig. 5e).90 NPC exhibited an excellent degradation rate for the removal of phenol by PMS activation (98.0% within 30 min), which was attributed to the fact that the doped P could provide more active sites and further regulated the charge distribution of the carbon network to accelerate the electron transport. Besides, Xie et al. prepared a boron and nitrogen co-doped porous carbon material with enhanced catalytic performance for PMS activation.91
The doping level and methods play an important role in the catalytic performance of porous carbon materials. Recent research indicated that excessive P concentration did not promote the catalytic performance of the catalyst due to the lack of complicated synergistic coupling effects. To sufficiently optimize the doping proportion of various heteroatoms, Ma et al. prepared novel N, P, and S tri-doped hollow carbon shells (NPSC) by using poly(cyclotriphosphazene-co-4,4′-sulfonyldiphenol) (PZS) as the shell precursor and ZIF-67 as the additional N source.92 Notably, the key of this method was that the doping level of P was manipulated by using different ratios of ZIF-67 to PZS. The synergistic effects of nonmetallic atoms were investigated and it was found high S content, limited P content, and rich N-doping in porous carbon materials were conducive to the breakage of O–O bonds in PMS and the redistribution of electrons in the carbon networks. Besides, the spin density, polarizability, and structural defects could also be regulated by doping heteroatoms (N, P, and S), which also could facilitate the breaking of the O–O bonds from PMS.
It has been demonstrated that the integration of g-C3N4 and carbon materials can broaden the light response range, which is beneficial to the production of photogenerated electrons and enhancement of photocatalytic performance with PMS activation. Therefore, coupling ZIF-derived porous carbon materials with g-C3N4 is a feasible way to promote the photocatalytic activity of g-C3N4 with PMS activation. The ZIF-derived N-doping carbon (ZIF-NC)/g-C3N4 composite synthesized by Gong's group exhibited superior photocatalytic performance to degrade BPA with PMS.93 In this composite, ZIF-NC was in close contact with the g-C3N4 interface, forming the ZIF-NC/g-C3N4 heterostructure. The formed heterostructure can shorten the distance of electron transport and provide additional surface area for the photocatalytic reaction. The possible mechanism of the photocatalytic reaction with PMS is illustrated in Fig. 5d. Upon visible light irradiation, the photogenerated electrons can transfer to the surface of ZIF-NC and g-C3N4 to yield SO4˙− and ˙O2− to rapidly degrade BPA. Simultaneously, the photogenerated holes in the valence band of g-C3N4 can directly react with BPA.
Recently, hollow nanomaterials with an inner cavity and porous thin wall have displayed outstanding catalytic performance in PMS activation due to their additional active sites, larger surface area, and better mass and charge transport.96 Khan et al. prepared a hollow Co3O4/C composite by a top-down etching strategy and two-step heat treatment method (Fig. 7a).97 The results showed that compared with other reported Co3O4 catalysts, the hollow Co3O4/C exhibited superior catalytic performance under comparable conditions, owing to the unique hollow structure that accelerated the mass transport for the catalytic reaction. Although the preparation of ZIF-derived carbon-coated Co3O4 (Co3O4/C) catalysts was an effective method to solve the problem of poor water stability of catalysts for PMS activation, it remained a challenge that Co3O4 nanoparticles easily agglomerate during catalysis.94,98 The growth of nanomaterials on large supports is the most promising strategy to resolve this problem. Therefore, Chen et al. mounted ZIF-derived carbon-coated Co3O4 nanoparticles onto porous biochar (BC) from Eichhornia crassipes to obtain a novel catalyst with high catalytic activity and good stability to degrade BPA (Fig. 7b).99 In this catalytic system, 100% of BPA was removed within less than 30 min. The high catalytic performance was attributed to the rich functional groups and high porosity of BC (Fig. 7c). The rich functional groups could interact with nanoparticles to enhance their dispersion and stability,100,101 while the highly porous structure could control the size of nanoparticles.102
Furthermore, the complex shell structures can make full use of the advantages of cavity structure and exhibit excellent catalytic activities.103 Chen et al. prepared Co3O4@Co–Fe oxide double-shelled nanocages (DSNCs) by pyrolysis of the precursor composed of ZIF-67 cores and Co–Fe PBA shells (Fig. 6).104 The results revealed that this catalyst reached about 99% removal of acid fuchsin (AF) by PMS activation within 20 min. In addition, yolk–shell nanoparticles (YSNs) with a cavity between the core and the shell have been widely researched for their unique physicochemical properties.105–108 In the process of degradation, metal ions provided electrons to PMS to generate SO4˙− and ˙OH, while they received electrons from the lattice oxygen in the PMS to maintain the oxidation reduction cycles of metal ions. Zhang et al. prepared a yolk–shell nanoreactor composed of a carbon-loaded Co3O4 core and SiO2 shell (Fig. 7d).109 This catalyst exhibited significantly improved catalytic activity toward BPA, and the degradation rate was more than 5 times compared with Co3O4. In this work, BPA and PMS could diffuse to the cavity through a silica shell of about 10 nm. During the reaction, mass transfer was derived from a concentration gradient that could be attributed to the confinement effect of the yolk–shell nanostructure, while the transfer of electrons from the catalyst to PMS was accelerated by the presence of graphitic carbon, and thus the reaction kinetics were increased. It was worth noting that the SiO2 shell not only protected the Co3O4/C core from external erosion, but also reduced the leaching of cobalt ions.
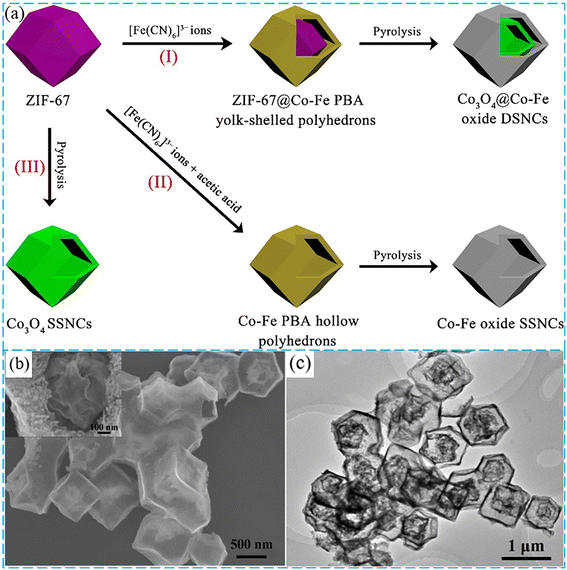 | ||
| Fig. 6 (a) Schematic illustration of routes towards MOF-derived double-shelled Co3O4@Co–Fe oxide, single-shelled Co–Fe oxide and Co3O4 nanocages. (b) SEM image of Co3O4@Co–Fe oxide DSNCs. (c) TEM image of Co3O4@Co–Fe oxide DSNCs. Reproduced with permission.104 Copyright 2020, Elsevier. | ||
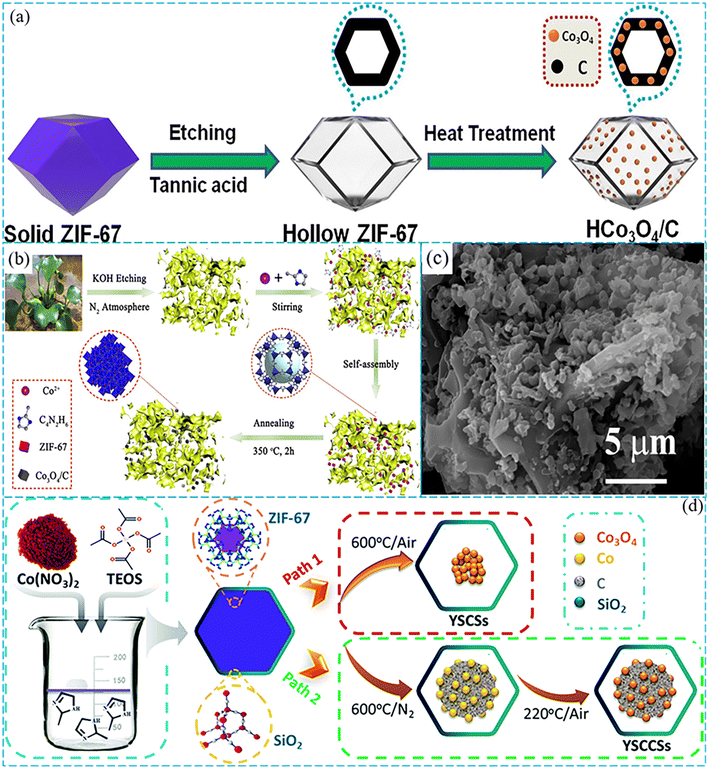 | ||
| Fig. 7 (a) Illustration of the synthesis procedure of ZIF-67, hollow ZIF-67, and HCo3O4/C. Reproduced with permission.97 Copyright 2019, Elsevier. (b) Schematic illustration of the fabrication process of Co3O4/C-BC. (c) SEM image of Co3O4/C-BC. Reproduced with permission.99 Copyright 2021, Elsevier. (d) Illustration of the preparation process of ZIF-67@SiO2, YSCSs and YSCCSs. Reproduced with permission.109 Copyright 2018, The Royal Society of Chemistry. | ||
Apart from monometallic oxides, ZIFs have also been used as precursors for the synthesis of multiple metallic oxides (e.g., AB2O4) for improved catalytic performance. For example, Fang et al. prepared mixed metal Co–Zn oxide (CoZnO-PC) by pyrolysis of embedded polyvinylpyrrolidone (PVP) encapsulated Co, Zn-bimetal centered ZIF and used them to degrade BPA.110 The results displayed that the ZnCoOx-based composites exhibited higher catalytic performance than the Co3O4-based composites because the electron transfer synergy between Co and Zn in the composites could significantly accelerate the PMS activation of the composites.111 The encapsulation of PVP in the ZIFs not only provided more N to the resulting carbon materials, but also prevented the collapse of the porous structure during the pyrolysis process.112 Moreover, metal oxide nanoparticles could be uniformly dispersed within the porous carbon framework without aggregation.113 More importantly, the “nanocages” constructed from porous carbon could also prevent the metal ions from leaching into the water.114 In another work, Zhao et al. prepared Zn doped Co oxides (ZnCoOx) by pyrolysis of Zn doped ZIF-67 in air.115 The results showed that 1O2 could be generated from oxygen vacancies in ZnCoOx, which could be adjusted by the doping level of Zn. The generated 1O2 played the most important role in the removal of contaminants, while SO4˙− played the partial role.
