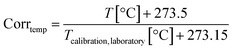Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: A submersible probe with in-line calibration and a symmetrical reference element for continuous direct nitrate concentration measurements
Tara
Forrest
 ,
Thomas
Cherubini
,
Stéphane
Jeanneret
,
Elena
Zdrachek
,
Polyxeni
Damala
and
Eric
Bakker
,
Thomas
Cherubini
,
Stéphane
Jeanneret
,
Elena
Zdrachek
,
Polyxeni
Damala
and
Eric
Bakker
 *
*
Department of Inorganic and Analytical Chemistry, University of Geneva, Quai Ernest-Ansermet 30, CH-1211 Geneva, Switzerland. E-mail: eric.bakker@unige.ch
First published on 6th June 2023
Abstract
Correction for ‘A submersible probe with in-line calibration and a symmetrical reference element for continuous direct nitrate concentration measurements' by Tara Forrest et al., Environ. Sci.: Processes Impacts, 2023, 25, 519–530, https://doi.org/10.1039/D2EM00341D.
An incorrect value of the nitrate levels was quoted on page 519 of the original article. Lines 19–22 of the abstract on page 519 should read as follows:
“The nitrate levels measured using the symmetrical reference element over this period were estimated at 44.0 ± 3.5 μM and agreed well with the values obtained with ion chromatography (38.2 ± 2.1 μM) used as the reference method.”
An incorrect value of the diamond powder suspensions size was quoted on page 521 of the original article. Lines 4–6 of the laboratory electrochemical equipment subsection of the experimental on page 521 should read as follows:
“Before use they were polished using different diamond powder suspensions (Ø 6 − 3 − 1 − 0.25 μm).”
There was a typographical error on lines 6–9 in the laboratory electrochemical equipment subsection of the experimental on page 521 which should read as follows:
“Electropolymerisation of the transducing layer was performed in a three electrode system with a PGSTAT 101 (Metrohm Autolab, B.V., Utrecht, The Netherlands) controlled by the Nova 1.8 software.”
There was a typographical error on lines 6–9 in the electrode preparation subsection of the experimental in the left-hand column on page 522 which should read as follows:
“The transducing layer was then prepolarised by applying a constant potential to a solution of 0.03 M of KTPFPB in acetonitrile to reach an oxidation ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 between PEDOT-C140/PEDOT-C14+.36”
50 between PEDOT-C140/PEDOT-C14+.36”
There was a typographical error on lines 29–32 in the electrode preparation subsection of the experimental in the right-hand column on page 522 which should read as follows:
It can be seen in Fig. S4 and Table S1† that the proposed ion-selective electrodes discriminate against sulfate and chloride, which are the main anions that might interfere in freshwater samples.”
There was a typographical error on line 11 (right-hand column) in the calibration and measurement principle sub-section of the experimental on page 524 which should read as follows:
“…the concentration of nitrate in the calibrant and KpotI,j is the selectivity coefficient.”
There was a typographical error in the equation in the calibration and measurement principle sub-section of the experimental on page 525 which should read as follows:
There was an incorrect placement of the sentence on lines 38–39 in the right-hand column of the results and discussion on page 525. This sentence should be located after the sentence on lines 18–22 in the left-hand column of the results and discussion of page 526 as follows:
“The resulting junction potential calculated between the two solutions was lower than 0.3 mV and can be neglected considering that the expected potential shift between both solutions corresponds to −38.0 mV. The liquid junction potential was calculated according to Henderson's eqn (8).”
There was a typographical error of the sentence on lines 17–19 in the right-hand column of the results and discussion on page 526 which should read as follows:
“Cycle by cycle data extracted from Fig. 5 can be found in Table S3† for the symmetrical reference and Table S4† for the Ag/AgCl reference element.”
There was a typographical error of the sentence on lines 135–136 in the right-hand column of the results and discussion on page 526 which should read as follows:
“The details of the calibration cycle can be found in Fig. S6 and Table S5.†”
An incorrect value of average nitrate concentration was quoted on page 528 of the original article. Lines 1–2 in the left-hand column of the results and discussion section on page 528 should read as follows:
“The average nitrate concentration measured by ion chromatography is 38.2 ± 2.1 μM.”
An incorrect value of average nitrate concentration was quoted on page 528 of the original article. Lines 10–13 in the left-hand column of the results and discussion section on page 528 should read as follows:
“The average nitrate concentration observed using the probe for the same times at which these samples were taken was 44.0 ± 3.5 μM when measured against the symmetrical reference element.”
An incorrect value of average nitrate concentration was quoted on page 528 of the original article. Lines 13–14 in the left-hand column of the results and discussion section on page 528 should read as follows:
“This value increases to 77.4 ± 6.4 μM when using Ag/AgCl as the reference, which is unacceptable.”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |