Probabilistic health risk assessment of zinc oxide nanoparticles from consumer products in adult populations†
Yunsong
Mu
 *a,
Xiang
Li
a,
Peihan
Chen
b,
Chengfang
Pang
*a,
Xiang
Li
a,
Peihan
Chen
b,
Chengfang
Pang
 c,
Fengchang
Wu
c,
John P.
Giesy
def,
Huazhen
Chang
c,
Fengchang
Wu
c,
John P.
Giesy
def,
Huazhen
Chang
 a and
Fangang
Zeng
*a
a and
Fangang
Zeng
*a
aSchool of Environment & Natural Resources, Renmin University of China, Beijing 100872, China. E-mail: muyunsong@ruc.edu.cn; zengfg@ruc.edu.cn
bCollege of Political Science and Law, Capital Normal University, Beijing 100048, China
cState Key Laboratory of Environmental Criteria and Risk Assessment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
dDepartment of Veterinary Biomedical Sciences, University of Saskatchewan, Saskatoon, Saskatchewan, Canada
eDepartment of Integrative Biology and Center for Integrative Toxicology, Michigan State University, East Lansing, MI, USA
fDepartment of Environmental Science, Baylor University, One Bear Place #97266, Waco, TX, 76798-7266 USA
First published on 3rd November 2022
Abstract
Zinc oxide nanoparticles (n-ZnO) are one of the most ever-increasing utilized nanomaterials in consumer products. Due to their antibacterial properties and superior efficiency in absorbing ultraviolet radiation, they are widely used as additives in food packaging and sunscreens. There is thus a need for scientific understanding of risks to the health of adult populations associated with n-ZnO. However, due to inadequate data in relation to characterizing hazards and exposure, there is a substantial uncertainty in risk assessment. In the present study, probabilistic approaches, including Monte Carlo and bootstrap methods, were integrated to assess the relative uncertainties and risks of n-ZnO to the health of males and females. Two major exposure pathways, oral from food packaging and percutaneous from sunscreen-based comestics, were evaluated by considering the uncertainty and variability involved in the exposure assessment. Given the cumulative uncertainties of all the extrapolation factors, the results showed that the individual margin of exposure (IMoE) of n-ZnO exhibited a minimal risk through oral exposure, with a minimum value of 786 for males and 96.2 for females (5th centile). However, within the entire range of IMoE values by Monte Carlo simulation through dermal exposure, the IMoE values in 11.45% of exposure scenarios for males and 18.87% for females were lower than the upper limit of the acceptable risk (IMoE ≤ 1). Intra-species, inter-species, and subacute-to-chronic extrapolation factors in the hazard assessment process contributed up to 97% of the uncertainty. These findings provided a scientific basis for understanding risks to the health of adult populations that could help allow regulatory acceptance of consumer products containing n-ZnO and highlighted the need for additional studies on hazard and exposure assessments of nanotechnologies.
Environmental significanceNanomaterials are being used to drive the development and production of new technologies as a result of their infinite sizes, shapes, types and compositions. Meanwhile, the release of these materials into the environment poses a potential hazard to human health. In addition, their innovative and varied properties pose challenges to existing tools. Therefore, it is useful to assess the potential risks of n-ZnO to human health to guarantee that nanotechnology-based consumer products are used in a secure and sustainable way. This paper puts forward an approach to introduce probability into health risk assessment and bridge the gap resulting from inadequate data of human health on nanomaterials. |
1. Introduction
According to a statistical report by the Nanotechnology Consumer Products Inventory in 2015, due to the rapid development of nanotechnology, more than 1814 nanomaterial-based consumer products have been transferred from the workbench of the laboratory to the shelves of stores, a 30-fold increase from the 54 products listed in 2005.1,2 There are currently 5224 nano-related products in The Nanodatabase developed by the Technical University of Denmark (DTU) Environment, the Danish Ecological Council and the Danish Consumer Council (https://www.nanodb.dk/).3 Among the commonly utilized nanomaterials, zinc oxide nanoparticles (n-ZnO), which exhibit superior anti-bacterial and anti-ultraviolet properties, are used in a broad range of applications, including personal care products, food additives, drug delivery vehicles, packaging and biosensors.4,5 There are continuous concerns about the environmental release of n-ZnO and their potential hazards to the health of humans. For instance, several in vivo and in vitro studies indicated that n-ZnO caused adverse effects when migrating through cells, blood and tissues (e.g. liver, lungs, and brain).6 The mucosal cells of the human gastrointestinal tract and excrement containing n-ZnO influenced the biological diversity of human intestinal flora and indirectly affected human health.7 However, existing health risk assessments of nanomaterials in humans (HHRA) mainly focused on nano-TiO2 in sunscreen products,8 nano-Ag in sterilization sprays,9 flame retardant material-coated multi-walled carbon nanotubes,10 nano-scale copper oxide (CuO) and basic copper carbonate (Cu2(OH)2CO3) in both ionic and micronized wood.11–13 The risk assessment associated with n-ZnO is still inadequate, thus hampering further regulatory acceptance of these nanomaterials.14 It is thus necessary to assess the risks of n-ZnO based on the recent development in research on characterizing exposures and potential effects of nanomaterials.Hazards of n-ZnO to human health have been extensively studied in recent years.15–17 Several studies suggested that n-ZnO can cause cytotoxicity, neurotoxicity, mutagenicity and oxidative stress damage to the liver and pancreas.18–20 N-ZnO had the potential to generate reactive oxygen species (ROS) in rats after 14 days of exposure, which resulted in mitochondrial-mediated apoptosis. Moreover, accumulation of n-ZnO in the liver at a concentration of 300 mg kg−1 wet mass could damage cells, with serum alanine aminotransferase (ALT) of 98 U L−1 and alkaline phosphatase (ALP) of 624 U L−1.21 Feeding male and female rats 536.8 mg kg−1 n-ZnO for 90 days induced moderate or mild pancreatitis.22 The effects of n-ZnO on human health were also manifested in damage to the microbiome of the gut, which not only led to dose-dependent changes in the composition and diversity of the intestinal flora but also changed their key functional pathways.23 The proposed toxic mechanism of n-ZnO was by free Zn2+ produced by dissolution, but some influencing factors, such as particle size, surface charge, and environmental conditions (pH and dissolved organic matter), should be taken into account.24–28 Therefore, the risk assessment of n-ZnO is still technically feasible if the uncertainties in the hazard assessment can be completely considered.