ZIF derived metal/C nanocomposites were first reported by Lin's group in 2015. They directly prepared magnetic Co graphene (MCG) nanocomposites by carbonizing the ZIF-67/GO composite for PMS activation (Fig. 10a).121 The transmission electron microscopy (TEM) image indicated that monoliths of MCG were assembled from reduced graphene oxide (RGO) sheets with Co-containing NPs, in which Co was in the form of cobalt oxide (Fig. 10b). Compared with the carbonized ZIF-67, MCG exhibited higher catalytic activity because the interfacial interaction between RGO and cobalt oxide improved the electron transport ability and chemical reaction sites. In another report, Li′s group similarly reported a Co/C nanocomposite by pyrolysis of ZIF-67 and activated carbon (AC).122 The results showed that this nanocomposite in the removal of RhB not only had enhanced adsorption capacity and catalytic performance, but also had good stability and recovery. Besides, Lei et al. used cow manure biochar as a carrier to prepare Co nanoparticles on carbon at different pyrolysis temperatures, and they revealed that the crystallinity and graphite degree increased with the increase of temperature for enhanced catalytic properties.123
Encapsulating metal species under carbon shells is an effective strategy to protect metal nanoparticles from leaching and endow carbon materials with magnetic properties. Li et al. synthesized magnetic N-doped carbon (Co@N-C) derived from ZIF-67 by pyrolysis and acid washing (Fig. 10c).124 As shown in Fig. 10d, the Co nanoparticles were uniformly distributed in the carbon shells and the leaching was restricted. Besides, the presence of cobalt not only endowed the nanocomposite with magnetic properties, but also contributed to the electron migration for the catalytic reaction.125–127 The results displayed that Co@N-C exhibited excellent catalytic activity for the degradation of BPA due to the reasonable structure design and processing. Additionally, a confinement pyrolysis strategy to obtain N-doped hierarchical carbon (NDHC) catalysts for activation of PMS processes was also proposed in another report (Fig. 8).128 After simple pyrolysis of phenolic resin (PR) coated ZIF particles, NDHC with rich active N sites, well-distributed hierarchical pore structure and high graphitization were prepared. During the carbonation process, the phenolic resin could limit the aggregation of Co nanoparticles and promote the formation of a smaller Co nanoparticle. The results showed that NDHC exhibited superior catalytic performance for the removal of BPA (nearly 98% in 5 min), which was attributed to the high graphitization degree promoting the electron transport. As shown in Fig. 8(a), both radical process and non-radical process appeared in the degradation system, but the non-radical process with 1O2 as the ROS was the major route. Additionally, Pang et al. synthesized a cobalt-based catalyst with rich carbon and nitrogen by electrospinning and calcination, which could largely reduce cobalt leaching.129
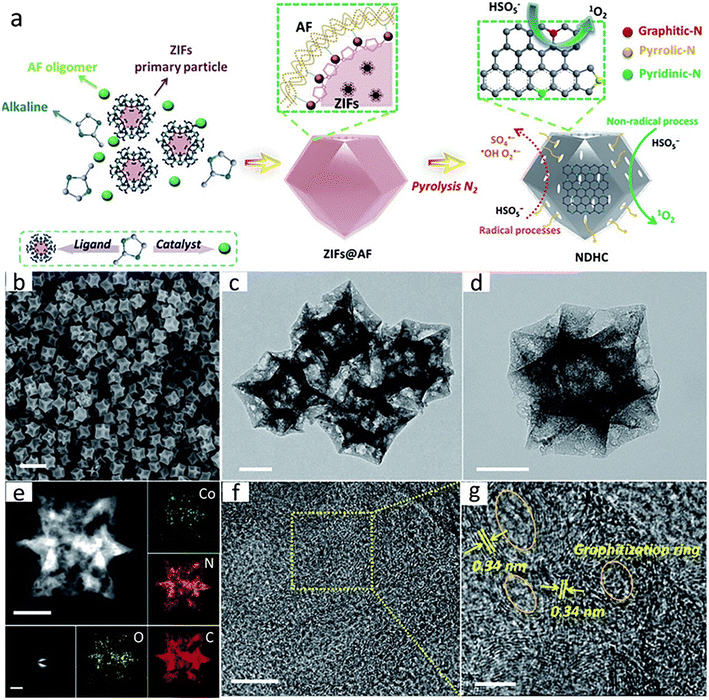 | ||
| Fig. 8 (a) Illustration of the preparation process of NDHC, (b) SEM image of NDHC-9, (c) TEM image of NDHC-9, (d) enlarged TEM image of NDHC-9, (e) the enlarged HAADF-STEM image, element maps and (f) and (g) HRTEM images of NDHC. Scale bar, 1 μm (b); 200 nm (c), (d) and (e); 5 nm insert (e); 10 nm (f); 5 nm (g). Reproduced with permission.128 Copyright 2019, The Royal Society of Chemistry. | ||
Metal sulfides with good electrical conductivity and low electronegativity have attracted increasing attention in PMS activation. For example, Zhu et al. prepared GO nanosheet-supported hollow Co sulfide nanocatalysts (CoS@GN) for the degradation of PBA by a facile self-templated ligand exchange step of ZIF-67@GN and a following thermal annealing step (Fig. 10e).130 The critical roles of the graphene carrier in regulating the types and action sites of radicals were explored firstly. The results indicated that adsorption and electrical conductivity of GO allowed the immediate reaction of SO4˙− with the contaminant once it was generated, which restricted the diffusion of SO4˙− on the catalyst surface and the production of ˙OH. Furthermore, to overcome the difficult recovery of nanoscale catalysts from water after use, they constructed a CoS@GN-coated membrane reactor using inert polytetrafluoroethylene (PTFE) (Fig. 10f). The results suggested that the reactor system possessed good reusability and system robustness.
Similarly, Zhang et al. prepared Co, N, and S co-doped dual-shelled hollow carbon nanocages (Co-NC-CoS) by encapsulating ZIF-67 with trithiocyanuric acid (TCA) and carbonizing it.131 During pyrolysis, the strong synergy between TCA and Co2+ might lead to the partial dissolution of ZIF-67, with different degrees of inner and outer layers of the core–shell catalyst shrinkage, which contributed to the formation of a dual-shelled structure with a hollow interior. The results suggested that Co-NC-CoS exhibited good degradation ability for various organic contaminants due to its yolk–shell structure and S doping. On the one hand, the inner cavities of yolk–shell particles could facilitate mass and charge transfer as well as the exposure of active sites. On the other hand, as an electron donor, the doped S2− with high reducibility promoted the conversion of Co3+ to Co2+ in the absence of PMS. The generated Co2+ would continue to react with PMS to generate SO4˙− for the removal of contaminants.
The role of the yolk–shell structure was further investigated in the catalytic process. Zhang et al. proposed a controllable etching strategy to synthesize a yolk–shell nanoreactor for the selective degradation of multicomponent contaminants (Fig. 9a).132 The YSCCNs exhibited improved BPA degradation ability, which was 23.1% and 45.4% higher than that of hollow carbon/Co nanoreactors (HCCNs) and solid carbon/Co nanoreactors (SCCNs) in the humus acid (HA)/BPA system. The possible synergistic mechanism is illustrated in Fig. 9b, and the excellent catalytic performance could be attributed to the synergetic effects of the shell layer (size-exclusion) and core/shell (confinement effect). In this YSCCN, the selective shell layer was able to intercept macromolecules and traverse small molecules, and the cavity between the core and shell of yolk–shell nanoparticles (YSNs) could play a specific role in separation, storage and confinement.133 Besides, Chen et al. prepared Co/Zn co-doped carbonaceous catalysts by pyrolysis of core–shell ZIF-67@ZIF-8.134 The narrow shell and blockage of the interfacial pores of catalysts could prevent the vaporization of Zn species. They revealed the Co/Zn synergy where Zn could regulate the electron distribution of Co and facilitate PMS adsorption for the generation of 1O2.
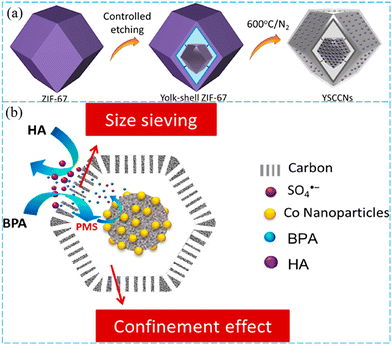 | ||
| Fig. 9 (a) Schematic illustration of the preparation process and selective removal mechanism of YSCCNs. (b) The possible synergistic mechanisms of BPA degradation on the YSCCNs. Reproduced with permission.132 Copyright 2020, American Chemical Society. | ||
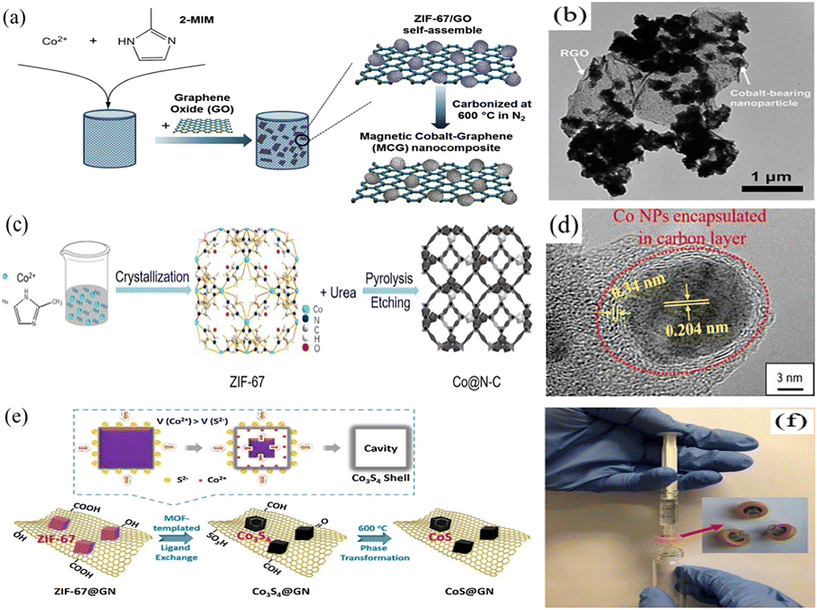 | ||
| Fig. 10 (a) Scheme of synthesis of the MCG nanocomposite. (b) TEM image of single-sheet MCG. Reproduced with permission.121 Copyright 2015, The Royal Society of Chemistry. (c) Schematic illustration of the synthesis procedure of magnetic N-doped carbon, Co@N-C. (d) HRTEM images of Co@N-C. Reproduced with permission.124 Copyright 2018, Elsevier. (e) Schematic diagram of the synthetic route of graphene-supported hollow cobalt sulfide nanocrystals. (f) Photograph of the CoS@GN-60-coated membrane reactor. Reproduced with permission.130 Copyright 2019, Elsevier. | ||
In recent years, single-atom catalysts (SACs) have been proven to be ideal choices for PMS activation due to their high atomic efficiencies and high surface energies. ZIF-8 is an ideal precursor for the synthesis of SACs, because various metals such as Mn, Fe, Co or Cu can be introduced to replace the evaporated Zn sites at high temperatures. He et al. prepared Fe–N–C catalysts for phenol degradation by pyrolysis of the Fe@ZIF precursor under inert atmosphere.135 During the pyrolysis, the FeNx sites were formed by the coordination between Fe species and pyridinic N, and the content of which depended on the Fe doping level. Impressively, the catalytic performance for contaminant degradation was improved by increasing the FeNx content. The well-distributed pores in Fe–N–C catalysts caused by the evaporation of Zn species contributed to the exposure of FeNx sites and the mass transfer.81 In addition, FeNx sites played the pivotal role in the catalytic process. On the one hand, the synergistic effects of Fe and N regulated the electron structure on the surface of Fe–N–C catalysts. On the other hand, the combination of PMS and electron-deficient FeNx sites with rapid electron transfer resulted in the successive activation of PMS to produce of 1O2. However, ZIF precursors tended to shrink and collapse during pyrolysis, which reduced the catalytic activity. Zhang et al. demonstrated that the use of SiO2 as a protective layer on the precursor is a feasible strategy to overcome this disadvantage.136 Besides, Hua et al. used anionic polyacrylamide (APAM) as a carrier to provide additional N and facilitate the uniform dispersion of the metals.137 Zou et al. introduced P into Co-based SACs derived from ZIF-8-Co and found that the doping of P could adjust the electronic structure and reactivity of Co, which contributed to the enhanced catalytic performance.138 Likewise, crystalline carbon nanotube interconnected Fe/Fe3C-doped nanoporous carbonitride139 and zero-valent Co–Fe encapsulated in nitrogen-doped porous carbon nanocomposites140 were also synthesized for PMS activation, both of which exhibited good performance for the degradation of multiple contaminants. The past research on the activation of PMS for degradation of organic contaminants by ZIF derivatives is summarized in Table 2.
