Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An immiscible phase-separation electrolyte and interface ion transfer electrochemistry enable zinc/lithium hybrid batteries with a 3.5 V-class operating voltage†
Ao
Chen
a,
Yaqin
Zhang
 a,
Qing
Li
a,
Guojing
Liang
a,
Shuo
Yang
a,
Zhaodong
Huang
b,
Qi
Yang
b,
Hong
Hu
a,
Xinliang
Li
a,
Ze
Chen
a,
Jun
Fan
a,
Qing
Li
a,
Guojing
Liang
a,
Shuo
Yang
a,
Zhaodong
Huang
b,
Qi
Yang
b,
Hong
Hu
a,
Xinliang
Li
a,
Ze
Chen
a,
Jun
Fan
 *a and
Chunyi
Zhi
*a and
Chunyi
Zhi
 *abcde
*abcde
aDepartment of Materials Science and Engineering, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong, China. E-mail: junfan@cityu.edu.hk; cy.zhi@cityu.edu.hk
bHong Kong Center for Cerebro-Cardiovascular Health Engineering (COCHE), Shatin, NT, HKSAR, China
cCentre for Advanced Nuclear Safety and Sustainable Development, City University of Hong Kong, 83 Tat Chee Avenue, Kowloon, Hong Kong 999077, China
dHong Kong Institute for Clean Energy, City University of Hong Kong, Kowloon 999077, Hong Kong
eHong Kong Institute for Advanced Study, City University of Hong Kong, Kowloon, Hong Kong 999077, China
First published on 27th July 2023
Abstract
The low-cost and high safety of zinc batteries have attracted tremendous attention in recent years. However, with conventional electrolytes, the operating voltages of zinc batteries are normally below 2 V. Hybrid batteries represented by commercial zinc–nickel batteries (zinc–proton hybrid batteries) employ H+ as the charge carrier to obtain a high operating voltage and electrochemical performance. Nevertheless, the preparation of other alkaline zinc-based hybrid batteries with high electrochemical performance is difficult to achieve due to the narrow cathodic window and incompatibility of cathode materials with the alkaline electrolyte. Herein, we develop a unique phase-separation electrolyte (PSE) consisting of a completely immiscible aqueous phase and an oil phase. The alkaline aqueous electrolyte can take advantage of the low electrode potential of the zinc anode. The interface ion transfer electrochemistry in the PSE can further boost the operating voltage by ∼0.35 V. Accordingly, our developed zinc/lithium hybrid batteries deliver an unprecedented average operating voltage of 3.41 V and a high energy density of 362.4 W h kganode+cathode−1 (N/P is assumed to be 1), approaching the voltage of lithium-ion batteries. More interestingly, the liquid–liquid interface in the PSE can entirely intercept the propagation of zinc dendrites, benefiting from the completely blocked diffusion of Zn2+ into the oil phase. Zinc/lithium hybrid batteries with LiMn2O4 cathodes deliver an excellent cycle performance over 600 cycles with a 99.6% coulombic efficiency. To further demonstrate practicality, we fabricated a full cell with a commercial-level cathode mass loading of 18.3 mg cm−2, achieving a zinc-based battery with a high voltage of 2.56 V and a high areal capacity of 2.14 mA h cm−2.
Broader contextLimited by the electrochemical stability window (ESW) of conventional single aqueous electrolytes and cathode materials, the average operating voltage of zinc batteries is below 2 V. Hybrid batteries with flexible operability can deliver superior electrochemical performance compared to the traditional battery systems. Herein, we initiate a novel phase-separation electrolyte (PSE) with an ESW of 4.6 V, enabling the high voltage cathode materials to be used in zinc-based batteries. The alkaline zinc/lithium hybrid batteries employing lithium ions as the charge carrier achieved an unprecedented average operating voltage of 3.41 V. Interface ion transfer electrochemistry was found to increase the operating voltage by an additional ∼0.35 V in the PSE. The investigation of employing unconventional electrolytes in zinc-based batteries provides a new pathway to develop battery systems with high electrochemical performances. |
Introduction
Charge carriers are fundamental components of batteries that determine battery chemistry and performance.1 In conventional rechargeable metal anode batteries, the corresponding metal ions serve as the charge carriers and shuttle between the anode and cathode, which requires the cathode materials to allow the reversible intercalation of the metal ions. Thus, the selectivity of cathode materials is severely limited. Recently, hybrid batteries without anode metal ions as the charge carriers have attracted tremendous attention, which has initiated a promising approach for designing batteries with better electrochemical performance and extending the selection of cathode materials. For instance, Na–Li hybrid batteries,2–4 Mg–Li hybrid batteries,5–8 Mg–Na hybrid batteries, and calcium-ion-based hybrid batteries exhibited a high operating voltage and stable cycling performance.9–16Aqueous rechargeable zinc-based batteries with the advantages of low-cost, facile manufacturing, and superior safety have been developed via employing alkaline, neutral or mild acidic electrolytes, and are the most potential candidates in large-scale and safe energy storage systems. For aqueous zinc batteries with neutral or mild acidic electrolytes, previous reports demonstrate that both H+ and Zn2+ usually act as the charge carriers to intercalate and deintercalate into cathode materials.17–22 However, due to the high electrode potential of zinc in a neutral or mild acidic electrolyte (−0.76 V versus the standard hydrogen electrode (SHE)), the narrow electrochemical stability window (ESW), and the limitation of available cathode materials, zinc batteries usually exhibit a working voltage of below 1.5 V, which vastly limits their applications. Moreover, with Zn2+ as the high charge ion, most cathode materials cannot allow its reversible intercalation. Therefore, developing hybrid batteries with non-zinc ions as charge carriers is effective to improve the electrochemical performance of zinc-based batteries. For example, high-voltage materials LiMn2O4,23 LiVPO4F,24 and CuHCF could be used as the cathodes25 when Li+ ions or Na+ ions worked as charge carriers in zinc-based hybrid batteries. Nevertheless, the operating voltages were still below 2 V, which is far lower than that of commercial lithium-ion batteries (LIBs) (>3 V). In alkaline electrolyte, the electrochemical potential of zinc is about −1.21 V versus the SHE, which can potentially lead to a higher voltage of zinc-based hybrid batteries. Typically, the traditional commercialized alkaline nickel–zinc battery is a representative Zn2+/H+ hybrid battery. H+ serves as the charge carrier and the anodic reaction is the conversion between Zn and ZnO, and moreover, H+ is reversibly intercalated into the Ni(OH)2 cathode. However, because of the narrow cathodic window and low reduction potential of Ni(OH)2 in alkaline electrolyte, the operating voltage of the battery is also about 1.7 V. In addition, due to the poor compatibility of alkaline electrolyte with most cathode materials, the development of high-voltage zinc-based hybrid batteries based on alkaline electrolytes remains a challenge.
Hence, to take advantage of the low electrochemical potential of zinc in alkaline electrolytes, multi-chamber structured batteries have been developed recently.26–28 These devices usually consist of alkaline/neutral/acid electrolyte chambers, which could broaden the ESW to be ∼3.2 V and achieve working voltages of over 2 V by assembling hybrid batteries. However, the ESWs of multi-chamber alkaline/acid electrolytes are still not sufficiently wide. More importantly, single-phase aqueous electrolytes including alkaline, neutral, and acid electrolytes are inclined to be miscible, leading to a poor stability of the batteries. In addition, the cathodes need to be immersed in the strong acid electrolyte, resulting in their decomposition. Accordingly, many commonly used high voltage materials such as LiMn2O4, LiNi0.5Mn1.5O4 and LiCoMnO4 cannot work, which limits the further improvement of the operating voltage of zinc batteries.
On the other hand, the growth of zinc dendrites is one of the main failure modes of zinc batteries. In particular, the growth of dendrites in alkaline electrolytes is much more severe than that in acid or neutral electrolytes. Although much effort has been made to constrain the growth of zinc dendrites, they are generally not effective for zinc anodes in alkaline electrolytes.29–32 An ultimate way to prevent dendrites approaching the cathode is to create an electrolyte structure with no Zn2+ ions existing at the cathode side, which has never been demonstrated so far.