Humans can be exposed to n-ZnO through oral ingestion, inhalation, and percutaneous contact during occupational exposures or daily consumption through non-occupational exposures. Aerosol particles with n-ZnO are produced during the spraying and polishing process, and can reach the alveoli through breathing and cause adverse lung reactions or aggravate existing lung diseases in workers.29 They are also widely used in consumer products, such as sunscreens and food packaging owing to their superior absorption of ultraviolet rays (UVs) and antibacterial properties.30 Other characteristics of n-ZnO particles include good aggregate performance, which suggests that they cannot penetrate into deep skin and present a well-tolerated performance for the skin.31,32 Collectively, existing studies showed that adding n-ZnO to sunscreens was more beneficial than harmful to human health with respect to protecting the skin from aging and carcinomatous changes caused by UV radiation.33 However, there are still concerns related to the toxic effects of n-ZnO particles. Kim et al. compared the toxic effects of four metal oxide particles (Al2O3, CeO2, TiO2, and ZnO) on epithelial cells in lungs of humans, A549 cancer, and L-132 cells. N-ZnO were found to produce a strong cytotoxic effect in terms of cell proliferation, cell viability, membrane integrity, and colony formation.34 Food packaging is also an important vector for oral intake of n-ZnO. Some applications in food packaging feature a coating of n-ZnO, which releases Zn2+ to inhibit growth of bacteria. This prolongs the quality guarantee period of the food, but it can also result in exposure of consumers.35 The media of exposure, including release of Zn2+ from n-ZnO, physiological differences (e.g. age and sex) of those exposed, and intake capacity or frequency make the HHRA of n-ZnO more complicated and challenging.
Based on an existing deterministic framework for HHRA,36 the aim of this study was to comprehensively apply a probabilistic HHRA of n-ZnO in adult populations exposed to primary nanomaterial-based consumer products. Uncertainties were explicitly considered to allow for robust risk management decisions. Considering the particularity of percutaneous exposure, independent assessment processes were made for males and females. Due to insufficient data for the HHRA, multiple extrapolation factors and estimated values were inevitably introduced, which produced great uncertainties for the results of the risk assessment. Herein, the present study applied probability approaches (e.g. Monte Carlo and bootstrap) to quantify the associated uncertainties and to visualize the distributions of risks. This approach can be applied to promote understanding for regulatory bodies to govern the uses of nanomaterials in consumer products.
2. Materials and methods
2.1 Problem formulation
Nanomaterial, as defined by the European Commission, is a term used to describe a natural, incidental or manufactured material consisting of solid particles that are present either on their own or as identifiable constituent particles in aggregates or agglomerates, where 50% or more of these particles in the number-based size distribution fulfil at least one of the following conditions: (a) one or more external dimensions of the particle are in the size range 1 nm to 100 nm; (b) the particle has an elongated shape, such as a rod, fiber or tube, where two external dimensions are smaller than 1 nm and the other dimension is larger than 100 nm; (c) the particle has a plate-like shape, where one external dimension is smaller than 1 nm and the other dimensions are larger than 100 nm.37,38 N-ZnO are usually synthesized and manufactured as nanoparticles.39 The diameter of n-ZnO particles is inversely proportional to the surface area, with smaller particles providing superior performance for absorbing UVs.40 During interactions with bacterial surfaces, n-ZnO can release Zn2+ and thus have effective antibacterial properties, which allows for extensive applications in food packaging and delivery of biopharmaceuticals.41–43 To provide decision makers with valuable information and improve the regulatory acceptance of these products, in the present study, a probabilistic approach was used to perform an HHRA of n-ZnO from major consumer products, including sunscreen-based cream, lipstick and packaging for solid and liquid food.2.2 Hazard assessment
2.2.2.1 Oral toxicity test. Estimation of the benchmark dose (BMD) was performed by applying the dose–response models available in the Benchmark Dose Software (BMDS) developed by the United States Environmental Protection Agency (U.S. EPA) and the National Institute for Occupational Safety and Health (NIOSH) of the United States. The BMD was calculated with the standard deviation (SD) of BMR type I as the benchmark response factor (BMRF), a 95% confidence interval, a normal distribution type and a non-constant type of variance which is decided by the different variances of each dose group. Dose–response curves were fitted by several functions, including exponential, Hill, polynormal, power and linear. Goodness of fit was evaluated by calculating the Akaike information criterion (AIC). More than 100
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 iterations were calculated and the resulting CED distributions were divided by inter-species (AFinter), intra-species (AFintra), and sub-acute-chronic assessment factors (AFsubacute-chronic) to derive individual human critical effect dose (ICED) distributions (eqn (1)). The mean and SD of CEDanimal were predicted by means of the bootstrapping technique with the R project for statistical computing (Version 4.1.2).
000 iterations were calculated and the resulting CED distributions were divided by inter-species (AFinter), intra-species (AFintra), and sub-acute-chronic assessment factors (AFsubacute-chronic) to derive individual human critical effect dose (ICED) distributions (eqn (1)). The mean and SD of CEDanimal were predicted by means of the bootstrapping technique with the R project for statistical computing (Version 4.1.2).| ICED = CEDanimal/(AFinter × AFintra × AFsubacute-chronic) | (1) |
| AFinter = AFinter1 × AFinter2 | (2) |
| AFinter1 = [human bw/animal bw]1−scaling power | (3) |
Variability in sensitivities among individual humans was represented by AFintra, which was defined as a lognormal distribution with a specific geometric mean (GM) and a geometric standard deviation (GSD). Due to the lack of estimates of n-ZnO, default AFintra values were used in this study.46 AFsubacute-chronic was estimated by a lognormal distribution, which was empirically derived from subacute and chronic data.47
Another recommended approach to compute the RfD(o) by the U.S. EPA was used as a reference approach, which is shown in eqn (4):48
| RfD = NOAEL (or LOAEL or BMDL or BMD)/UFs | (4) |
2.2.2.2 Dermal toxicity test. The main evidence for transdermal penetration in relation to ZnO nanoparticles is systemic availability as shown by detection of the stable isotope Zn in blood/urine.49 In most cases, the toxicological database does not include detailed testing on all possible routes of administration, with possibly significant differences in factors such as mode of action and bioavailability. Consideration is given to potential differences in absorption or metabolism resulting from different routes of exposure, and whenever appropriate data are available, the quantitative impacts of these differences on the risk assessment are delineated.50 The U.S. EPA assumes that oral toxicity data can be transformed into dermal risk assessment when toxicity data on dermal absorption are not available. The dermal dose was calculated as follows (eqn (5)):51,52
| RfD(d) = RfD(o) × Absorption factor | (5) |
This can be calculated by eqn (6):
| BMD(d) = BMD(o) × Absorption factor | (6) |
Estimation through dermal exposure to n-ZnO from sunscreen products was calculated using eqn (7):53
| Vsunscreen = (DA × EV × ED × EF × SA)/(BW × AT) | (7) |
Estimation of oral exposure to n-ZnO from food packaging was performed using eqn (8).53
| Vfood = (C × IR × EF × ED)/(BW × AT) | (8) |
| IEXP = (U1V1 + U2V2 + U3V3…… + UnVn) | (9) |
| IMoE = ICED/IEXP | (10) |
To validate the results from the probabilistic risk assessment (PRA), another deterministic approach was also introduced by the U.S. EPA. The margin of exposure (MoE) is used to determine if exposure to a chemical can be expected to cause an adverse effect. The MoE is calculated by dividing the toxicological point of departure (POD) by the estimated daily dose to which humans will be exposed, as shown in eqn (11).55
| MoE = POD/Daily Dose | (11) |
The POD is the lower confidence bound on the lowest experimental dose that showed an effect in a dose–response study. The dose is determined from dose–response data and marks the beginning of extrapolation to determine the risk associated with environmentally relevant human exposures. Commonly, it is the NOAEL from an animal toxicity study in the laboratory, which represents the dose at which no adverse effects were observed in laboratory animals.
After calculating the MoE from the POD and daily dose, the U.S. EPA evaluates the risk from exposure to a chemical by comparing the calculated MoE to a target MoE.55 To explain the incomplete characterization in the n-ZnO toxicity studies, an additional uncertainty factor (UFD), which accounts for the incomplete characterization of nanotoxicities, together with AFinter and AFintra, should be introduced. In order to extrapolate chronic toxicity (90 days) from subacute toxicity (14 days), a three-fold assessment factor was used to account for greater toxic potency to the liver and bioaccumulation. Based on these considerations, a target MoE was estimated using eqn (12).
| target MoE = AFinter × AFintra × UFD × Tchronic/Tsubacute | (12) |
If calculated MoE > target MoE: risk is not of concern and mitigation is not required; if calculated MoE ≤ target MoE: risk is of concern and mitigation is required. In this case, mitigation measures such as engineering controls and/or personal protective equipment are employed until the calculated MoE exceeds the target MoE.
3. Results and discussion
3.1 Hazard identification
Searches of multiple bibliographic databases resulted in 13 in vivo studies (ESI,† Table S1). According to an expert scoring panel44 and a weight-of-evidence approach,45 the quality of data was evaluated and a toxicity study with the greatest quality scores was selected for hazard assessment of n-ZnO (ESI,† Tables S2–S4).56 Healthy Sprague Dawley rats of both sexes aged between 8 and 9 weeks with body masses of 180–220 g were randomly divided into six groups, with five females and five males in each group, and then exposed to 20 nm n-ZnO with distilled water. The range of actual particle sizes was 15–120 nm, with a mean size of particles of 63 nm. Rats were not fed overnight before the test and then fed 5, 50, 300, 1000 or 2000 mg kg−1 bw. The toxicity in vivo was determined based on Guideline 423 of the Organization for Economic Cooperation and Development. Two weeks later, rats of both sexes were euthanized to measure the biochemical indicators of blood serum.56 All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of the “Organization for Economic Co-operation and Development (OECD) Guideline 423 (OECD, 2001)” and approved by the Animal Ethics Committee of the “International Institute of Biotechnology and Toxicology (IIBAT)”. Due to the particle size of n-ZnO in daily consumer products being generally less than 100 nm,57 toxicity endpoints with an average particle size of 63 nm were used for further analysis (ESI,† Table S5).3.2 Dose response assessment
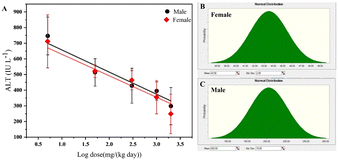
Changes in the total number of inflammatory cells in the liver can be considered as a critical endpoint for assessment,12 in which alanine aminotransferase (ALT) activity is the most sensitive indicator of liver inflammatory changes.58 A linear model was used to fit the logarithm of toxicity data with the activity of ALT, which was estimated to be associated with CEDanimal values of 43.5 mg kg−1 d−1 for females and 202.0 mg kg−1 d−1 for males (Fig. 2A). Bootstrap sampling generated a normal CEDanimal probability distribution defined by SD values of 2 and 19. The 5th to 95th percentiles of CEDanimal span from 39.14 to 47.54 mg kg−1 d−1 for females (Fig. 2B) and from 162.1 to 238.2 mg kg−1 d−1 for males (Fig. 2C).Based on CEDanimal, extrapolation factors were applied to estimate the distribution of ICED (ESI,† Table S6). Specifically, the AFinter1 accounted for the difference between the mean body mass of a healthy Sprague Dawley rat (200 g) and that of an adult Chinese person (the average weight is 60 kg).53 Considering the scaling power of the AFinter1 was defined within the range of 0.65–0.75, 0.70 was determined with a SD of 0.033. The AFinter1 was defined as a log-normal distribution with a geometric mean (GM) of 5.54 and a geometric standard deviation (GSD) of 1.2. The AFinter2 was estimated as a log-normal distribution with a GM of 1.00 and a GSD of 2.00 and AFintra was a log-normal distribution with a GM of 0.60 and a GSD of 1.60. The AFsubacute-chronic was defined as a log-normal distribution with a GM of 4.10 and a GSD of 4.40. These values were empirically derived from subacute and chronic data.47
3.3 Exposure assessment
In 2020, global production of metal oxide nanoparticles, specifically those incorporated into skincare products, was estimated to be 1000 tons per year.60 The general population is also exposed to n-ZnO-based food packaging with antibacterial properties.61,62 Therefore, the assessment of incorporated included four sources of exposure, namely, sunblocking cream (P1), sunscreen-based lipstick (P2), solid food packaging (P3) and liquid food packaging (P4) (Table 1). The total released n-ZnO per day from each product was 7.62 ± 1.71 μg cm−2 (sunblocking cream),62 0.9 mg kg−1 (sunscreen-based lipstick),59 15.51 ± 0.160 mg kg−1 (solid food packaging),41 and 0.16 ± 0.007 μg L−1 (liquid food packaging).63| P1 | P2 | P3 | P4 | |
|---|---|---|---|---|
| a Based on expert judgement. b 25%: the maximum percentage of nano materials allowed to be added to consumer goods. | ||||
| Release setup | Sunblocking cream | Sunscreen-based lipstick | Solid food (frozen fish packaging) | Liquid food (orange juice packaging) |
| Exposure route | Dermal | Oral/dermal | Oral | Oral |
| Daily release | 7.62 ± 1.71 μg cm−2 | ≥0.9 mg kg−1 | 15.51 ± 0.160 mg kg−1 | 0.16 ± 0.007 μg l−1 |
| Test duration (d) | 1 | — | 14 | 28 |
| Daily intake in females kg−1 body mass (mg kg−1 d−1) | 0.9030 ± 0.2027 | 0.90*25%b | 0.00262 ± 0.000027 | 5.4 × 10−8 ± 2.36 × 10−9 |
| Probability of intake percentage in femalesa | 5–6–7% | Oral: 10–20–30% dermal: 4.5–5–5.5% | 40–50–60% | 40–50–60% |
| Daily intake in males per kg body mass (mg kg−1 d−1) | 0.1272 ± 0.0287 | — | 0.00229 ± 0.0000236 | 4.72 × 10−8 ± 2.07 × 10−10 |
| Probability of intake percentage in malesa | 4–5–6% | — | 40–50–60% | 40–50–60% |
According to the SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and Their Safety Evaluation (SCCS/1416/11), the daily exposure to sunscreen-based lipstick is 0.9 mg kg−1 d−1. It is expected that exposure to sunscreen-based lipstick that contains high SPF factors (e.g. ZnO nanomaterials) is less than exposure to ‘regular’ sunscreen-based lipstick. Typically, these products are used only in specific time frames, e.g. during outdoor holidays. The value of 0.9 mg kg−1 d−1 is considered a conservative one.59 In terms of n-ZnO intake, the probability of intake from sunblocking cosmetics was less than indirect oral intake through food packaging. This result is consistent with the fact that there is little evidence of systemic exposure from penetration of skin by the metal oxide nanoparticles used in sunblocking cream. However, a question has been raised for injured skin. In addition, with respect to differences between sexes in utilizing sunscreens, the frequency of utilization was set to two times per day for males and three times per day for females, which was recommended by the DHI Water and Environment, Denmark.64 With reference to the basic exposure parameters provided in a Chinese document named ‘Exposure Factors Handbook of Chinese Population (Adults)’, the standard body mass of adult males is recommended to be 65 kg, with the exposed area with sunblocking cream being 0.44 m2, which consists of 0.12 m2 on the face, 0.24 m2 on arms and 0.08 m2 on hands. By comparison, the body mass of adult females is 56.8 kg, with the exposed area with sunblocking cream being 0.91 m2, which consists of 0.12 m2 on the face, 0.24 m2 on arms, 0.08 m2 on hands and 0.47 m2 on legs.65 Daily intake of solid and liquid food was recommended to be 0.25 kg and 0.25 L, respectively. The average daily intake of n-ZnO was estimated to be 0.1272 ± 0.0287 mg kg−1 d−1 for males and 0.9056 ± 0.2027 mg kg−1 d−1 for females (Table 1). This study formulated a worst case involving a person exposed to all these products simultaneously and exposed to the maximum amount of released n-ZnO.