| Catalysts | Contaminants | Optimal experiment terms | Removal efficiency | Reusability | Ref. |
|---|---|---|---|---|---|
| Hollow carbon nanofibers (HCNFs) | Tetracycline (TC) | TC = 50 mg L−1, catalyst = 0.2 g L−1, PMS = 0.5 g L−1, T = 25 °C, pH = 3.2 | 80% (20 min) | Catalytic activity decreased after the first run | 86 |
| N-doped hierarchical carbon (NDHC) | Bisphenol A (BPA) | BPA = 10 mg L−1, catalyst = 0.15 g L−1, PMS = 0.2 g L−1, T = 35 °C, pH = 5.65 | 98% (5 min) | Catalytic activity decreased after the first run | 128 |
| Nitrogen-doped and magnetic carbons (Co@N-C) | Bisphenol A (BPA) | BPA = 20 mg L−1, catalyst = 0.1 g L−1, PMS = 0.25 mM | 100% (10 min) | The degradation efficiency decreased in the second and third runs, respectively | 124 |
| N, P, and S tri-doped hollow carbon shells (NPSC) | Bisphenol A (BPA) | BPA = 25 mg L−1, catalyst = 0.06 g L−1, PMS = 0.4 g L−1, T = 20 °C | 90.1% (30 min) | Apparent decreased efficiency is observed for the used catalyst | 92 |
| Nitrogen doped porous carbons (NPCs) | Phenol | Phenol = 20 mg L−1, catalyst = 0.2 g L−1, PMS = 1.6 mM, T = 25 °C | 100% (50 min) | The NPC possesses good stability for repeated use | 84 |
| N-doped 3D hierarchically porous carbon | Phenol | Phenol = 20 mg L−1, catalyst = 0.1 g L−1, PMS = 0.5 g L−1, T = 30 °C | 95.4% (60 min) | The adsorption and catalytic degradation performance decreased after using | 87 |
| Core/shell structured N–P co-doped porous carbons | Phenol | Phenol = 50 mg L−1, catalyst = 0.05 g L−1, PMS = 0.3 g L−1, pH = 7.0 | 98% (30 min) | Slight loss of the catalytic activity after 4 recycles | 90 |
| Hollow carbon supported ultrafine Co3O4 nanoparticles (HCo3O4/C) | Bisphenol A (BPA) | BPA = 87.6 μM, catalyst = 0.1 g L−1, PMS = 325.3 μM, T = 25 °C, pH = 9.0 | 97% (4 min) | A slight decrease of the catalytic activity was observed after the 5th run | 97 |
| Yolk–shell Co3O4/C@SiO2 nanoreactors (YSCCSs) | Bisphenol A (BPA) | BPA = 20 mg L−1, catalyst = 0.1 g L−1, PMS = 0.1 g L−1, T = 25 °C, pH = 5.65 | 95% (5 min) | The recycled catalyst still remained highly active after 5 cycles | 109 |
| Co3O4@Co–Fe oxide double-shelled nanocages (DSNCs) | Acid fuchsin (AF) | AF = 15 mg L−1, catalyst = 0.1 g L−1, PMS = 0.3 g L−1, T = 25 °C | 99.1% (20 min) | The catalyst displayed good reusability over a ten-cycle run | 104 |
| CoZnO incorporated porous carbon composites (CoZnO-PC) | Bisphenol A (BPA) | BPA = 0.02 mM, catalyst = 0.1 g L−1, PMS = 2.0 mM, T = 25 °C | 100% (5 min) | The catalyst exhibited excellent degradation performance after five cycles | 110 |
| Co3O4/C-BC | Bisphenol A (BPA) | BPA = 20 mg L−1, catalyst = 0.3 g L−1, PMS = 1 mmol L−1, T = 30 °C, pH = 7 | 100% (30 min) | Maintaining high catalytic performance after six cycles | 99 |
| Magnetic cobalt–graphene (MCG) | Acid yellow (AY) | AY = 50 mg L−1, catalyst = 500 mg L−1, PMS = 90 mg L−1, T = 25 °C, pH = 3.8 | 100% (30 min) | The regeneration efficiency remained over 50 cycles | 121 |
| Co/N co-doped polyhedron carbonaceous catalyst (Co@N-C-2) | Orange II | Orange II = 80 mg L−1, catalyst = 100 mg L−1, PMS = 300 mg L−1 | 98.1% (15 min) | Apparent decreased efficiency is observed for the used catalyst | 155 |
| Co sites embedded in carbon nitride catalyst (CoCN) | Bisphenol A (BPA) | BPA = 20 mg L−1, V0 = 50 mL, PMS = 200 mg L−1, T = 25 °C | 100% (2 min) | The catalytic efficiency did not decrease significantly after six recoveries | 156 |
| Dual-shelled Co, N, and S codoped hollow carbon nanocages | 4-NP | 4-NP = 60 mg L−1, catalyst = 10 mg, PMS = 200 mg, T = 28 °C | 100% (20 min) | The catalyst was slightly decreased after 4 cycles | 157 |
| Nanocomposites prepared from zero-valent Co–Fe encapsulated in N–C nanoparticles | 4-Chlorophenol (4-CP) | 4-CP = 50 mg L−1, catalyst = 89 mg L−1, PMS = 1.1 g L−1, T = 30 °C | 99.1% (30 min) | Without a significant drop in catalytic performance during the five consecutive cycle tests | 158 |
| N-doped porous carbon encapsulated magnetic Co nanoparticles (Co@NC-800) | Tetracycline (TC) | TC = 30 mg L−1, catalyst = 0.2 g L−1, PMS = 0.2 g L−1 | 90.1% (3 min) | The removal efficiency was almost unchanged after being reused 4 times | 159 |
| Magnetic recoverable and micro-nanostructural catalyst (Co@N-PC) | Methylene blue (MB) | MB = 30 mg L−1, catalyst = 10 mg L−1, PMS = 0.5 mM, T = 25 °C | 100% (30 min) | Slight loss of the catalytic activity after 4 recycles | 160 |
| P-doped Co-based SACs (ZIF-CoN3P-C) | Sulfadiazine | Sulfadiazine = 30 mg L−1, catalyst = 0.05 g L−1, PMS = 1 mM, T = 20 °C | 98.4% (5 min) | The catalyst retained its catalytic performance after five test cycles | 138 |
| Fe/N-co-doped porous carbon (Fe/N-PC) material | Aflatoxin B1 (AFB1) | Sulfadiazine = 2.5 mg L−1, catalyst = 0.25 mg, PMS = 1.0 mmol L−1, pH = 7 | 99.88% (30 min) | The degradation was maintained at levels above 75% over three cycles | 136 |
| Yolk–shell Co/C nanoreactors (YSCCNs) | Bisphenol A (BPA)/humus acid (HA) | BPA = 20 mg L−1, HA = 10 and 20 ppm, catalyst = 0.1 g L−1, PMS = 0.15 g L−1, pH = 8.23 | 100% (15 min) | Catalytic activity slightly decreased after 5 cycles | 161 |
3. The application of ZIFs/PMS in wastewater treatment
In actual wastewater, the effect of pH on the degradation efficiency must be considered. When the net charge on the catalyst surface is zero, the pH in the water at this point is called the isoelectric point. When pH > pHpzc (point-of-zero), the negative charge on the catalyst surface can result in electrostatic repulsion between the catalyst surface and the PMS, leading to the decline of degradation. Besides, PMS may self-decompose without producing SO4˙− under alkaline conditions.59 When pH < pHpzc, the positive charge on the catalyst surface can lead to the formation of CoOH+ and attract H+ to stabilize on PMS, which may inhibit the PMS activation.97 Therefore, relatively speaking, N-doped carbon catalysts without metal are more suitable for catalytic degradation of contaminants at lower pH. Studies have shown that the Co@N-C/PMS system could accelerate the degradation of Orange II in river water due to the strong buffer capacity against pH drop in river water.155There are many kinds of inorganic anions (Cl−, HCO3−, and H2PO4−) and natural organic matter present in wastewater, which can inhibit the catalytic efficiency to different degrees. Therefore, it is necessary to investigate their influences on contaminant degradation. Cl− is widely present in natural water bodies and generally inhibits the degradation system of organic contaminants as Cl− can consume ˙OH and SO4˙−. However, excessive Cl− can produce a large amount of HOCl and Cl2, which has beneficial effects on the degradation of contaminants. H2PO4− can form a complex with Co2+ in the catalyst through a chelation reaction, which can occupy the active radical sites. Besides, H2PO4− can also consume radicals in water bodies. HCO3− has no significant effect on the final degradation removal efficiency, but the addition of HCO3− can slightly inhibit the degradation and buffer the change of pH values.97 Natural organic matter represented by humic acid contains many hydroxyl and carboxyl groups which usually inhibit the degradation of contaminants by quenching radicals in solution and binding with the active sites of metals.54 The HCo3O4/C/PMS system has been applied on river water and the results suggested that the rate constant of BPA degradation decreased significantly, which was attributed to the high concentration of ions and natural organic matter in the river water.97
4. Catalytic mechanisms
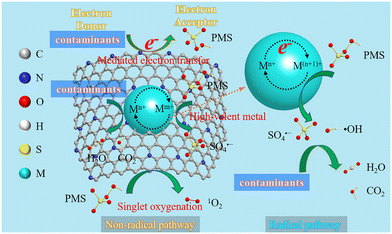
Up to now, the possible mechanisms of the generation of ROS by PMS activation have been explored by using radical quenching experiments, electron paramagnetic resonance (EPR) analyses, and theoretical calculations. In general, the degradation mechanisms of PMS activation to remove organic contaminants by ZIF-based materials include radical and nonradical pathways. Pristine ZIFs, ZIF composites, and ZIF-derived metal-oxides degrade contaminants by PMS activation mainly through the radical pathway, while ZIF-derived metal/carbon hybrids and carbon materials are commonly dominant through the nonradical pathway. In general, electron-deficient contaminants are easily degraded by radicals such as SO4˙− and ˙OH through the free radical pathway, while electron-rich species with the ability of donating electrons such as phenolic and dyes can be rapidly degraded through the nonradical pathway.141 Therefore, the catalysts for the corresponding degradation pathways can be selected according to the nature of contaminants and designed rationally based on factors such as conductivity and carbon to nitrogen ratio. It is worth noting that the leading role of radical and non-radical pathways in the process of contaminant degradation is still unclear, and it is mainly affected by various conditions, such as the catalyst active site, oxidant type, pH value, and reaction rate of the pollutants with ROS.4.1 Role of the radical pathway
Generally, radicals including SO4˙−, ˙OH and O2˙− are considered as the main active species during PMS activation. Electron transfer is the basis of the radical pathway, in which transition metal ions in ZIF-based catalysts can act as electron donors for the breakage of the O–O bonds and the production of SO4˙− and/or ˙OH. SO4˙− is a selective ROS that is conducive to one-electron oxidation. It can easily oxidize aromatic compounds with ring activating groups, such as p-hydroxybenzoic acid, while aromatic compounds with ring deactivating groups, such as nitrobenzene, show poor activity. In contrast, ˙OH, as a relatively non-selective ROS, shows degrading activity against most organic pollutants.130 In addition, some peroxides appearing in the reaction may lead to the generation of O2˙− by decomposing or reacting with ˙OH.142 However, the participation of O2˙− in the production of 1O2 makes it difficult to determine whether O2˙− is merely an intermediate or an ROS that degrades pollutants.The introduction of heteroatoms can break up the inert pristine carbon network and generate some new charged sites which make it easy for catalysis.143 Specifically, the electronegativity of N (3.04) and S (2.58) is higher than that of C (2.55), so these electron-rich atoms with lone-paired electrons can extract electrons from adjacent carbon atoms. Similarly, electron-deficient P atoms with lower electronegativity (2.19) can also act as electron donors to produce more active sites in carbon networks.144 Moreover, heteroatom modification (N, P, and S) helps to modulate the spin density, polarizability, and structural defects of PMS, thereby accelerating the breaking of O–O bonds in PMS.92
Moreover, ZIF-based photocatalysts can be used for photocatalysis with PMS through a unique radical process. When the energy of light illuminating the photocatalyst is greater than or equal to the energy gap, photogenerated electrons are transitioned from the valence band to the conduction band and can react to generate radicals. Meanwhile, photogenerated holes with oxidation ability are also generated on the valence band. The formed ROS can degrade the organic contaminants quickly together with photogenerated holes from the valence band.145,146
4.2 Role of the non-radical pathway
Both radical and non-radical pathways occur in the PMS activation of ZIF-derived metal/C hybrid nanocomposites and metal-free carbon materials. Graphitic N in the catalyst can greatly break up the inert carbon networks, which may significantly improve the conductivity of carbon catalysts and induce the non-radical pathway.116 Meanwhile, the amorphous carbon transfers electrons directly to the oxidant to accelerate the production of radicals.85 The generation of 1O2, high-valent metals and mediated electron transfer are the dominant ways to induce the non-radical pathway.As a non-radical ROS, 1O2 with milder oxidative potential can selectively oxidize electron-rich organic compounds. Graphitic N and carbonyl groups (C–O) are the vital active sites for the generation of 1O2 through nucleophilic addition and mediating a peroxide intermediate. Also, the recombination of ROS and the self-decomposition of PMS are two ways for the generation of 1O2. Besides, Co–Nx or FeNx in some catalysts acts as the active sites to directly adsorb PMS to form carbon-persulfate* complexes (*PMS) for the formation of S2O82−, SO4− and 1O2 by PMS activation.135,147 Several studies have made other speculations about the mechanism of 1O2 generation on metal oxides. 1O2 is generated from oxygen activated by oxygen vacancies148 and oxygen in the lattice.149 Due to the strong Lewis acidity of oxygen vacancies, PMS could be adsorbed onto the surface of the catalysts and decomposed into O2. The oxygen vacancies might be converted to lattice oxygen by oxygen migration, completing the cycle of O2−/O2. Transition metals could accept electrons from lattice oxygen and promote their oxidation–reduction cycle. The produced active oxygen from the reaction can be coupled with PMS to produce 1O2. Besides, oxygen vacancies can be easily converted to active oxygen.