Herein, we developed a phase-separation electrolyte (PSE) and for the first time achieved an unprecedented average operating voltage of up to 3.41 V for alkaline aqueous hybrid batteries. The PSE is composed of alkaline aqueous and N-methyl-N-butylpyrrolidinium bis(trifluoromethylsulfonyl)imide (Py14TFSI) electrolytes, which can autonomically form separated aqueous phases and oil phases due to their completely immiscible features. By employing Li+ as the charge carrier, those cathode materials that do not allow reversible intercalation of Zn2+ can be matched with zinc anodes. In addition, experiments, and molecular dynamics (MD) simulations reveal that the PSE has the capability of additionally boosting the working voltage by ∼0.35 V via interface ion transfer electrochemistry. Moreover, in the PSE, Zn2+ ions are completely confined in the aqueous electrolyte, which thoroughly prevents the zinc dendrites from reaching the cathode. As a result, with the LiNi0.5Mn1.5O4 cathode, a record-high average operating voltage of 3.41 V was achieved for zinc/lithium hybrid batteries.
Phase-separation electrolytes
In contrast to the conventional single-phase electrolyte of zinc batteries, the PSE consists of completely immiscible water and oil components for the Zn anode and cathode materials, respectively, as shown in Fig. 1(a) and the ESI,† Fig. S1. The aqueous electrolyte component contains 3 M LiOH, 0.3 M ZnO and 2 M LiTFSI, enabling the reversible plating/stripping of Zn in the alkaline electrolyte. The LiTFSI dissolved in the Py14TFSI as the oil phase component of the PSE, which is totally immiscible with the aqueous phase component. To decrease the viscosity of the Py14TFSI, highly fluorinated ether (HFE) of 1,1,2,2-tetrafluoroethyl-2,2,3,3-tetrafluoropropylether that is also immiscible with aqueous electrolytes was employed as the diluent, considering its high stability against alkali compared with the conventional ester solvents (ESI,† Fig. S2). The ionic conductivity was significantly increased from 0.34 (Py14TFSI) to 1.23 mS cm−1 (Py14TFSI-HFE) (ESI,† Fig. S3). The inset in Fig. 1(b) shows the clearly separated two phases autonomically formed in the PSE.Linear sweep voltammetry (LSV) shows that the pure Py14TFSI decomposes over 2.5 V (vs. Ag/AgCl) (ESI,† Fig. S4a), whereas HFE has no obvious decomposition voltage (ESI,† Fig. S4b). After introducing the HFE, the cathodic limitation of the Py14TFSI-HFE electrolyte is extended to 2.9 V (vs. Ag/AgCl) (Fig. 1(b)). Although the PSE contains an aqueous component, the cathodic window of Py14TFSI-HFE in the PSE was unaffected due to the intrinsically immiscible character. The alkaline aqueous electrolyte presents a hydrogen evolution reaction (HER) below −1.6 V (vs. SHE). Therefore, the PSE exhibits a remarkable ESW of ∼4.6 V (Fig. 1(c)). More importantly, the 2.9 V cathodic window of the PSE enables nearly all current high-potential cathode materials to be usable in zinc batteries (Fig. 1(d)), which forms the foundation of zinc batteries with a super high voltage output.33–46
High operating voltage aqueous zinc/lithium hybrid batteries based on PSEs