The probability of intake from consuming different products was defined as a triangular distribution (ESI,† Table S6). For oral exposure, the predicted mean of IEXP for females was 0.046 mg kg−1 d−1, ranging from a 5th centile of 0.030 mg kg−1 d−1 to a 95th centile of 0.064 mg kg−1 d−1. By comparison, the predicted mean of IEXP for males (0.0011 mg kg−1 d−1) was lower, ranging from a 5th centile of 0.0010 mg kg−1 d−1 to a 95th centile of 0.0013 mg kg−1 d−1 (ESI,† Fig. S5). Through the dermal exposure pathway, the predicted mean of IEXP for females was 0.0065 mg kg−1 d−1, ranging from a 5th centile of 0.0045 mg kg−1 d−1 to a 95th centile of 0.0087 mg kg−1 d−1. By comparison, the predicted mean of IEXP for males was 0.0006 mg kg−1 d−1, ranging from a 5th centile of 0.0004 mg kg−1 d−1 to a 95th centile of 0.0009 mg kg−1 d−1 (ESI,† Fig. S7).
3.4 Characterization of risk
The forecast of ICED, IEXP, and IMoE in different sexes and exposure pathways was performed by the Crystal Ball software (ESI,† Tables S7–S10). The probability distribution functions of IMoE were fitted by a sigmoidal-logistic model, with acceptable coefficients of correlation (R2 ≥ 0.999) (Fig. 3 and Table S11†). | ||
| Fig. 3 The cumulative probabilities of individual margins of exposure (IMoE). The curves were fitted by a sigmoidal-logistic model (ESI,† Table S10). The red area shows unacceptable risk where IMoE is lower than 1. The minimum IMoE through oral exposure within the 5–95% interval was greater than that through dermal exposure, for both males and females. Females exhibit a greater probability (18.87%) than males (11.45%) to have risks to human health through dermal exposure. | ||
The higher values of IMoE indicated a lower level of risk. Herein, the IMoE through oral exposure was always higher than 1 (minimum = 786.2 for males and minimum = 96.23 for females within the 5–95% interval), indicating no likely risk to humans associated with lip products and food packaging containing n-ZnO (ESI,† Fig. S6). By comparison, the IMoE through dermal exposure was always partially lower than 1 (minimum = 0.3941 for males and minimum = 0.1800 for females within the 5–95% interval), triggering a non-acceptable dermal risk of 18.87% for females and 11.45% for males (ESI,† Fig. S7). Therefore, it is still necessary to reduce the frequency of n-ZnO sunscreen-based product use and to find safe alternatives to reduce intake of n-ZnO.
Nevertheless, the minimum IMoE values in females through oral exposure were 0.04 within the 0–5% interval (<1) (ESI,† Fig. S8), indicating that n-ZnO posed a potential risk of liver damage to a very small number of the general population.
3.5 Uncertainty analysis
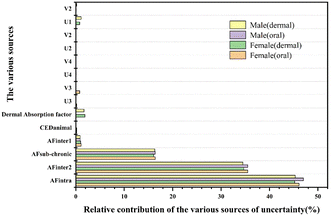
The contributions of the sources of uncertainty are mainly from AFintra, AFinter2 and AFsubacute-to-chronic, which account for 96% of the total uncertainty (Fig. 4). The dermal absorption factor contributes more than 1% of the overall uncertainty (1.9% for females and 1.7% for males), while the uncertainty due to the variability of intake behaviour (Vn and Un) was minor (0 to 0.5%).3.6 Contribution of uncertainty in the hazard assessment
The determination of the toxicity endpoint was a foundation for the HHRA of n-ZnO. Among the reported chronic toxic effects of n-ZnO, injury to the liver was the most concerning toxicity endpoint.56 Activities of ALT in liver cells were 1000- to 3000-fold greater than that in serum, and a 1% death of liver cells can double the activity of ALT in blood serum.66,67 Therefore, ALT was selected as a sensitive biomarker of damage to liver cells. N-ZnO exhibited toxicity to mice at lesser doses, indicating an inverse dose-dependent increase observed for activities of ALT in blood serum in individuals exposed to n-ZnO (Fig. 2A). Compared to micro-sized zinc oxide, incidences of microscopic lesions in liver were greater at lesser doses of n-ZnO (≤2000 mg kg−1 bw), which was consistent with the finding of Seok et al.22 The three extrapolation factors, AFintra, AFinter, and AFsubacute-chronic, contributed more to the uncertainty of the IMoE, and uncertainties in estimations of exposure were comparatively less (<4%).12,46 To reduce the overall uncertainty in the step of hazard assessment, toxicity tests on more organisms are needed to provide solid information for exploring the dose–response relationships. Deriving empirical parameters from other substances reduces the accuracy of risk assessment. Importantly, several transdermal experiments are also needed to further explore the dermal mechanism of nanoparticles.3.7 Contribution of uncertainty in the exposure assessment
Some uncertainties mainly came from the variability of exposure pathways and the estimated probability for the intake of n-ZnO. The predicted distribution of IEXP for females within the 5–95% interval ranged from 0.0300 to 0.0640 through oral exposure and from 0.0045 to 0.0087 through dermal exposure, while it ranged from 0.0010 to 0.0013 through oral exposure and from 0.0004 to 0.0009 through dermal exposure for males (ESI,† Fig. S3 and S7). However, there was no significant difference between the sexes through food packaging.The exposure assessment was conducted for worst case scenarios, when many measures (e.g. rubbish/washing off the sunscreen by accident, wiping off lipstick when they eat) that may reduce their intake concentrations were not considered. Therefore, one source of uncertainty in the exposure assessment is the proportion of absorbed sunscreen. Particle size is another potential impact factor to determine the dissolution kinetics. Other sources of uncertainty in the exposure assessment may result from the fact that only external doses were considered in the present study and the uptake and translocation in the organism were not included.