| SO52− + HSO5− → SO42− + HSO4− + 1O2 | (3) |
| SO4˙− + HSO5− → SO5˙− + HSO4− | (4) |
| 2SO5˙− + H2O → 2HSO5− + 1O2 | (5) |
| O2˙− + OH˙ → OH− + 1O2 | (6) |
| 2O2˙− + 2H+ → H2O2 + 1O2 | (7) |
On the other hand, another typical non-radical pathway in the catalytic reaction is the mediated electron transfer. The mediated electrons are transferred from the contaminant (electron donor) to the catalyst (electron transfer intermediate) and then to PMS (electron acceptor). The electron density of positively charged adjacent carbon configurations can be modulated to produce electron transfer intermediates that accelerate the electron transport from the catalyst to PMS. The electron transfer intermediates can transfer electrons from the contaminant directly to PMS to break the O–O bond. In general, this unique non-radical pathway by mediated electron transfer relies on good electrical conductivity, high graphitization and N-doping degree in the catalysts.
In addition to the above two non-radical pathways, the recently discovered high-valent metal species are another effective pathway for the SR-AOPs. These active species are produced by the reaction of reduced metal ions (such as Fe(II), Ag(I) and Co(II)) with persulfate for direct oxidation of organic pollutants. For example, FeSA–N–C obtained by one-step pyrolysis of Fe-doped ZIF could selectively oxidize organic pollutants by the generated high-valent iron–oxygen species (FeIV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and FeV
O and FeV![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O).135 However, it should be noted that these high-valence metal intermediates are very unstable, and this mechanism can only be proved by some indirect evidence or theoretical calculations.
O).135 However, it should be noted that these high-valence metal intermediates are very unstable, and this mechanism can only be proved by some indirect evidence or theoretical calculations.
At present, it is still challenging to quantify the contribution of the mechanism in the non-radical pathway. Adding a chemical probe is a promising method, but there is still a lack of viable quenching methods to quantify the contribution. More attention should be paid to tackle this issue. Finally, the different mechanisms of ZIF-based materials in PMS activation are schematically illustrated in Fig. 11.
5. Reusability and stability of ZIF-based materials in PMS activation
Good reusability and stability contribute to the long-term practical applications of ZIF-based materials. Some methods including scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared (FTIR) and X-ray photoelectron spectroscopy (XPS) can be compared to evaluate the structural stability.150 It was found that the structure and properties of ZIF-based materials did not change significantly and they maintained good stability after several recycles. Composite fabrication is an effective method to improve stability, where the integration of ZIFs and other functional materials can acquire new functions such as hydrophobicity and hydrostability. Impressively, the shell in the catalysts with core–shell structure (such as SiO2 shell, carbon shell, etc.) can inhibit metal ion leaching. For example, the yolk–shell Co3O4/C@SiO2 nanoreactor exhibited high degradation performance and stability, decomposing 95% of BPF within 5 min.109 In addition, the catalyst maintained highly activity even after 5 cycles (90% of BPF was decomposed within 7 min).It has been proven that the catalytic performance of carbonaceous catalysts after several cycles will significantly decrease. For example, HCNFs decomposed 80% of TC within 20 min and NDHC decomposed 98% of BPF within 5 min, but their catalytic activity significantly decreased to 52.1% and 35%, respectively, after the first run.86,128 By investigating the changes in catalyst surface composition after cycling, it can be concluded that the decrease of efficiency might be due to the coverage of active sites by intermediates. Interestingly, thermal annealing could restore the catalytic performance by regenerating C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and graphitic N contents in the catalysts. The catalytic activity of HCNFs was almost completely recovered after thermal annealing. Considering the decrease of cobalt content and the relatively high energy cost in the thermal treatment, other more feasible methods such as solvent desorption151 and alkaline washing152 to recover the activity of carbonaceous catalysts were also worth trying.
O and graphitic N contents in the catalysts. The catalytic activity of HCNFs was almost completely recovered after thermal annealing. Considering the decrease of cobalt content and the relatively high energy cost in the thermal treatment, other more feasible methods such as solvent desorption151 and alkaline washing152 to recover the activity of carbonaceous catalysts were also worth trying.
6. Conclusions and perspectives
As described in this review, ZIFs, ZIF composites and ZIF derivatives as activators for PMS exhibited excellent catalytic performance in eliminating organic contaminants and overcame the deficiencies of traditional materials.162,163 Compared with powdery ZIF-based catalysts, ZIF-based macroscopical materials including aerogels and fiber filters have the advantages of easy separation, performance, industrialization and cost in PMS activation. The easy separation property of ZIF-based materials is a guarantee for industrial application in PMS activation. However, further applications of these materials in water treatment have some drawbacks such as the leaching of toxic metal ions. How to inhibit metal dissolution is the most critical problem in the ZIF-based PMS activation reaction. Although great progress has been made in the application of ZIF-based materials for PMS activation, related research is still at a primary stage and the technology needs further improvements. The future prospects in the field of ZIF-based PMS activation are as follows:1. The ultimate goal of ZIF-based PMS activation research is industrialization. ZIF-based materials still appear to be quite expensive compared to the easily available carbon materials. It is necessary to improve the cost-effectiveness of using ZIFs to reduce manufacturing costs and enable mass production.
2. Most of the current studies have been carried out in a laboratory setting to simulate wastewater. However, many co-existing substances in water could consume a lot of free radicals and reduce the degradation efficiency of target contaminants. Therefore, it is imperative to study effective methods to prepare ZIF-derived nanostructures with controllable pore structures to achieve the selective removal of multicomponent contaminants.
3. Studies have shown that the structure of ZIF-based catalysts is a critical factor to determine the catalytic performance. Some special shell structure can prevent the collapse. Also, some hollow structures, hierarchical structures and nanotubes with a high surface area and porosity can enhance the catalytic performance. Therefore, it is necessary to explore effective preparation methods to regulate the structure of catalysts and further improve the application of the ZIF-based catalysts in PMS.
4. Experiments to date have shown that most ZIF-based catalysts have good stability, but the stability of some catalysts is still unsatisfactory. Composite fabrication and thermal annealing are popular methods to further improve stability. For example, ZIFs can be encapsulated into other materials to prevent hydrolysis and collapse. Due to the special requirements of different synthesis strategies, these targeted approaches may be worth further investigating.
5. Existing studies have shown that the integration of ZIFs with functional materials can combine their advantages to provide superior performance. For example, when combined with magnetic metal oxides, the composites offer the advantage of easy recovery. When combined with suitable photocatalytic materials, the light absorption properties of ZIF composites can be readily adjusted for efficient utilization of solar light. Therefore, it is very promising to explore the possibility of combining with more functional materials in future investigations.
6. According to reported studies, the most investigated contaminants are dyes, phenol and BPA. The degradation efficiency of emerging contaminants such as pharmaceuticals and personal care products (PPCPs), endocrine disrupters and fluoroquinolone antibiotics needs more research.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This study was financially supported by the Program for the National Natural Science Foundation of China (51909084, 51909085) and the Fundamental Research Funds for the Central Universities (531118010247).References
- L. J. Xu, X. M. Zhang, J. G. Han, H. Gong, L. Meng, X. Mei, Y. Sun, L. Y. Qi and L. Gan, Degradation of emerging contaminants by sono-Fenton process with in situ generated H2O2 and the improvement by P25-mediated visible light irradiation, J. Hazard. Mater., 2020, 391, 10 CrossRef PubMed.
- M. H. Hsu, C. J. Tsai and A. Y. C. Lin, Mechanism and pathways underlying the self-sensitized photodegradation of methotrexate under simulated solar irradiation, J. Hazard. Mater., 2019, 373, 468–475 CrossRef CAS PubMed.
- Q. H. Ji, S. Tabassum, S. Hena, C. G. Silva, G. X. Yu and Z. J. Zhang, A review on the coal gasification wastewater treatment technologies: past, present and future outlook, J. Cleaner Prod., 2016, 126, 38–55 CrossRef CAS.
- J. J. Liu, B. Pemberton, J. Lewis, P. J. Scales and G. J. O. Martin, Wastewater treatment using filamentous algae-A review, Bioresour. Technol., 2020, 298, 15 Search PubMed.
- Y. Z. Lin, L. B. Zhong, S. Dou, Z. D. Shao, Q. Liu and Y. M. Zheng, Facile synthesis of electrospun carbon nanofiber/graphene oxide composite aerogels for high efficiency oils absorption, Environ. Int., 2019, 128, 37–45 CrossRef CAS PubMed.
- H. D. Liu, M. Cheng, Y. Liu, G. X. Zhang, L. Li, L. Du, B. Li, S. Xiao, G. F. Wang and X. F. Yang, Modified UiO-66 as photocatalysts for boosting the carbon-neutral energy cycle and solving environmental remediation issues, Coord. Chem. Rev., 2022, 458, 33 CrossRef.
- G. F. Wang, D. L. Huang, M. Cheng, S. Chen, G. X. Zhang, L. Du, L. Lei, Y. S. Chen, R. J. Li and Y. Liu, An insight into the bridging role of Co3O4 in MOF-derived binary metal oxide modified sheet-like g-C3N4 for photo-assisted peroxymonosulfate activation, Environ. Sci.: Nano, 2022, 9, 4393–4410 RSC.
- G. F. Wang, D. L. Huang, M. Cheng, S. Chen, G. X. Zhang, L. Lei, Y. S. Chen, L. Du, R. J. Li and Y. Liu, Metal-organic frameworks template-directed growth of layered double hydroxides: A fantastic conversion of functional materials, Coord. Chem. Rev., 2022, 460, 22 CrossRef.
- Y. Liu, H. Cheng, M. Cheng, Z. F. Liu, D. L. Huang, G. X. Zhang, B. B. Shao, Q. H. Liang, S. H. Luo, T. Wu and S. Xiao, The application of Zeolitic imidazolate frameworks (ZIFs) and their derivatives based materials for photocatalytic hydrogen evolution and pollutants treatment, Chem. Eng. J., 2021, 417, 21 Search PubMed.
- L. Du, W. H. Xu, S. B. Liu, X. Li, D. L. Huang, X. F. Tan and Y. G. Liu, Activation of persulfate by graphitized biochar for sulfamethoxazole removal: The roles of graphitic carbon structure and carbonyl group, J. Colloid Interface Sci., 2020, 577, 419–430 CrossRef CAS PubMed.
- H. D. Liu, M. Cheng, Y. Liu, J. Wang, G. X. Zhang, L. Li, L. Du, G. F. Wang, S. Z. Yang and X. Y. Wang, Single atoms meet metal-organic frameworks: collaborative efforts for efficient photocatalysis, Energy Environ. Sci., 2022, 15, 3722–3749 RSC.
- Y. X. Chen, M. Cheng, C. Lai, Z. Wei, G. X. Zhang, L. Li, C. S. Tang, L. Du, G. F. Wang and H. D. Liu, The Collision between g-C3N4 and QDs in the Fields of Energy and Environment: Synergistic Effects for Efficient Photocatalysis, Small, 2023, 19(14), 2205902 CrossRef CAS PubMed.
- Y. J. Liu, Z. F. Liu, G. M. Zeng, M. Chen, Y. L. Jiang, B. B. Shao, Z. G. Li and Y. Liu, Effect of surfactants on the interaction of phenol with laccase: Molecular docking and molecular dynamics simulation studies, J. Hazard. Mater., 2018, 357, 10–18 CrossRef CAS PubMed.
- D. Huang, G. Zhang, J. Yi, M. Cheng, C. Lai, P. Xu, C. Zhang, Y. Liu, C. Zhou, W. Xue, R. Wang, Z. Li and S. Chen, Progress and challenges of metal-organic frameworks-based materials for SR-AOPs applications in water treatment, Chemosphere, 2021, 263, 127672 CrossRef CAS PubMed.
- G. X. Zhang, D. L. Huang, M. Cheng, L. Lei, S. Chen, R. Z. Wang, W. J. Xue, Y. Liu, Y. S. Chen and Z. H. Li, Megamerger of MOFs and g-C3N4 for energy and environment applications: upgrading the framework stability and performance, J. Mater. Chem. A, 2020, 8, 17883–17906 RSC.
- Q. K. Shi, S. Deng, Y. L. Zheng, Y. L. Du, L. Li, S. Z. Yang, G. X. Zhang, L. Du, G. F. Wang, M. Cheng and Y. Liu, The application of transition metal-modified biochar in sulfate radical based advanced oxidation processes, Environ. Res., 2022, 212, 15 Search PubMed.
- W. H. Fu, J. Yi, M. Cheng, Y. Liu, G. X. Zhang, L. Li, L. Du, B. Li, G. F. Wang and X. F. Yang, When bimetallic oxides and their complexes meet Fenton-like process, J. Hazard. Mater., 2022, 424, 15 Search PubMed.
- W. J. Xiao, M. Cheng, Y. Liu, J. Wang, G. X. Zhang, Z. Wei, L. Li, L. Du, G. F. Wang and H. D. Liu, Functional Metal/Carbon Composites Derived from Metal-Organic Frameworks: Insight into Structures, Properties, Performances, and Mechanisms, ACS Catal., 2023, 13, 1759–1790 CrossRef CAS.
- M. Cheng, G. M. Zeng, D. L. Huang, C. Lai, Y. Liu, C. Zhang, J. Wan, L. Hu, C. Y. Zhou and W. P. Xiong, Efficient degradation of sulfamethazine in simulated and real wastewater at slightly basic pH values using Co-SAM-SCS /H2O2 Fenton-like system, Water Res., 2018, 138, 7–18 CrossRef CAS PubMed.
- Y. F. Rao, F. M. Han, Q. Chen, D. Wang, D. Xue, H. Wang and S. Y. Pu, Efficient degradation of diclofenac by LaFeO3-Catalyzed peroxymonosulfate oxidation-kinetics and toxicity assessment, Chemosphere, 2019, 218, 299–307 CrossRef CAS PubMed.
- W. D. Oh, Z. L. Dong and T. T. Lim, Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects, Appl. Catal., B, 2016, 194, 169–201 CrossRef CAS.
- C. He, W. Xia, C. Y. Zhou, D. L. Huang, C. Zhang, B. Song, Y. Yang, J. Li, X. Xu, Y. N. Shang and L. Du, Rational design to manganese and oxygen co-doped polymeric carbon nitride for efficient nonradical activation of peroxymonosulfate and the mechanism insight, Chem. Eng. J., 2022, 430, 11 CrossRef.
- C. Y. Zhou, E. Almatrafi, X. F. Tang, B. B. Shao, W. Xia, B. Song, W. P. Xiong, W. J. Wang, H. Guo, S. Chen and G. M. Zeng, Investigation on the structure-performance of phthalic acid carboxyl position and carbon nitride towards efficient photocatalytic degradation of organic pollutants, Sep. Purif. Technol., 2022, 286, 10 Search PubMed.
- X. F. Tang, C. Y. Zhou, W. Xia, Y. T. Liang, Y. X. Zeng, X. Y. Zhao, W. P. Xiong, M. Cheng and Z. W. Wang, Recent advances in metal-organic framework-based materials for removal of fluoride in water: Performance, mechanism, and potential practical application, Chem. Eng. J., 2022, 446, 17 Search PubMed.
- S. Guerra-Rodriguez, E. Rodriguez, D. N. Singh and J. Rodriguez-Chueca, Assessment of Sulfate Radical-Based Advanced Oxidation Processes for Water and Wastewater Treatment: A Review, Water, 2018, 10, 20 Search PubMed.
- X. X. Zheng, X. J. Niu, D. Q. Zhang, M. Y. Lv, X. Y. Ye, J. L. Ma, Z. Lin and M. L. Fu, Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review, Chem. Eng. J., 2022, 429, 22 CrossRef.
- X. Zhao, Y. X. Wang, D. S. Li, X. H. Bu and P. Y. Feng, Metal-Organic Frameworks for Separation, Adv. Mater., 2018, 30, 34 Search PubMed.
- L. E. Kreno, K. Leong, O. K. Farha, M. Allendorf, R. P. Van Duyne and J. T. Hupp, Metal-Organic Framework Materials as Chemical Sensors, Chem. Rev., 2012, 112, 1105–1125 CrossRef CAS PubMed.
- M. Yoon, R. Srirambalaji and K. Kim, Homochiral Metal-Organic Frameworks for Asymmetric Heterogeneous Catalysis, Chem. Rev., 2012, 112, 1196–1231 CrossRef CAS PubMed.
- J. A. Mason, M. Veenstra and J. R. Long, Evaluating metal-organic frameworks for natural gas storage, Chem. Sci., 2014, 5, 32–51 RSC.
- P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Ferey, R. E. Morris and C. Serre, Metal-Organic Frameworks in Biomedicine, Chem. Rev., 2012, 112, 1232–1268 CrossRef CAS PubMed.
- W. Xia, A. Mahmood, R. Q. Zou and Q. Xu, Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion, Energy Environ. Sci., 2015, 8, 1837–1866 RSC.
- S. Chaemchuen, N. A. Kabir, K. Zhou and F. Verpoort, Metal-organic frameworks for upgrading biogas via CO2 adsorption to biogas green energy, Chem. Soc. Rev., 2013, 42, 9304–9332 RSC.
- B. Y. Guan, X. Y. Yu, H. B. Wu and X. W. Lou, Complex Nanostructures from Materials based on Metal-Organic Frameworks for Electrochemical Energy Storage and Conversion, Adv. Mater., 2017, 29, 20 Search PubMed.
- R. Banerjee, A. Phan, B. Wang, C. Knobler, H. Furukawa, M. O'Keeffe and O. M. Yaghi, High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture, Science, 2008, 319, 939–943 CrossRef CAS PubMed.
- R. Banerjee, H. Furukawa, D. Britt, C. Knobler, M. O'Keeffe and O. M. Yaghi, Control of Pore Size and Functionality in Isoreticular Zeolitic Imidazolate Frameworks and their Carbon Dioxide Selective Capture Properties, J. Am. Chem. Soc., 2009, 131, 3875-+ CrossRef CAS PubMed.
- K. S. Park, Z. Ni, A. P. Cote, J. Y. Choi, R. D. Huang, F. J. Uribe-Romo, H. K. Chae, M. O'Keeffe and O. M. Yaghi, Exceptional chemical and thermal stability of zeolitic imidazolate frameworks, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 10186–10191 CrossRef CAS PubMed.
- C. X. Duan, Y. Yu and H. Hu, Recent progress on synthesis of ZIF-67-based materials and their application to heterogeneous catalysis, Green Energy Environ., 2022, 7, 3–15 CrossRef CAS.
- G. H. Zhong, D. X. Liu and J. Y. Zhang, The application of ZIF-67 and its derivatives: adsorption, separation, electrochemistry and catalysts, J. Mater. Chem. A, 2018, 6, 1887–1899 RSC.
- G. P. Anipsitakis and D. D. Dionysiou, Radical generation by the interaction of transition metals with common oxidants, Environ. Sci. Technol., 2004, 38, 3705–3712 CrossRef CAS PubMed.
- K. Y. A. Lin and H. A. Chang, Zeolitic Imidazole Framework-67 (ZIF-67) as a heterogeneous catalyst to activate peroxymonosulfate for degradation of Rhodamine B in water, J. Taiwan Inst. Chem. Eng., 2015, 53, 40–45 CrossRef CAS.
- J. K. Cong, F. Lei, T. Zhao, H. Liu, J. M. Wang, M. T. Lu, Y. W. Li, H. Xu and J. K. Gao, Two Co-zeolite imidazolate frameworks with different topologies for degradation of organic dyes via peroxymonosulfate activation, J. Solid State Chem., 2017, 256, 10–13 CrossRef CAS.
- B. Yao, S.-K. Lua, H.-S. Lim, Q. Zhang, X. Cui, T. J. White, V. P. Ting and Z. Dong, Rapid ultrasound-assisted synthesis of controllable Zn/Co-based zeolitic imidazolate framework nanoparticles for heterogeneous catalysis, Microporous Mesoporous Mater., 2021, 314, 110777 CrossRef CAS.
- J. K. Zareba, M. Nyk and M. Samoc, Co/ZIF-8 Heterometallic Nanoparticles: Control of Nanocrystal Size and Properties by a Mixed-Metal Approach, Cryst. Growth Des., 2016, 16, 6419–6425 CrossRef CAS.
- Y. Fang, B. Y. Qian, Y. Yang, Y. Song, Z. G. Yang and H. P. Li, Purification of high-arsenic groundwater by magnetic bimetallic MOFs coupled with PMS: Balance of catalysis and adsorption and promotion mechanism of PMS, Chem. Eng. J., 2022, 432, 10 CrossRef.
- F. Ling, X. F. Xiao, Y. J. Li and W. F. Li, A Zn/Co bimetal zeolitic imidazolate framework material as a catalyst to activate persulfates to degrade tylosin in aqueous solutions, New J. Chem., 2022, 46, 18917–18925 RSC.
- A. T. Gu, P. Wang, K. W. Chen, E. D. Miensah, C. H. Gong, Y. Jiao, P. Mao, K. Chen, J. L. Jiang, Y. Liu and Y. Yang, Core-shell bimetallic Fe-Co MOFs to activated peroxymonosulfate for efficient degradation of 2-chlorophenol, Sep. Purif. Technol., 2022, 298, 14 CrossRef.
- K. Zhang, D. D. Sun, C. Ma, G. L. Wang, X. L. Dong and X. X. Zhang, Activation of peroxymonosulfate by CoFe2O4 loaded on metal-organic framework for the degradation of organic dye, Chemosphere, 2020, 241, 11 Search PubMed.
- L. Hu, G. Deng, W. Lu, Y. Lu and Y. Zhang, Peroxymonosulfate activation by Mn3O4/metal-organic framework for degradation of refractory aqueous organic pollutant rhodamine B, Chin. J. Catal., 2017, 38, 1360–1372 CrossRef CAS.
- M. Chen, N. Wang, X. Wang, Y. Zhou and L. Zhu, Enhanced degradation of tetrabromobisphenol A by magnetic Fe3O4@ZIF-67 composites as a heterogeneous Fenton-like catalyst, Chem. Eng. J., 2021, 413, 127539 CrossRef CAS.
- Z. G. Zhu, C. H. Ji, L. L. Zhong, S. Liu, F. Y. Cui, H. L. Sun and W. Wang, Magnetic Fe-Co crystal doped hierarchical porous carbon fibers for removal of organic pollutants, J. Mater. Chem. A, 2017, 5, 18071–18080 RSC.
- C. D. Qi, X. T. Liu, Y. Li, C. Y. Lin, J. Ma, X. W. Li and H. J. Zhang, Enhanced degradation of organic contaminants in water by peroxydisulfate coupled with bisulfite, J. Hazard. Mater., 2017, 328, 98–107 CrossRef CAS PubMed.