To investigate the electrochemical behaviors of zinc/lithium hybrid batteries based on the PSE, four high-potential materials of LiFePO4, LiMn2O4, LiNi0.5Mn1.5O4 and LiCoMnO4 were employed as cathodes. The reversible electrochemical behavior and galvanostatic voltage profiles of the Zn|PSE|LiFePO4 battery are shown in the ESI,† Fig. S5a and Fig. 2(a), respectively. The well-defined and symmetrical redox peaks of cyclic voltammetry (CV) correspond to the typical ultra-flat discharge and charge plateaus of the LiFePO4. Benefiting from the PSE, even with the LiFePO4 cathode, the Zn|PSE|LiFePO4 can achieve a high open-circuit voltage (OCV) of 2.27 V and average working voltage of 1.85 V at 0.2C, respectively, which exceeds most reported high-voltage zinc batteries. It also delivered a specific capacity of 165.2 mA h g−1, corresponding to a high energy density of 307.3 W h kgcathode−1. Fig. 2(b) exhibits the charge–discharge profiles of a Zn|PSE|LiMn2O4 cell. The cell exhibits a high OCV of up to 2.87 V with two high discharge plateaus at ∼2.7 V and ∼2.5 V, corresponding to two well-defined anodic peaks of CV curves (ESI,† Fig. S5b). It delivers a high average operating voltage of 2.56 V and a specific capacity of 122 mA h g−1. Furthermore, the Zn|PSE|LiNi0.5Mn1.5O4 cell exhibits an OCV up to 3.35 V and discharge plateaus at about 3.2 V (Fig. 2(c), corresponding to the CV shown in the ESI,† Fig. S5c), consistent with the typical discharge behavior of LiNi0.5Mn1.5O4. Benefiting from the high discharge plateaus, the average operating voltage could reach an unprecedented 3.15 V at 0.2C and a high energy density of 396 W h kgcathode−1 is obtained. Fig. 2(d) shows the charge–discharge profiles of a Zn|PSE|LiCoMnO4 cell. The Zn|PSE|LiCoMnO4 battery discharges from 3.52 V and delivers a high plateau at 3.3 V, corresponding well to the reduction peak of CV (ESI,† Fig. S5d). The average discharge voltage is up to 3.10 V, which is slightly lower than that of the Zn|PSE|LiNi0.5Mn1.5O4 battery due to more capacity being discharged at 2.0–3.2 V. Apparently, benefiting from the immiscible design of the PSE, the aqueous zinc/lithium hybrid batteries exhibited excellent compatibility for a variety of cathode materials and for the first time achieved a landmark average operating voltage of over 3 V.Water-in-salt (WiS) electrolytes with extended ESWs were widely used to achieve high voltages for zinc-based hybrid batteries.23,24 For comparison, we tested the electrochemical performance of zinc batteries with the same cathode materials in the WiS electrolyte. As shown in Fig. 2(e), the Zn|WiS|LiFePO4 battery exhibited flat discharge/charge plateaus corresponding well to the CV profile (ESI,† Fig. S6a). Nevertheless, it only demonstrated a 1.09 V working voltage in the 1 M zinc trifluoromethylsulfonate (Zn(OTF)2) in 21 M LiTFSI electrolyte at 0.2C, which is far below the achieved 1.85 V in the PSE. Similarly, the CV result (ESI,† Fig. S6b) and galvanostatic discharge profile (Fig. 2(e)) demonstrate that the Zn|WiS|LiMn2O4 battery with the WiS electrolyte exhibited two discharge plateaus at 1.9 and 1.7 V, respectively, and the average discharge voltage is only 1.76 V. As for the LiNi0.5Mn1.5O4, although the WiS electrolyte possesses an extended electrochemical window of ∼3.1 V (ESI,† Fig. S7), obvious decomposition of electrolyte could be observed after charging to 2.3 V (Fig. 2(e)). This is due to the strong catalytic effect of Ni4+, leading to the oxygen evolution. The Zn|WiS|LiCoMnO4 battery also shows inevitable decomposition of water due to the catalytic effects of Co4+(Fig. 2(e)). Accordingly, the sharp contrast indicates the PSE can make the best advantage of the electrode potential of Zn in an alkaline environment and avoid the negative effects of alkaline electrolyte on cathode materials, rendering the alkaline zinc/lithium hybrid batteries with remarkable voltage outputs. In addition, the unconventional PSE with the 4.6 V electrochemical window provides excellent flexibility in selecting high potential cathode materials.
Since the concentration of alkali will affect the electrode potential of zinc due to the Nernst shift, the working voltage of the zinc/lithium hybrid batteries with PSEs can be regulated via adjusting the aqueous phase components of the PSE. As shown in Fig. 2(f), after improving the concentration of alkali, even the Zn‖LiFePO4 cell can achieve an average discharge voltage of 2.13 V at 0.2C. For the Zn‖LiMn2O4 battery in the concentrated alkaline PSE, the two discharge plateaus were further boosted to 2.95 V and 2.75 V, respectively, and it delivered a 3.12 V OCV and a high operating voltage of 2.85 V. The discharge plateau of Zn‖LiNi0.5Mn1.5O4 in the concentrated alkaline PSE reaches an unprecedented 3.5 V, and the average operating voltage is enhanced to 3.41 V, which is almost twice that of most high-voltage zinc batteries and comparable with rechargeable LIBs. Similarly, the discharge plateau and average operating voltage of the Zn‖LiCoMnO4 battery were improved to 3.6 V and 3.37 V, respectively (Fig. 2(f)).
Energy storage mechanism of cathode materials in the PSE
It should be noted that our investigation and previous reports demonstrated that the Zn‖LiFePO4 and the Zn‖LiNixMn2−xO4 can cycle in the Li+/Zn2+ hybrid aqueous electrolytes. In this case, Li+ instead of Zn2+ intercalates to cathodes.23,47,48 However, in an organic electrolyte, we found the Zn‖LiFePO4 and Zn‖LiNixMn2−xO4 are not reversible anymore. As shown in Fig. 3(a), in a 0.4 M LiTFSI/0.2 M Zn(TFSI)2 based Py14TFSI-HFE electrolyte, only 15% of the theoretical capacity was delivered and the battery failed after 5 cycles, demonstrating the irreversible intercalation of Zn2+. A similar phenomenon was observed for the Zn‖LiMn2O4 battery with the same electrolyte (Fig. 3(b)).To explain this phenomenon, radial distribution functions (RDFs) were employed to analyze the priority of Li+ and Zn2+ ions into cathode materials in aqueous and organic electrolytes. As shown in Fig. 3(c), H2O molecules mainly occupy the first coordination shell of both Li+ and Zn2+ in a 0.2 M Zn(OTF)2/0.4 M LiTFSI aqueous electrolyte. The coordination numbers of Zn2+ are higher than those of Li+ and the interaction of H2O molecules with Zn2+ is stronger than that of Li+, revealed by the larger g(r). This result suggests that Li+ will preferentially intercalate the cathode materials. Even in the 1 M Zn(OTF)2/21 M Zn TFSI based WiS electrolyte (Fig. 3(d)), due to the high molar ratio of salt, H2O molecules and TFSI− occupy the first coordination shell of both Li+ and Zn2+. However, compared to Zn2+, the larger g(r) indicates that Li+ ions will still preferentially intercalate to the cathode materials. The same results can be obtained even by substituting the Zn(OTF)2 with Zn(TFSI)2 (ESI,† Fig. S8). In sharp contrast, for the organic Py14TFSI-HFE electrolyte (Fig. 3(e)), the first coordination shell was enriched with TFSI− and HFE molecules for both Li+ and Zn2+. The larger g(r) of Li+ indicates the interaction between Li+ and electrolyte is stronger, demonstrating that Zn2+ instead of Li+ will preferentially intercalate the cathode materials. Therefore, both the experimental and simulation results reveal that the irreversible intercalation of Zn2+ is the reason leading to the failure of LiFePO4 and LiNixMn2−xO4 cathodes in organic electrolytes. The hybrid batteries that lithium ions serve as the charge carriers is the only approach to use these kinds of materials.