The exposure from drinking water was not taken into consideration. After flocculation/sedimentation, microfiltration and ultrafiltration treatment, the concentration of n-ZnO detected in ultimate drinking water samples was lower than 55 μg L−1.68,69 Therefore, compared to other exposure pathways, the intake of n-ZnO from drinking water can be neglected.
3.8 Contribution of uncertainty in the risk characterization
A contribution to the uncertainty in the IMoE estimates is expressed in Fig. 4, which explains possible determinants for all of the exposure scenarios. 96% of the variation in the IMoE distributions was influenced by uncertainties in AFinter, AFintra, and AFsubacute-chronic, while the remaining uncertainty was mainly from the dermal exposure. In addition to the incompleteness of the nanotoxicity database, the effects of n-ZnO due to their physicochemical properties (e.g. particle size, surface area, charge) were ignored after consuming nano products.44,70The minimum IMoE values for females and males through oral exposure were 0.04 and 3.098 within the 0–5% interval (<1) (ESI,† Tables S8 and S10), indicating that n-ZnO posed a potential risk of liver damage to a very small number of females through consuming lip products.
3.9 Comparison between the IMoE, deterministic margin of exposure (MoE), margin of safety (MoS) and low tier PRA
By comparing the BMDL with exposure data (ESI,† Fig. S4 and S7), the values of MoE for both sexes were calculated using eqn (13)–(16).71Through oral exposure:
 | (13) |
 | (14) |
Through dermal exposure:
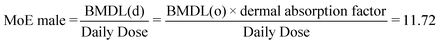 | (15) |
 | (16) |
The target MoE was calculated to be 6429 in eqn (12). The values of MoE through both oral and dermal exposures were less than the target MoE, which indicates that the risk of n-ZnO from consumer products is unacceptable. Compared to the probabilistic IMoE assessment, the deterministic MOE approach overestimated the risk, which indicates that MoE is apparently a more conservative approach. The different conclusions are mainly from great uncertainties of these assessment factors. For example, the factor UFD that accounts for the incomplete characterization of nanotoxicities contributed a great uncertainty to obtain the target MoE. Therefore, to reduce the uncertainty of UFD, more toxicity tests of n-ZnO need to be performed.
The risk of n-ZnO in sunscreen and lipstick is further assessed by an MoS method from the SCCS, where the MoS value of n-ZnO was 7.4 in sunscreen and 3.7 in lipstick.59 If the calculated MoS value is more than 1, the risk is identified to be acceptable. The finding is consistent with the result of the probabilistic IMoE assessment for most scenarios.
A low tier probabilistic analysis was performed using the APROBA-Plus tool released by the WHO International Programme on Chemical Safety.72 It applies lognormal uncertainty distributions to different aspects of the hazard assessment (e.g. point of departure, assessment factors), resulting in a confidence interval for the human dose associated with specified protection goals, as well as a probabilistic health-based guidance value. The uncertainty in exposure, by including the option to insert a quick and approximate estimate of the exposure uncertainty, is then graphically compared with the probabilistic outcome from the hazard characterization.73 The approximate approach based on lognormal distributions is much quicker and easier to apply, and simulations indicate that the resulting confidence bounds are a reasonable approximation to those obtained in the fully probabilistic approach.
The exposure assessment was divided into four groups, oral exposure for males, dermal exposure for males, oral exposure for females and dermal exposure for females. The plot in Fig. S10† shows the exposure uncertainty on the x-axis and the HDMI uncertainty on the y-axis. HDMI is depicted as a solid (vertical) line representing the confidence interval. For individual exposure scenarios, the results showed that when the whole ellipse is in the green area of the plot, then the exposure most likely is below HDMI, and the protection goals are most likely to be met. This is consistent with our independent assessment results. But the APROBA-Plus approach could not take uncertainties of cumulative exposure into account.
Therefore, among the three approaches, the IMoE approach presents incomparable advantages that we can obtain critical information on risks from a probabilistic perspective.
3.10 Advantages and limitations
Emerging nanotechnologies with high uncertainty challenge existing governance processes to identify, assess, and manage risks. The conventional deterministic risk-based paradigm is not essential for decision makers to evaluate the impact on human health since quantitative data for risk assessment remain incomplete or limited. A PRA approach is introduced to avoid overestimating risks due to the multiplication of several uncertainty factors in deriving point estimates of toxicity values. It is critical to use a higher tier PRA when a deterministic assessment cannot provide sufficient information for decision-makers. In the present case study on n-ZnO, the overall viability and uncertainty came from certain extrapolation factors when performing hazard and exposure assessment. Therefore, obtaining more intra-species and inter-species toxicity data is critical to improving the accuracy of the risk assessment. It is also worth noting that information about consumer exposure characteristics, e.g. gender, age range, weight, and exposure route of concern, needs to be collected in a scientifically rigorous manner.Herein, although the probabilistic HHRA presented the uncertainty that was needed in supporting a conservative assessment of risks, it should be further developed in three areas, including the following: 1) the n-ZnO products chosen to be included in this HHRA exclude some additional products, such as cosmetics, reusable face masks, food supplements, and liquid food supplements. More work is needed to fill incomplete data for exclusive consumer products with nano-sized ZnO. 2) Risk assessment of n-ZnO in children, who might have greater consumption to body weight ratios, is not a focus of the present study. 3) Relationships between the physico-chemical properties of n-ZnO (size, geometric configuration, and aggregation) and their toxic effects are highly desirable to balance the development of dose–response assessment with the intended use.
4. Conclusions
With the ever-increasing number of nano-enabled consumer products, a risk governance approach is required to quantitatively estimate risks to human health and associated uncertainties. Traditionally, risk assessments have been deterministic and “conservative,” relying on multiple safety factors and often overestimating risks. The framework of the high tier PRA developed here provides a foundation for evaluating potential risks associated with product use based on hazards and exposure, and allows for dealing with input information in a scientifically rigorous manner and supporting democratic decision making in governing emerging technologies. Future work could include multi-criteria decision analysis to characterize and balance the risks, benefits, costs, and societal implications, and investigation of probabilistic exposure models capable of linking to physiologically-based pharmacokinetic (PBPK) models to provide better estimates of the exposure ranges, dose, and risk in individuals in the population. Eventually, a robust decision-support tool based on such work should be developed for a broader suite of nanomaterials. These approaches will facilitate better and more cost-effective risk governance of emerging technologies.Author contributions
Y. S. M. and X. L. contributed equally to this work. Y. S. M. and F. C. W. conceived the project and designed the model. P. H. C. and C. F. P. collected and analyzed the data. X. L., P. H. C., C. F. P., and H. Z. C. performed statistical analysis and validated the QSAR-SSD model. Y. S. M. and F. G. Z. wrote the paper. J. P. G. revised the paper and gave constructive suggestions.Conflicts of interest
The authors have no conflict of interest to declare.Acknowledgements
This research was supported by the Fundamental Research Funds for the Central Universities and the Research Funds of the Renmin University of China (21XNLG25 and 2020030262). The authors would like to thank Dr. Kenneth M. Y. Leung (City University of Hong Kong) and three anonymous reviewers for providing comments on prior drafts. Language editing service was provided by Editage, Cactus Communications Inc. Prof. Giesy was supported by the Canada Research Chairs Program, the 2012 “High Level Foreign Experts” (#GDT20143200016) program provided by the State Administration of Foreign Experts Affairs, P.R. China to Nanjing University, the Einstein Professorship Program of the Chinese Academy of Sciences, and a Distinguished Visiting Professorship in the Department of Environmental Sciences, Baylor University in Waco, TX, USA.Notes and references
- M. E. Vance, T. Kuiken, E. P. Vejerano, S. P. McGinnis, M. F. Hochella, Jr., D. Rejeski and M. S. Hull, Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory, Beilstein J. Nanotechnol., 2015, 6, 1769–1780 CrossRef CAS PubMed.