- J. Zou, J. Ma, L. W. Chen, X. C. Li, Y. H. Guan, P. C. Xie and C. Pan, Rapid Acceleration of Ferrous Iron/Peroxymonosulfate Oxidation of Organic Pollutants by Promoting Fe(III)/Fe(II) Cycle with Hydroxylamine, Environ. Sci. Technol., 2013, 47, 11685–11691 CrossRef CAS PubMed.
- J. Cui, T. Liu, Q. Zhang, T. Wang and X. Hou, Rapid microwave synthesis of Fe3O4-PVP@ZIF-67 as highly effective peroxymonosulfate catalyst for degradation of bisphenol F and its mechanism analysis, Chem. Eng. J., 2021, 404, 126453 CrossRef CAS.
- Y. G. Sun and Y. N. Xia, Shape-controlled synthesis of gold and silver nanoparticles, Science, 2002, 298, 2176–2179 CrossRef CAS PubMed.
- W. J. Ma, N. Wang, T. Z. Tong, L. J. Zhang, K. Y. A. Lin, X. J. Han and Y. C. Du, Nitrogen, phosphorus, and sulfur tri-doped hollow carbon shells derived from ZIF-67@poly (cyclotriphosphazene-co-4,4 '-sulfonyldiphenol) as a robust catalyst of peroxymonosulfate activation for degradation of bisphenol A, Carbon, 2018, 137, 291–303 CrossRef CAS.
- V. F. Puntes, K. M. Krishnan and A. P. Alivisatos, Colloidal nanocrystal shape and size control: The case of cobalt, Science, 2001, 291, 2115–2117 CrossRef CAS PubMed.
- A. Filankembo and M. P. Pileni, Is the template of self-colloidal assemblies the only factor that controls nanocrystal shapes?, J. Phys. Chem. B, 2000, 104, 5865–5868 CrossRef CAS.
- Z. Wu, Y. Wang, Z. Xiong, Z. Ao, S. Pu, G. Yao and B. Lai, Core-shell magnetic Fe3O4@Zn/Co-ZIFs to activate peroxymonosulfate for highly efficient degradation of carbamazepine, Appl. Catal., B, 2020, 277, 119136 CrossRef CAS.
- X. Wu, D. D. Sun, H. C. Ma, C. Ma, X. X. Zhang and J. Hao, Activation of peroxymonosulfate by magnetic CuFe2O4@ZIF-67 composite catalyst for the study on the degradation of methylene blue, Colloids Surf., A, 2022, 637, 12 Search PubMed.
- Y. Y. Zhang, S. Yuan, X. Feng, H. W. Li, J. W. Zhou and B. Wang, Preparation of Nanofibrous Metal-Organic Framework Filters for Efficient Air Pollution Control, J. Am. Chem. Soc., 2016, 138, 5785–5788 CrossRef CAS PubMed.
- X. J. Ma, Y. T. Chai, P. Li and B. Wang, Metal-Organic Framework Films and Their Potential Applications in Environmental Pollution Control, Acc. Chem. Res., 2019, 52, 1461–1470 CrossRef CAS PubMed.
- C. H. Wang, H. Y. Wang, R. Luo, C. Liu, J. S. Li, X. Y. Sun, J. Y. Shen, W. Q. Han and L. J. Wang, Metal-organic framework one-dimensional fibers as efficient catalysts for activating peroxymonosulfate, Chem. Eng. J., 2017, 330, 262–271 CrossRef CAS.
- C. L. Zhang and S. H. Yu, Nanoparticles meet electrospinning: recent advances and future prospects, Chem. Soc. Rev., 2014, 43, 4423–4448 RSC.
- M. W. Kim, H. Yoon, T. Y. Ohm, H. S. Jo, S. An, S. K. Choi, H. Park, S. S. Al-Deyab, B. K. Min, M. T. Swihart and S. S. Yoon, Nanotextured cupric oxide nanofibers coated with atomic layer deposited ZnO-TiO2 as highly efficient photocathodes, Appl. Catal., B, 2017, 201, 479–485 CrossRef CAS.
- J. Ding, Y. F. Bu, M. Ou, Y. Yu, Q. Zhong and M. H. Fan, Facile decoration of carbon fibers with Ag nanoparticles for adsorption and photocatalytic reduction of CO2, Appl. Catal., B, 2017, 202, 314–325 CrossRef CAS.
- M. Rose, B. Bohringer, M. Jolly, R. Fischer and S. Kaskel, MOF Processing by Electrospinning for Functional Textiles, Adv. Eng. Mater., 2011, 13, 356–360 CrossRef CAS.
- Y. C. Chou, C. L. Shao, X. H. Li, C. Y. Su, H. C. Xu, M. Y. Zhang, P. Zhang, X. Zhang and Y. C. Liu, BiOCl nanosheets immobilized on electrospun polyacrylonitrile nanofibers with high photocatalytic activity and reusable property, Appl. Surf. Sci., 2013, 285, 509–516 CrossRef CAS.
- C. Wang, P. Cheng, Y. Yao, Y. Yamauchi, X. Yan, J. Li and J. Na, In-situ fabrication of nanoarchitectured MOF filter for water purification, J. Hazard. Mater., 2020, 392, 122164 CrossRef CAS PubMed.
- Q. Liao, X. P. Su, W. J. Zhu, W. Hua, Z. Q. Qian, L. Liu and J. M. Yao, Flexible and durable cellulose aerogels for highly effective oil/water separation, RSC Adv., 2016, 6, 63773–63781 RSC.
- J. D. Feng, S. T. Nguyen, Z. Fan and H. M. Duong, Advanced fabrication and oil absorption properties of super-hydrophobic recycled cellulose aerogels, Chem. Eng. J., 2015, 270, 168–175 CrossRef CAS.
- B. F. Martins, P. V. O. de Toledo and D. F. S. Petri, Hydroxypropyl methylcellulose based aerogels: Synthesis, characterization and application as adsorbents for wastewater pollutants, Carbohydr. Polym., 2017, 155, 173–181 CrossRef CAS PubMed.
- W. Ren, J. Gao, C. Lei, Y. Xie, Y. Cai, Q. Ni and J. Yao, Recyclable metal-organic framework/cellulose aerogels for activating peroxymonosulfate to degrade organic pollutants, Chem. Eng. J., 2018, 349, 766–774 CrossRef CAS.
- M. He, J. F. Yao, Q. Liu, Z. X. Zhong and H. T. Wang, Toluene-assisted synthesis of RHO-type zeolitic imidazolate frameworks: synthesis and formation mechanism of ZIF-11 and ZIF-12, Dalton Trans., 2013, 42, 16608–16613 RSC.
- C.-H. Wu, W.-C. Yun, T. Wi-Afedzi and K.-Y. A. Lin, ZIF-67 supported on marcoscale resin as an efficient and convenient heterogeneous catalyst for Oxone activation, J. Colloid Interface Sci., 2018, 514, 262–271 CrossRef CAS PubMed.
- L. Peng, X. Gong, X. Wang, Z. Yang and Y. Liu, In situ growth of ZIF-67 on a nickel foam as a three-dimensional heterogeneous catalyst for peroxymonosulfate activation, RSC Adv., 2018, 8, 26377–26382 RSC.
- D. Y. Chen, Q. Bai, T. T. Ma, X. F. Jing, Y. Y. Tian, R. Zhao and G. S. Zhu, Stable metal-organic framework fixing within zeolite beads for effectively static and continuous flow degradation of tetracycline by peroxymonosulfate activation, Chem. Eng. J., 2022, 435, 11 Search PubMed.
- C. C. Wang, J. R. Li, X. L. Lv, Y. Q. Zhang and G. S. Guo, Photocatalytic organic pollutants degradation in metal-organic frameworks, Energy Environ. Sci., 2014, 7, 2831–2867 RSC.
- J. Luo, Y. Dai, X. Xu, Y. Liu, S. Yang, H. He, C. Sun and Q. Xian, Green and efficient synthesis of Co-MOF-based/g-C3N4 composite catalysts to activate peroxymonosulfate for degradation of the antidepressant venlafaxine, J. Colloid Interface Sci., 2022, 610, 280–294 CrossRef CAS PubMed.
- H. Hu, L. Han, M. Z. Yu, Z. Y. Wang and X. W. Lou, Metal-organic-framework-engaged formation of Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages for efficient oxygen reduction, Energy Environ. Sci., 2016, 9, 107–111 RSC.
- Y. J. Chen, S. F. Ji, Y. G. Wang, J. C. Dong, W. X. Chen, Z. Li, R. A. Shen, L. R. Zheng, Z. B. Zhuang, D. S. Wang and Y. D. Li, Isolated Single Iron Atoms Anchored on N-Doped Porous Carbon as an Efficient Electrocatalyst for the Oxygen Reduction Reaction, Angew. Chem., Int. Ed., 2017, 56, 6937–6941 CrossRef CAS PubMed.
- W. J. Ma, Y. C. Du, N. Wang and P. Miao, ZIF-8 derived nitrogen-doped porous carbon as metal-free catalyst of peroxymonosulfate activation, Environ. Sci. Pollut. Res., 2017, 24, 16276–16288 CrossRef CAS PubMed.
- Y. Liu, W. Miao, X. Fang, Y. L. Tang, D. L. Wu and S. Mao, MOF-derived metal-free N-doped porous carbon mediated peroxydisulfate activation via radical and non-radical pathways: Role of graphitic N and C-O, Chem. Eng. J., 2020, 380, 10 Search PubMed.
- G. Wang, S. Chen, X. Quan, H. Yu and Y. Zhang, Enhanced activation of peroxymonosulfate by nitrogen doped porous carbon for effective removal of organic pollutants, Carbon, 2017, 115, 730–739 CrossRef CAS.
- W. Ma, N. Wang, Y. Fan, T. Tong, X. Han and Y. Du, Non-radical-dominated catalytic degradation of bisphenol A by ZIF-67 derived nitrogen-doped carbon nanotubes frameworks in the presence of peroxymonosulfate, Chem. Eng. J., 2018, 336, 721–731 CrossRef CAS.
- C. Wang, J. Kim, M. Kim, H. Lim, M. Zhang, J. You, J.-H. Yun, Y. Bando, J. Li and Y. Yamauchi, Nanoarchitectured metal–organic framework-derived hollow carbon nanofiber filters for advanced oxidation processes, J. Mater. Chem. A, 2019, 7, 13743–13750 RSC.
- X. Li, X. Yan, X. Hu, R. Feng, M. Zhou and L. Wang, Enhanced adsorption and catalytic peroxymonosulfate activation by metal-free N-doped carbon hollow spheres for water depollution, J. Colloid Interface Sci., 2021, 591, 184–192 CrossRef CAS PubMed.
- X. G. Duan, S. Indrawirawan, H. Q. Sun and S. B. Wang, Effects of nitrogen-, boron-, and phosphorus-doping or codoping on metal-free graphene catalysis, Catal. Today, 2015, 249, 184–191 CrossRef CAS.
- W. J. Tian, H. Y. Zhang, H. Q. Sun, A. Suvorova, M. Saunders, M. Tade and S. B. Wang, Heteroatom (N or N-S)-Doping Induced Layered and Honeycomb Microstructures of Porous Carbons for CO2 Capture and Energy Applications, Adv. Funct. Mater., 2016, 26, 8651–8661 CrossRef CAS.
- X. Li, L. Ye, Z. Ye, S. Xie, Y. Qiu, F. Liao, C. Lin and M. Liu, N, P co-doped core/shell porous carbon as a highly efficient peroxymonosulfate activator for phenol degradation, Sep. Purif. Technol., 2021, 276, 119286 CrossRef CAS.
- J. L. Xie, X. Luo, L. Chen, X. B. Gong, L. R. Zhang and J. Tian, ZIF-8 derived boron, nitrogen co-doped porous carbon as metal-free peroxymonosulfate activator for tetracycline hydrochloride degradation: Performance, mechanism and biotoxicity, Chem. Eng. J., 2022, 440, 14 CrossRef.