In our PSE, due to the strong coordination between Zn2+ and OH− (the stability constant of Zn(OH)42− is about 10−14),49 Zn2+ ions are completely confined in the aqueous electrolyte. The entering of Zn2+ ions to Py14TFSI-HFE is effectively blocked at the interface. As shown in Fig. 3(f), the inductively coupled plasma (ICP) results show that Zn2+ ions hardly exist in the oil-phase electrolyte, which enables the selected four cathode materials be cycled with the PSE electrolyte since only Li+ ions serve as the charge carriers in the Py14TFSI-HFE components of the PSE. The Li+ ions deintercalation and intercalation reactions in the cathode materials in the PSE during the charge–discharge process were also verified by X-ray diffraction (XRD) measurement. As shown in Fig. 3(g) and (h), the ex situ XRD patterns of LiMn2O4 were collected between 1.8 and 2.9 V during the charge–discharge process. The Bragg peaks ranging from 10 and 70° exhibit a continuous shift during the entire charge–discharge process, which is consistent with contraction and expansion of the unit cells of LiMn2O4 during Li extraction and insertion,50–52 indicating no Zn2+ inserted (Fig. 3(h)). The typical (111), (311) and (400) peaks gradually broaden during charge and recover to the standard LiMn2O4 structure with the intercalation of Li+, indicating a reversible ion intercalation/deintercalation with the PSE (Fig. 3(i) and (j)). Similarly, the ex situ XRD results of LiFePO4, LiNi0.5Mn1.5O4 and LiCoMnO4 also exhibited the same reversible phase transformation in the PSE as in the conventional organic electrolytes (ESI,† Note S1 and Fig. S9–S11).
Another merit of our PSE is its ability to completely avoid Zn dendrites approaching the cathode side of the battery. The growth of zinc dendrites is a great challenge for zinc batteries because it will lead to the short circuit and battery failure. In particular, the growth of zinc dendrites in alkaline solution is much more severe than that in neutral or acid electrolyte. While many strategies have been proposed to suppress dendrites growth in mild electrolytes, they are generally not effective at all when the electrolytes are switched to alkaline ones. In our PSE, since Zn2+ ions hardly exist in the organic Py14TFSI-HFE component, the plating of zinc can only be processed in the aqueous phase component during charge, suggesting that the zinc dendrites cannot grow across the water–oil interface. To verify our inference, the zinc anode was put across the water–oil interface and carried out the electrochemical measurements. As shown in Fig. 3(k), after 10 cycles, the plating of zinc only occurred in the aqueous phase component of the PSE, and the zinc metal in the oil-phase electrolyte maintains a smooth surface. Scanning electron microscopy (SEM) images reveal the surface morphologies of the zinc anode. As shown in Fig. 3(l), obvious zinc dendrites and nanosheets can be observed for the zinc anode immersed in the aqueous phase component of the PSE. In sharp contrast, the images of the zinc anode in the organic Py14TFSI-HFE component of the PSE demonstrate a pristine surface with no zinc plated, which confirms our inference that the zinc dendrites cannot grow across the water–oil interface (Fig. 3(m)), completely avoiding the dendrite induced short circuit.
Interface induced voltage enhancement by the PSE
The developed PSE electrolyte endows alkaline zinc/lithium batteries with unprecedent voltage outputs. However, detailed calculations reveal a deviation of theoretical voltages from the obtained voltages, that is, the obtained voltages are even higher than the theoretical voltages if only the potential difference of the cathode and anode is considered. As shown in the ESI,† Fig. S12a and b, the symmetric redox peak of LiFePO4 is located at 3.33 V (vs. Li+/Li) in the Py14TFSI-HFE electrolyte, and the average discharge voltage of the LiFePO4 cathode is ∼0.3 V (vs. SHE) at 0.2C. Considering the oxidation potential of the zinc anode in 3 M LiOH electrolyte is –1.23 V (vs. SHE) (ESI,† Fig. S13), accordingly, the theoretical average operating voltage of the Zn‖LiFePO4 should be ∼1.53 V, which is far below the experimental result of 1.85 V. Similarly, for the LiMn2O4 cathode, two redox peaks located at 4.1 and 3.9 V (vs. Li+/Li), correspond to the two well-defined