- J. Park, S. Ham, M. Jang, J. Lee, S. Kim, S. Kim, K. Lee, D. Park, J. Kwon, H. Kim, P. Kim, K. Choi and C. Yoon, Spatial-temporal dispersion of aerosolized nanoparticles during the use of consumer spray products and estimates of inhalation exposure, Environ. Sci. Technol., 2017, 51, 7624–7638 CrossRef CAS.
- S. F. Hansen, O. F. H. Hansen and M. B. Nielsen, Advances and challenges towards consumerization of nanomaterials, Nat. Nanotechnol., 2020, 15, 964–965 CrossRef CAS PubMed.
- L. Voss, P. E. J. Saloga, V. Stock, L. Bohmert, A. Braeuning, A. F. Thunemann, A. Lampen and H. Sieg, Environmental impact of ZnO nanoparticles evaluated by in vitro simulated digestion, ACS Appl. Nano Mater., 2020, 3, 724–733 CrossRef CAS.
- S. M. Youn and S. J. Choi, Food additive zinc oxide nanoparticles: dissolution, interaction, fate, cytotoxicity, and qral toxicity, Int. J. Mol. Sci., 2022, 23, 6074–6092 CrossRef CAS PubMed.
- B. Wang, W. Y. Feng, M. Wang, T. C. Wang, Y. Q. Gu, M. T. Zhu, H. Ouyang, J. W. Shi, F. Zhang, Y. L. Zhao, Z. F. Chai, H. F. Wang and J. Wang, Acute toxicological impact of nano- and submicro-scaled zinc oxide powder on healthy adult mice, J. Nanopart. Res., 2007, 10, 263–276 CrossRef.
- H. Bouwmeester, M. V. D. Zande and M. A. Jepson, Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2017, 1–12 Search PubMed.
- U.S. EPA, Nanomaterial case studies: nanoscale titanium dioxide in water treatment and in topical sunscreen (final), Report EPA/600/R-09/057F, Research Triangle Park, NC, 2010 Search PubMed.
- U.S. EPA, Nanomaterial case study: nanoscale silver in disinfectant spray (external review draft), Washington, DC, 2010 Search PubMed.
- U.S. EPA, Nanomaterial case study: a comparison of multiwalled carbon nanotube and decabromodiphenyl ether flame-retardant coatings applied to upholstery textiles (draft), Research Triangle Park, NC, 2012 Search PubMed.
- V. Cazzagon, E. Giubilato, L. Pizzol, C. Ravagli, S. Doumett, G. Baldi, M. Blosi, A. Brunelli, C. Fito, F. Huertas, A. Marcomini, E. Semenzin, A. Zabeo, I. Zanoni and D. Hristozov, Occupational risk of nano-biomaterials: Assessment of nano-enabled magnetite contrast agent using the BIORIMA Decision Support System, NanoImpact, 2022, 25, 100373 CrossRef CAS PubMed.
- D. Hristozov, L. Pizzol, G. Basei, A. Zabeo, A. Mackevica, S. F. Hansen, I. Gosens, F. R. Cassee, W. de Jong, A. J. Koivisto, N. Neubauer, A. Sanchez Jimenez, E. Semenzin, V. Subramanian, W. Fransman, K. A. Jensen, W. Wohlleben, V. Stone and A. Marcomini, Quantitative human health risk assessment along the lifecycle of nano-scale copper-based wood preservatives, Nanotoxicology, 2018, 12, 747–765 CrossRef CAS PubMed.
- L. Pizzol, D. Hristozov, A. Zabeo, G. Basei, W. Wohlleben, A. J. Koivisto, K. A. Jensen, W. Fransman, V. Stone and A. Marcomini, SUNDS probabilistic human health risk assessment methodology and its application to organic pigment used in the automotive industry, NanoImpact, 2019, 13, 26–36 CrossRef.
- K. Schilling, B. Bradford, D. Castelli, E. Dufour, J. F. Nash, W. Pape, S. Schulte, I. Tooley, J. van den Bosch and F. Schellauf, Human safety review of "nano" titanium dioxide and zinc oxide, Photochem. Photobiol. Sci., 2010, 9, 495–509 CrossRef CAS.
- H. C. Chuang, H. T. Juan, C. N. Chang, Y. H. Yan, T. H. Yuan, J. S. Wang, H. C. Chen, Y. H. Hwang, C. H. Lee and T. J. Cheng, Cardiopulmonary toxicity of pulmonary exposure to occupationally relevant zinc oxide nanoparticles, Nanotoxicology, 2014, 8, 593–604 CrossRef CAS.
- B. M. Johnson, J. A. Fraietta, D. T. Gracias, J. L. Hope, C. J. Stairiker, P. R. Patel, Y. M. Mueller, M. D. McHugh, L. J. Jablonowski, M. A. Wheatley and P. D. Katsikis, Acute exposure to ZnO nanoparticles induces autophagic immune cell death, Nanotoxicology, 2015, 9, 737–748 CrossRef CAS.
- S. Keerthana and A. Kumar, Potential risks and benefits of zinc oxide nanoparticles: a systematic review, Crit. Rev. Toxicol., 2020, 50, 47–71 CrossRef CAS PubMed.
- X. Hu, S. Cook, P. Wang and H. M. Hwang, In vitro evaluation of cytotoxicity of engineered metal oxide nanoparticles, Sci. Total Environ., 2009, 407, 3070–3072 CrossRef CAS PubMed.
- G. Yan, Y. Huang, Q. Bu, L. Lv, P. Deng, J. Zhou, Y. Wang, Y. Yang, Q. Liu, X. Cen and Y. Zhao, Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats, J. Environ. Sci. Health, Part A: Toxic/Hazard. Subst. Environ. Eng., 2012, 47, 577–588 CrossRef CAS PubMed.
- T. Kong, S. H. Zhang, C. Zhang, J. L. Zhang, F. Yang, G. Y. Wang, Z. J. Yang, D. Y. Bai, M. Y. Zhang, J. Wang and B. H. Zhang, Long-term effects of unmodified 50 nm ZnO in mice, Biol. Trace Elem. Res., 2019, 189, 478–489 CrossRef CAS PubMed.
- V. Sharma, P. Singh, A. K. Pandey and A. Dhawan, Induction of oxidative stress, DNA damage and apoptosis in mouse liver after sub-acute oral exposure to zinc oxide nanoparticles, Mutat. Res., 2012, 745, 84–91 CAS.