- W. Ma, N. Wang, T. Tong, L. Zhang, K.-Y. A. Lin, X. Han and Y. Du, Nitrogen, phosphorus, and sulfur tri-doped hollow carbon shells derived from ZIF-67@poly (cyclotriphosphazene-co-4, 4′-sulfonyldiphenol) as a robust catalyst of peroxymonosulfate activation for degradation of bisphenol A, Carbon, 2018, 137, 291–303 CrossRef CAS.
- Y. Gong, X. Zhao, H. Zhang, B. Yang, K. Xiao, T. Guo, J. J. Zhang, H. X. Shao, Y. B. Wang and G. Yu, MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation, Appl. Catal., B, 2018, 233, 35–45 CrossRef CAS.
- J. Y. Pu, J. Q. Wan, Y. Wang and Y. W. Ma, Different Co-based MOFs templated synthesis of Co3O4 nanoparticles to degrade RhB by activation of oxone, RSC Adv., 2016, 6, 91791–91797 RSC.
- J. Hu, M. Chen, X. S. Fang and L. W. Wu, Fabrication and application of inorganic hollow spheres, Chem. Soc. Rev., 2011, 40, 5472–5491 RSC.
- M. Marmier, G. Cecot, B. F. E. Curchod, P. Pattison, E. Solari, R. Scopelliti and K. Severin, Surface functionalization of dinuclear clathrochelates via Pd-catalyzed cross-coupling reactions: facile synthesis of polypyridyl metalloligands, Dalton Trans., 2016, 45, 8422–8427 RSC.
- M. Abdul Nasir Khan, P. Kwame Klu, C. Wang, W. Zhang, R. Luo, M. Zhang, J. Qi, X. Sun, L. Wang and J. Li, Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation, Chem. Eng. J., 2019, 363, 234–246 CrossRef CAS.
- R. H. Li, J. J. Wang, L. A. Gaston, B. Y. Zhou, M. L. Li, R. Xiao, Q. Wang, Z. Q. Zhang, H. Huang, W. Liang, H. T. Huang and X. F. Zhang, An overview of carbothermal synthesis of metal-biochar composites for the removal of oxyanion contaminants from aqueous solution, Carbon, 2018, 129, 674–687 CrossRef CAS.
- X.-L. Chen, F. Li, M. Zhang, B. Liu, H. Chen and H. Wang, Highly dispersed and stabilized Co3O4/C anchored on porous biochar for bisphenol A degradation by sulfate radical advanced oxidation process, Sci. Total Environ., 2021, 777, 145794 CrossRef CAS PubMed.
- L. W. Chen, D. H. Ding, C. Liu, H. Cai, Y. Qu, S. J. Yang, Y. Gao and T. M. Cai, Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration, Chem. Eng. J., 2018, 334, 273–284 CrossRef CAS.
- C. S. Tang, M. Cheng, C. Lai, L. Li, X. F. Yang, L. Du, G. X. Zhang, G. F. Wang and L. Yang, Recent progress in the applications of non-metal modified graphitic carbon nitride in photocatalysis, Coord. Chem. Rev., 2023, 474, 21 CrossRef.
- J. L. Wang and S. Z. Wang, Preparation, modification and environmental application of biochar: A review, J. Cleaner Prod., 2019, 227, 1002–1022 CrossRef CAS.
- J. T. Zhang, H. Hu, Z. Li and X. W. Lou, Double-Shelled Nanocages with Cobalt Hydroxide Inner Shell and Layered Double Hydroxides Outer Shell as High-Efficiency Polysulfide Mediator for Lithium-Sulfur Batteries, Angew. Chem., Int. Ed., 2016, 55, 3982–3986 CrossRef CAS PubMed.
- Q. Chen, X. Zhang, S. Li, J. Tan, C. Xu and Y. Huang, MOF-derived Co3O4@Co-Fe oxide double-shelled nanocages as multi-functional specific peroxidase-like nanozyme catalysts for chemo/biosensing and dye degradation, Chem. Eng. J., 2020, 395, 125130 CrossRef CAS.
- Z. Li and H. C. Zeng, Armored MOFs: Enforcing Soft Microporous MOF Nanocrystals with Hard Mesoporous Silica, J. Am. Chem. Soc., 2014, 136, 5631–5639 CrossRef CAS PubMed.
- Q. Yue, J. L. Li, Y. Zhang, X. W. Cheng, X. Chen, P. P. Pan, J. C. Su, A. A. Elzatahry, A. Alghamdi, Y. H. Deng and D. Y. Zhao, Plasmolysis-lnspired Nanoengineering of Functional Yolk-Shell Microspheres with Magnetic Core and Mesoporous Silica Shell, J. Am. Chem. Soc., 2017, 139, 15486–15493 CrossRef CAS PubMed.
- C. Galeano, C. Baldizzone, H. Bongard, B. Spliethoff, C. Weidenthaler, J. C. Meier, K. J. J. Mayrhofer and F. Schuth, Carbon-Based Yolk-Shell Materials for Fuel Cell Applications, Adv. Funct. Mater., 2014, 24, 220–232 CrossRef CAS.
- M. B. Gawande, A. Goswami, T. Asefa, H. Z. Guo, A. V. Biradar, D. L. Peng, R. Zboril and R. S. Varma, Core-shell nanoparticles: synthesis and applications in catalysis and electrocatalysis, Chem. Soc. Rev., 2015, 44, 7540–7590 RSC.
- M. Zhang, C. Wang, C. Liu, R. Luo, J. Li, X. Sun, J. Shen, W. Han and L. Wang, Metal-organic framework derived Co3O4/C@SiO2 yolk-shell nanoreactors with enhanced catalytic performance, J. Mater. Chem., 2018, 6, 11226–11235 RSC.
- X. Fang, L. Gan, L. Wang, H. Gong, L. Xu, Y. Wu, H. Lu, S. Han, J. Cui and C. Xia, Enhanced degradation of bisphenol A by mixed ZIF derived CoZn oxide encapsulated N-doped carbon via peroxymonosulfate activation: The importance of N doping amount, J. Hazard. Mater., 2021, 419, 126363 CrossRef CAS PubMed.
- Y. J. Yao, Y. M. Cai, G. D. Wu, F. Y. Wei, X. Y. Li, H. Chen and S. B. Wang, Sulfate radicals induced from peroxymonosulfate by cobalt manganese oxides (CoxMn3-xO4) for Fenton-Like reaction in water, J. Hazard. Mater., 2015, 296, 128–137 CrossRef CAS PubMed.
- Z. Z. Liang, C. C. Zhang, H. T. Yuan, W. Zhang, H. Q. Zheng and R. Cao, PVP-assisted transformation of a metal-organic framework into Co-embedded N-enriched meso/microporous carbon materials as bifunctional electrocatalysts, Chem. Commun., 2018, 54, 7519–7522 RSC.
- L. J. Wang, P. H. Tang, J. Liu, A. B. Geng, C. Song, Q. Zhong, L. J. Xu and L. Gan, Multifunctional ZnO-porous carbon composites derived from MOF-74(Zn) with ultrafast pollutant adsorption capacity and supercapacitance properties, J. Colloid Interface Sci., 2019, 554, 260–268 CrossRef CAS PubMed.
- M. A. N. Khan, P. K. Klu, C. H. Wang, W. X. Zhang, R. Luo, M. Zhang, J. W. Qi, X. Y. Sun, L. J. Wang and J. S. Li, Metal-organic framework-derived hollow Co3O4/carbon as efficient catalyst for peroxymonosulfate activation, Chem. Eng. J., 2019, 363, 234–246 CrossRef.
- J. Zhao, F. Li, H. Wei, H. Ai, L. Gu, J. Chen, L. Zhang, M. Chi and J. Zhai, Superior performance of ZnCoOx/peroxymonosulfate system for organic pollutants removal by enhancing singlet oxygen generation: The effect of oxygen vacancies, Chem. Eng. J., 2021, 409, 128150 CrossRef CAS.
- Y. J. Yao, H. Chen, C. Lian, F. Y. Wei, D. W. Zhang, G. D. Wu, B. J. Chen and S. B. Wang, Fe, Co, Ni nanocrystals encapsulated in nitrogen-doped carbon nanotubes as Fenton-like catalysts for organic pollutant removal, J. Hazard. Mater., 2016, 314, 129–139 CrossRef CAS PubMed.
- J. Kang, H. Y. Zhang, X. G. Duan, H. Q. Sun, X. Y. Tan, S. M. Liu and S. B. Wang, Magnetic Ni-Co alloy encapsulated N-doped carbon nanotubes for catalytic membrane degradation of emerging contaminants, Chem. Eng. J., 2019, 362, 251–261 CrossRef CAS.
- J. Wang, Z. W. Liao, J. Ifthikar, L. R. Shi, Y. N. Du, J. Y. Zhu, S. Xi, Z. Q. Chen and Z. L. Chen, Treatment of refractory contaminants by sludge-derived biochar/persulfate system via both adsorption and advanced oxidation process, Chemosphere, 2017, 185, 754–763 CrossRef CAS PubMed.
- P. J. Duan, T. F. Ma, Y. Yue, Y. W. Li, X. Zhang, Y. A. Shang, B. Y. Gao, Q. Z. Zhang, Q. Y. Yue and X. Xu, Fe/Mn nanoparticles encapsulated in nitrogen-doped carbon nanotubes as a peroxymonosulfate activator for acetamiprid degradation, Environ. Sci.: Nano, 2019, 6, 1799–1811 RSC.
- W. D. Oh, S. K. Lua, Z. L. Dong and T. T. Lim, Performance of magnetic activated carbon composite as peroxymonosulfate activator and regenerable adsorbent via sulfate radical-mediated oxidation processes, J. Hazard. Mater., 2015, 284, 1–9 CrossRef CAS PubMed.
- K.-Y. Andrew Lin, F.-K. Hsu and W.-D. Lee, Magnetic cobalt–graphene nanocomposite derived from self-assembly of MOFs with graphene oxide as an activator for peroxymonosulfate, J. Mater. Chem. A, 2015, 3, 9480–9490 RSC.
- Y. Li, X. Yan, X. Hu, R. Feng and M. Zhou, Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water, Chem. Eng. J., 2019, 375, 122003 CrossRef CAS.
- Y. X. Lei, X. Guo, M. J. Jiang, W. Sun, H. He, Y. Chen, K. Thummavichai, O. Ola, Y. Q. Zhu and N. N. Wang, Co-ZIF reinforced cow manure biochar (CMB) as an effective peroxymonosulfate activator for degradation of carbamazepine, Appl. Catal., B, 2022, 319, 14 CrossRef.
- H. Li, J. Tian, Z. Zhu, F. Cui, Y.-A. Zhu, X. Duan and S. Wang, Magnetic nitrogen-doped nanocarbons for enhanced metal-free catalytic oxidation: Integrated experimental and theoretical investigations for mechanism and application, Chem. Eng. J., 2018, 354, 507–516 CrossRef CAS.
- J. Deng, D. H. Deng and X. H. Bao, Robust Catalysis on 2D Materials Encapsulating Metals: Concept, Application, and Perspective, Adv. Mater., 2017, 29, 23 Search PubMed.
- J. Deng, P. J. Ren, D. H. Deng and X. H. Bao, Enhanced Electron Penetration through an Ultrathin Graphene Layer for Highly Efficient Catalysis of the Hydrogen Evolution Reaction, Angew. Chem., Int. Ed., 2015, 54, 2100–2104 CrossRef CAS PubMed.
- Z. Z. Wei, Y. Q. Chen, J. Wang, D. F. Su, M. H. Tang, S. J. Mao and Y. Wang, Cobalt Encapsulated in N-Doped Graphene Layers: An Efficient and Stable Catalyst for Hydrogenation of Quinoline Compounds, ACS Catal., 2016, 6, 5816–5822 CrossRef CAS.
- M. Zhang, R. Luo, C. Wang, W. Zhang, X. Yan, X. Sun, L. Wang and J. Li, Confined pyrolysis of metal-organic frameworks to N-doped hierarchical carbon for non-radical dominated advanced oxidation processes, J. Mater. Chem. A, 2019, 7, 12547–12555 RSC.