- S. H. Seok, W. S. Cho, J. S. Park, Y. Na, A. Jang, H. Kim, Y. Cho, T. Kim, J. R. You, S. Ko, B. C. Kang, J. K. Lee, J. Jeong and J. H. Che, Rat pancreatitis produced by 13-week administration of zinc oxide nanoparticles: biopersistence of nanoparticles and possible solutions, J. Appl. Toxicol., 2013, 33, 1089–1096 CrossRef CAS PubMed.
- T. Zhang, X. Zhu, J. Guo, A. Z. Gu, D. Li and J. Chen, Toxicity assessment of nano-ZnO exposure on the human intestinal microbiome, metabolic functions, and resistome using an in vitro colon simulator, Environ. Sci. Technol., 2021, 55, 6884–6896 CrossRef CAS PubMed.
- A. Nicolosi, L. Cardoit, P. Pasquereau, C. Jaillet, M. Thoby-Brisson, L. Juvin and D. Morin, Acute exposure to zinc oxide nanoparticles critically disrupts operation of the respiratory neural network in neonatal rat, Neurotoxicology, 2018, 67, 150–160 CrossRef CAS PubMed.
- N. Singh, M. K. Das, R. Gautam, A. Ramteke and P. Rajamani, Assessment of intermittent exposure of zinc oxide nanoparticle (ZNP)-mediated toxicity and biochemical alterations in the splenocytes of male Wistar rat, Environ. Sci. Pollut. Res., 2019, 26, 33642–33653 CrossRef.
- H. Rokbani, F. Daigle and A. Ajji, Long- and short-term antibacterial properties of low-density polyethylene-based films coated with zinc oxide nanoparticles for potential use in food packaging, J. Plast. Film Sheeting, 2019, 35, 117–134 CrossRef CAS.
- J. Du, J. Tang, S. Xu, J. Ge, Y. Dong, H. Li and M. Jin, ZnO nanoparticles: recent advances in ecotoxicity and risk assessment, Drug Chem. Toxicol., 2020, 43, 322–333 CrossRef CAS.
- J. Yu and S. J. Choi, Particle size and biological fate of ZnO do not cause acute toxicity, but affect toxicokinetics and gene expression profiles in the rat livers after oral administration, Int. J. Mol. Sci., 2021, 22, 1698–1718 CrossRef CAS PubMed.
- M. R. Cooper, G. H. West, L. G. Burrelli, D. Dresser, K. N. Griffin, A. M. Segrave, J. Perrenoud and B. E. Lippy, Inhalation exposure during spray application and subsequent sanding of a wood sealant containing zinc oxide nanoparticles, J. Occup. Environ. Hyg., 2017, 14, 510–522 CrossRef CAS.
- K. B. Kim, Y. W. Kim, S. K. Lim, T. H. Roh, D. Y. Bang, S. M. Choi, D. S. Lim, Y. J. Kim, S. H. Baek, M. K. Kim, H. S. Seo, M. H. Kim, H. S. Kim, J. Y. Lee, S. Kacew and B. M. Lee, Risk assessment of zinc oxide, a cosmetic ingredient used as a UV filter of sunscreens, J. Toxicol. Environ. Health, Part B, 2017, 20, 155–182 CAS.
- E. Spisni, S. Seo, S. H. Joo and C. Su, Release and toxicity comparison between industrial- and sunscreen-derived nano-ZnO particles, Int. J. Environ. Sci. Technol., 2016, 13, 2485–2494 CrossRef CAS.
- R. Ridwan, T. Rihayat, S. Suryani, A. S. Ismi, N. Nurhanifa and S. Riskina, Combination of poly lactid acid zinc oxide nanocomposite for antimicrobial packaging application, International Conference on Innovation in Engineering and Vocational Education 2019 (Icieve 2019), Pts 1–4, 2020, vol. 830 Search PubMed.
- G. J. Nohynek and E. K. Dufour, Nano-sized cosmetic formulations or solid nanoparticles in sunscreens: A risk to human health?, Arch. Toxicol., 2012, 86, 1063–1075 CrossRef CAS PubMed.
- I. S. Kim, M. Baek and S. J. Choi, Comparative Cytotoxicity of Al2O3, CeO2, TiO2 and ZnO Nanoparticles to Human Lung Cells, J. Nanosci. Nanotechnol., 2010, 10, 3453–3458 CrossRef CAS PubMed.
- N. Bumbudsanpharoke and S. Ko, Nano-food packaging: an overview of market, migration research, and safety regulations, J. Food Sci., 2015, 80, R910–R923 CrossRef CAS PubMed.
- D. Romeo, B. Salieri, R. Hischier, B. Nowack and P. Wick, An integrated pathway based on in vitro data for the human hazard assessment of nanomaterials, Environ. Int., 2020, 137, 105505 CrossRef CAS.
- J. Potočnik, Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU), Off. J. Eur. Union, 2011, 275, 38–40 Search PubMed.
- European Commission, Commission recommendation of 10 June 2022 on the definition of nanomaterials (Text with EEA relevance) 2022/C 229/01, 2022, pp. 1–5.
- W. Moller, K. Felten, K. Sommerer, G. Scheuch, G. Meyer, P. Meyer, K. Haussinger and W. G. Kreyling, Deposition, retention, and translocation of ultrafine particles from the central airways and lung periphery, Am. J. Respir. Crit. Care Med., 2008, 177, 426–432 CrossRef PubMed.
- S. E. Cross, B. Innes, M. S. Roberts, T. Tsuzuki, T. A. Robertson and P. McCormick, Human skin penetration of sunscreen nanoparticles: in-vitro assessment of a novel micronized zinc oxide formulation, Skin Pharmacol. Physiol., 2007, 20, 148–154 CrossRef CAS PubMed.
- M. Heydari-Majd, B. Ghanbarzadeh, M. Shahidi-Noghabi, M. A. Najafi and M. Hosseini, A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets, Food Packag. Shelf Life, 2019, 19, 94–103 CrossRef.
- M. A. Emamhadi, M. Sarafraz, M. Akbari, V. N. Thai, Y. Fakhri, N. T. T. Linh and A. Mousavi Khaneghah, Nanomaterials for food packaging applications: A systematic review, Food Chem. Toxicol., 2020, 146, 111825 CrossRef CAS PubMed.
- X. Li, Y. Xing, Y. Jiang, Y. Ding and W. Li, Antimicrobial activities of ZnO powder-coated PVC film to inactivate food pathogens, Int. J. Food Sci. Technol., 2009, 44, 2161–2168 CrossRef CAS.
- C. Pang, D. Hristozov, A. Zabeo, L. Pizzol, M. P. Tsang, P. Sayre and A. Marcomini, Probabilistic approach for assessing infants' health risks due to ingestion of nanoscale silver released from consumer products, Environ. Int., 2017, 99, 199–207 CrossRef CAS PubMed.
- H. J. Klimisch, M. Andreae and U. Tillmann, A systematic approach for evaluating the quality of experimental toxicological and ecotoxicological data, Regul. Toxicol. Pharmacol., 1997, 25, 1–5 CrossRef CAS PubMed.
- B. G. H. Bokkers and W. Slob, Deriving a data-based interspecies assessment factor using the NOAEL and the benchmark dose approach, Crit. Rev. Toxicol., 2007, 37, 355–373 CrossRef CAS PubMed.
- H. J. Kramer, W. A. vanden Ham, W. Slob and M. N. Pieters, Conversion factors estimating indicative chronic no-observed-adverse-effect levels from short-term toxicity data, Regul. Toxicol. Pharmacol., 1996, 23, 249–255 CrossRef CAS PubMed.
- U.S. EPA, A review of the reference dose and reference concentration processes, U.S. Environmental Protection Agency, Report EPA/630/P-02/002F, Risk Assessment Forum, Washington, DC, 2002 Search PubMed.
- Danish Ministry of the Environment, Dermal absorption of nanomaterials part of the “Better control of nano” initiative 2012–2015, 2013 Search PubMed.
- U.S. EPA, Reference dose (RfD): Description and use in health risk assessments, Regul. Toxicol. Pharmacol., 1988, 8, 471–486 CrossRef PubMed.
- U.S. EPA, Risk Assessment Guidance for Superfund (RAGS), Volume I: Human health evaluation manual (Part E, Supplemental guidance for dermal risk assessment) interim, Office of Solid Waste Emergency Response, 2009 Search PubMed.
- P. R. Bushel, J. A. L. Bourdon, D. Krewski and S. Auerbach, Technical guide for applications of gene expression profiling in human health risk assessment of environmental chemicals, Regul. Toxicol. Pharmacol., 2015, 72, 292–309 CrossRef.
- X. L. Duan, Exposure Factors Handbook of Chinese Population, China Environmental Science Press, Beijing, 2013 Search PubMed.
- H. van der Voet, W. Slob and J. van Klaveren, Integration of probabilistic exposure assessment and probabilistic hazard characterization, Toxicol. Lett., 2007, 172, S109–S109 CrossRef.
- U.S. EPA, Conditional registration of HeiQ AGS-20 as a materials preservative in textiles, 2011 Search PubMed.
- S. Pasupuleti, S. Alapati, S. Ganapathy, G. Anumolu, N. R. Pully and B. M. Prakhya, Toxicity of zinc oxide nanoparticles through oral route, Toxicol. Ind. Health, 2012, 28, 675–686 CrossRef CAS PubMed.
- H. Genc, B. Barutca, A. T. Koparal, U. Ozogut, Y. Sahin and E. Suvaci, Biocompatibility of designed MicNo-ZnO particles: Cytotoxicity, genotoxicity and phototoxicity in human skin keratinocyte cells, Toxicol. In Vitro, 2018, 47, 238–248 CrossRef CAS PubMed.
- Q. Li, L. Chen and Y. Zhou, Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels, Sci. Rep., 2018, 8, 5224 CrossRef PubMed.
- S. C. O. C. Safety (SCCS), Opinion on zinc oxide (nano form) COLIPA S 76, 2012 Search PubMed.
- P. J. Borm, D. Robbins, S. Haubold, T. Kuhlbusch, H. Fissan, K. Donaldson, R. Schins, V. Stone, W. Kreyling, J. Lademann, J. Krutmann, D. Warheit and E. Oberdorster, The potential risks of nanomaterials: a review carried out for ECETOC, Part. Fibre Toxicol., 2006, 3, 11 CrossRef PubMed.
- V. D. Subramaniam, S. V. Prasad, A. Banerjee, M. Gopinath, R. Murugesan, F. Marotta, X. F. Sun and S. Pathak, Health hazards of nanoparticles: understanding the toxicity mechanism of nanosized ZnO in cosmetic products, Drug Chem. Toxicol., 2019, 42, 84–93 CrossRef CAS PubMed.
- A. M. Holmes, I. Kempson, T. Turnbull, D. Paterson and M. S. Roberts, Penetration of zinc into human skin after topical application of nano zinc oxide used in commercial sunscreen formulations, ACS Appl. Bio Mater., 2020, 3, 3640–3647 CrossRef CAS PubMed.
- A. Emamifar, M. Kadivar, M. Shahedi and S. Soleimanian-Zad, Evaluation of nanocomposite packaging containing Ag and ZnO on shelf life of fresh orange juice, Innovative Food Sci. Emerging Technol., 2010, 11, 742–748 CrossRef CAS.
- F. Stuer-Lauridsen, A. Kamper, P. Borling, G. I. Petersen and A. Baun, Survey of nanotechnological consumer products, 2007 Search PubMed.
- X. Duan, X. Zhao, B. Wang, Y. Chen and S. Cao, Highlights of the Chinese Exposure Factors Handbook, Highlights of the Chinese Exposure Factors Handbook(Adults), 2015 Search PubMed.
- Y. Luo, L. Ma, H. Zheng, L. Chen, R. Li, C. He, S. Yang, X. Ye, Z. Chen, Z. Li, Y. Gao, J. Han, G. He, L. Yang and Y. Wei, Discovery of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione, a readily available and orally active glitazone for the treatment of concanavalin A-induced acute liver injury of BALB/c mice, J. Med. Chem., 2010, 53, 273–281 CrossRef CAS PubMed.
- V. Forest, J. F. Hochepied and J. Pourchez, Importance of choosing relevant biological end points to predict nanoparticle toxicity with computational approaches for human health risk assessment, Chem. Res. Toxicol., 2019, 32, 1320–1326 Search PubMed.
- T. E. Abbott Chalew, G. S. Ajmani, H. Huang and K. J. Schwab, Evaluating nanoparticle breakthrough during drinking water treatment, Environ. Health Perspect., 2013, 121, 1161–1166 CrossRef PubMed.
- V. S. Sousa and M. Ribau Teixeira, Metal-based engineered nanoparticles in the drinking water treatment systems: A critical review, Sci. Total Environ., 2020, 707, 136077 CrossRef CAS PubMed.
- A. Spinazzè, A. Cattaneo, F. Borghi, L. Del Buono, D. Campagnolo, S. Rovelli and D. M. Cavallo, Probabilistic approach for the risk assessment of nanomaterials: A case study for graphene nanoplatelets, Int. J. Hyg. Environ. Health, 2019, 222, 76–83 CrossRef PubMed.
- U. S. EPA, Conditional Registration of HeiQ AGS-20 as a Materials Preservative in Textiles, December 1, 2011, pp. 1–51 Search PubMed.
- B. G. H. Bokkers, M. J. Mengelers, M. I. Bakker, W. A. Chiu and W. Slob, APROBA-Plus: A probabilistic tool to evaluate and express uncertainty in hazard characterization and exposure assessment of substances, Food Chem. Toxicol., 2017, 110, 408–417 CrossRef CAS PubMed.
- T. P. Pastoor, A. N. Bachman, D. R. Bell, S. M. Cohen, M. Dellarco, I. C. Dewhurst, J. E. Doe, N. G. Doerrer, M. R. Embry, R. N. Hines, A. Moretto, R. D. Phillips, J. C. Rowlands, J. Y. Tanir, D. C. Wolf and A. R. Boobis, A 21st century roadmap for human health risk assessment, Crit. Rev. Toxicol., 2014, 44(Suppl 3), 1–5 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2en00570k |
| This journal is © The Royal Society of Chemistry 2023 |