- G. W. Pang, M. Ji, Z. R. Li, Z. W. Yang, X. J. Qiu and Y. X. Zhao, Electrospinning of ZIF-67 Derived Co-C-N Composite Efficiently Activating Peroxymonosulfate to Degrade Dimethyl Phthalate, Water, 2022, 14, 17 Search PubMed.
- C. Zhu, F. Liu, C. Ling, H. Jiang, H. Wu and A. Li, Growth of graphene-supported hollow cobalt sulfide nanocrystals via MOF-templated ligand exchange as surface-bound radical sinks for highly efficient bisphenol A degradation, Appl. Catal., B, 2019, 242, 238–248 CrossRef CAS.
- X. Zhang, X. Yan, X. Hu, R. Feng, M. Zhou and L. Wang, Efficient removal of organic pollutants by a Co/N/S-doped yolk-shell carbon catalyst via peroxymonosulfate activation, J. Hazard. Mater., 2022, 421, 126726 CrossRef CAS PubMed.
- M. Zhang, C. Xiao, X. Yan, S. Chen, C. Wang, R. Luo, J. Qi, X. Sun, L. Wang and J. Li, Efficient Removal of Organic Pollutants by Metal–organic Framework Derived Co/C Yolk–Shell Nanoreactors: Size-Exclusion and Confinement Effect, Environ. Sci. Technol., 2020, 54, 10289–10300 CrossRef CAS PubMed.
- T. Zeng, X. L. Zhang, S. H. Wang, H. Y. Niu and Y. Q. Cai, Spatial Confinement of a Co3O4 Catalyst in Hollow Metal-Organic Frameworks as a Nanoreactor for Improved Degradation of Organic Pollutants, Environ. Sci. Technol., 2015, 49, 2350–2357 CrossRef CAS PubMed.
- Y. W. Chen, K. P. Cui, T. Liu, M. S. Cui, Y. Ding, Y. H. Chen, X. Chen, W. W. Li and C. X. Li, Enhanced degradation of sulfamethoxazole by non-radical-dominated peroxymonosulfate activation with Co/Zn co-doped carbonaceous catalyst: Synergy between Co and Zn, Sci. Total Environ., 2022, 850, 15 Search PubMed.
- J. J. He, Y. Wan and W. J. Zhou, ZIF-8 derived Fe-N coordination moieties anchored carbon nanocubes for efficient peroxymonosulfate activation via non-radical pathways: Role of FeNx sites, J. Hazard. Mater., 2021, 405, 15 Search PubMed.
- Y. Q. Zhang, Y. M. Sun, Y. Man, H. Yuan, R. Y. Zhao, G. Q. Xiang, X. M. Jiang, L. J. He and S. S. Zhang, Highly efficient adsorption and catalytic degradation of aflatoxin B-1 by a novel porous carbon material derived from Fe-doped ZIF-8, Chem. Eng. J., 2022, 440, 11 Search PubMed.
- B. L. Hua, L. Zheng, A. Adeboye and F. T. Li, Defect- and nitrogen-rich porous carbon embedded with Co NPs derived from self-assembled Co-ZIF-8 @ anionic polyacrylamide network as PMS activator for highly efficient removal of tetracycline hydrochloride from water, Chem. Eng. J., 2022, 443, 13 CrossRef.
- Y. B. Zou, J. H. Hu, B. Li, L. Lin, Y. Li, F. F. Liu and X. Y. Li, Tailoring the coordination environment of cobalt in a single-atom catalyst through phosphorus doping for enhanced activation of peroxymonosulfate and thus efficient degradation of sulfadiazine, Appl. Catal., B, 2022, 312, 12 CrossRef.
- Z. Shen, L. Fan, S. Yang, Y. Yao, H. Chen and W. Wang, Fe-based carbonitride as Fenton-like catalyst for the elimination of organic contaminants, Environ. Res., 2021, 198, 110486 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhang and X. Hu, Novel zero-valent Co-Fe encapsulated in nitrogen-doped porous carbon nanocomposites derived from CoFe2O4@ZIF-67 for boosting 4-chlorophenol removal via coupling peroxymonosulfate, J. Colloid Interface Sci., 2020, 575, 206–219 CrossRef CAS PubMed.
- X. G. Duan, H. Q. Sun and S. B. Wang, Metal-Free Carbocatalysis in Advanced Oxidation Reactions, Acc. Chem. Res., 2018, 51, 678–687 CrossRef CAS PubMed.
- Y. B. Wang, D. Cao and X. Zhao, Heterogeneous degradation of refractory pollutants by peroxymonosulfate activated by CoOx-doped ordered mesoporous carbon, Chem. Eng. J., 2017, 328, 1112–1121 CrossRef CAS.
- Y. Zhao, L. J. Yang, S. Chen, X. Z. Wang, Y. W. Ma, Q. Wu, Y. F. Jiang, W. J. Qian and Z. Hu, Can Boron and Nitrogen Co-doping Improve Oxygen Reduction Reaction Activity of Carbon Nanotubes?, J. Am. Chem. Soc., 2013, 135, 1201–1204 CrossRef CAS PubMed.
- Z. Yang, Z. Yao, G. F. Li, G. Y. Fang, H. G. Nie, Z. Liu, X. M. Zhou, X. Chen and S. M. Huang, Sulfur-Doped Graphene as an Efficient Metal-free Cathode Catalyst for Oxygen Reduction, ACS Nano, 2012, 6, 205–211 CrossRef CAS PubMed.
- J. Zhou, W. Liu and W. Cai, The synergistic effect of Ag/AgCl@ZIF-8 modified g-C3N4 composite and peroxymonosulfate for the enhanced visible-light photocatalytic degradation of levofloxacin, Sci. Total Environ., 2019, 696, 133962 CrossRef CAS PubMed.
- Y. Gong, X. Zhao, H. Zhang, B. Yang, K. Xiao, T. Guo, J. Zhang, H. Shao, Y. Wang and G. Yu, MOF-derived nitrogen doped carbon modified g-C3N4 heterostructure composite with enhanced photocatalytic activity for bisphenol A degradation with peroxymonosulfate under visible light irradiation, Appl. Catal., B, 2018, 233, 35–45 CrossRef CAS.
- Y. Chen, K. Cui, M. Cui, T. Liu, X. Chen, Y. Chen, X. Nie, Z. Xu and C.-X. Li, Insight into the degradation of tetracycline hydrochloride by non-radical-dominated peroxymonosulfate activation with hollow shell-core Co@NC: Role of cobalt species, Sep. Purif. Technol., 2022, 289, 120662 CrossRef CAS.
- L. Y. Wu, Y. B. Yu, Q. Zhang, J. M. Hong, J. Wang and Y. C. She, A novel magnetic heterogeneous catalyst oxygen-defective CoFe2O4-x for activating peroxymonosulfate, Appl. Surf. Sci., 2019, 480, 717–726 CrossRef CAS.
- C. X. Li, J. E. Wu, W. Peng, Z. D. Fang and J. Liu, Peroxymonosulfate activation for efficient sulfamethoxazole degradation by Fe3O4/beta-FeOOH nanocomposites: Coexistence of radical and non-radical reactions, Chem. Eng. J., 2019, 356, 904–914 CrossRef CAS.
- H. Li, J. Wan, Y. Ma, Y. Wang, X. Chen and Z. Guan, Degradation of refractory dibutyl phthalate by peroxymonosulfate activated with novel catalysts cobalt metal-organic frameworks: Mechanism, performance, and stability, J. Hazard. Mater., 2016, 318, 154–163 CrossRef CAS PubMed.
- Y. K. Long, Y. X. Huang, H. Y. Wu, X. W. Shi and L. Xiao, Peroxymonosulfate activation for pollutants degradation by Fe-N-codoped carbonaceous catalyst: Structure-dependent performance and mechanism insight, Chem. Eng. J., 2019, 369, 542–552 CrossRef CAS.
- G. L. Wang, X. W. Nie, X. J. Ji, X. Quan, S. Chen, H. Z. Wang, H. T. Yu and X. W. Guo, Enhanced heterogeneous activation of peroxymonosulfate by Co and N codoped porous carbon for degradation of organic pollutants: the synergism between Co and N, Environ. Sci.: Nano, 2019, 6, 399–410 RSC.
- M. Kohantorabi, S. Giannakis, G. Moussavi, M. Bensimon, M. R. Gholami and C. Pulgarin, An innovative, highly stable Ag/ZIF-67@GO nanocomposite with exceptional peroxymonosulfate (PMS) activation efficacy, for the destruction of chemical and microbiological contaminants under visible light, J. Hazard. Mater., 2021, 413, 125308 CrossRef CAS PubMed.
- J. Zhou, W. Liu and W. Cai, The synergistic effect of Ag/AgCl@ZIF-8 modified g-C3N4 composite and peroxymonosulfate for the enhanced visible-light photocatalytic degradation of levofloxacin, Sci. Total Environ., 2019, 696, 133926 CrossRef PubMed.
- H. Dai, W. Zhou and W. Wang, Co/N co-doped carbonaceous polyhedron as efficient peroxymonosulfate activator for degradation of organic pollutants: Role of cobalt, Chem. Eng. J., 2021, 417, 127921 CrossRef CAS.
- N. Liu, N. Lu, H. Yu, S. Chen and X. Quan, Degradation of aqueous bisphenol A in the CoCN/Vis/PMS system: Catalyst design, reaction kinetic and mechanism analysis, Chem. Eng. J., 2021, 407, 127228 CrossRef CAS.
- X. Zhang, X. Yan, X. Hu, R. Feng, M. Zhou and L. Wang, Efficient removal of organic pollutants by a Co/N/S-doped yolk-shell carbon catalyst via peroxymonosulfate activation, J. Hazard. Mater., 2022, 421, 126726 CrossRef CAS PubMed.
- Y. Zhou, Y. Zhang and X. Hu, Novel zero-valent Co-Fe encapsulated in nitrogen-doped porous carbon nanocomposites derived from CoFe2O4@ZIF-67 for boosting 4-chlorophenol removal via coupling peroxymonosulfate, J. Colloid Interface Sci., 2020, 575, 206–219 CrossRef CAS PubMed.
- J. Cao, Z. Yang, W. Xiong, Y. Zhou, Y. Wu, M. Jia, S. Sun, C. Zhou, Y. Zhang and R. Zhong, Peroxymonosulfate activation of magnetic Co nanoparticles relative to an N-doped porous carbon under confinement: Boosting stability and performance, Sep. Purif. Technol., 2020, 250, 117237 CrossRef CAS.
- Z. Wang, X. Wang, L. Wang, Y. Wei, Z. Zhao, K. Du, D. Chen, X. Li, C. Zhou, G. Liu and Y. Luo, ZIF-67-derived Co@N-PC anchored on tracheid skeleton from sawdust with micro/nano composite structures for boosted methylene blue degradation, Sep. Purif. Technol., 2021, 278, 119489 CrossRef.
- M. Zhang, C. Xiao, X. Yan, S. Chen, C. Wang, R. Luo, J. Qi, X. Sun, L. Wang and J. Li, Efficient Removal of Organic Pollutants by Metal-organic Framework Derived Co/C Yolk-Shell Nanoreactors: Size-Exclusion and Confinement Effect, Environ. Sci. Technol., 2020, 54, 10289–10300 CrossRef CAS PubMed.
- P. Zhang, P. Zhou, J. L. Peng, Y. Liu, H. Zhang, C. S. He, Z. K. Xiong, W. Liu and B. Lai, Insight into metal-free carbon catalysis in enhanced permanganate oxidation: Changeover from electron donor to electron mediator, Water Res., 2022, 219, 9 Search PubMed.
- Z. W. Wang, E. Almatrafi, H. Wang, H. Qin, W. J. Wang, L. Du, S. Chen, G. M. Zeng and P. Xu, Cobalt Single Atoms Anchored on Oxygen-Doped Tubular Carbon Nitride for Efficient Peroxymonosulfate Activation: Simultaneous Coordination Structure and Morphology Modulation, Angew. Chem., Int. Ed., 2022, 61, 10 Search PubMed.
| This journal is © The Royal Society of Chemistry 2023 |