discharge plateaus (ESI,† Fig. S12c and d). The average discharge voltage is ∼0.98 V (vs. SHE) for LiMn2O4 in the Py14TFSI-HFE electrolyte. This indicates that the two discharge plateaus of the Zn‖LiMn2O4 cell should be at 2.3 and 2.1 V, and the average operating voltage is ∼2.21 V, while it achieves 2.56 V in the PSE. Similarly, based on the CV measurements and galvanostatic discharge test results (ESI,† Fig. S12e–h), the high-potential LiNi0.5Mn1.5O4 and LiCoMnO4 material-based zinc/lithium hybrid batteries should have 2.8 and 2.75 V average working voltages, respectively, much less than the >3 V achieved.We note that all average operating voltages of the zinc/lithium hybrid batteries in the PSE are generally 0.35 V higher than the theoretical values. To clarify the mechanism of the voltage enhancement, MD studies were performed. Fig. 4(a) and (b) exhibit the distribution of Li+ and TFSI− ions near the liquid–liquid interface in the PSE in the static and discharge states, and the corresponding snapshots of the cross section of the liquid–liquid interface are shown in the ESI,† Fig. S14 and S15. In the static state, the density of Li+ ions reaches 3 nm−3 within 1 nm from the interface in the aqueous component of the PSE (Fig. 4(c)). During discharge, TFSI− and Li+ ions move towards the anode and cathode, respectively. Apparently, Li+ ions accumulate at the aqueous electrolyte side, while TFSI− ions also diffuse across the liquid–liquid interface and accumulate at the aqueous side of the PSE, which can be attributed to the different transport velocities of ions in the aqueous phase and oil phase components of the PSE. Accordingly, the number density of Li+ ions increases significantly within 1 nm from the interface and dramatically decreases within 2–4 nm from the interface during discharge (Fig. 4(c)). TFSI− ions also increase at the aqueous side compared to that in the static state (ESI,† Fig. S16). Therefore, the equilibrium state of the LiTFSI in the aqueous phase and oil phase components of the PSE is broken at the interface, leading to a remarkable concentration increase of LiTFSI in the aqueous phase component of PSE close to the interface. The concentration gradient will drive LiTFSI to spontaneously diffuse into the oil phase component of the PSE to maintain the balance.
Experimentally, a four electrode cell is a powerful tool to investigate ion transfer electrochemistry across the liquid–liquid interface. The CV result demonstrated that the ion transfer potential of Li+ from the aqueous component to the oil phase component of the PSE is about 0.38 V (Fig. 4(d)). In our PSE, since the Zn2+ ions are confined in the aqueous component, Li+ ions serve as the charge carriers during the charge–discharge process. The results indicate the solvation energy of Li+ in the oil phase component is higher than that in the aqueous component. Density functional theory (DFT) calculation also demonstrated that Li+ ions are in a high energy state in the oil phase component (ESI,† Fig. S17). Four H2O molecules interact with a Li+ ion in the first solvation sheath in the aqueous components, and TFSI− and two HFE molecules interact with a Li+ ion in the first solvation sheath in the oil phase component (Li+(TFSI−HFE2)). Since the cathode materials are placed in the oil phase component, the Li+ can directly desolvate from the high energy state and intercalate into the cathode materials (Fig. 4(e)), which contributes to the additional enhancement of the operating voltage. Meanwhile, according to the above MD results, the concentration gradient at the interface during discharge can drive the Li+ ions to diffuse into the oil phase electrolyte, leading to a stable voltage increase without breaking the ionic equilibrium in the PSE.
As shown in Fig. 4(f), with the addition of 0.35 V boosted by the interface in PSE, the average operating voltages of Zn‖LiFePO4, Zn‖LiMn2O4, Zn‖LiNi0.5Mn1.5O4 and Zn‖LiCoMnO4 with the concentrated alkaline PSE were significantly enhanced by 41.1%, 30.0%, 21.3% and 20.7%, respectively, compared with the corresponding theoretical values by only considering the potential difference of the anode and cathode. In addition, benefiting from the high operating voltages, the energy densities of four zinc/lithium hybrid batteries in our work were all over 300 W h kgcathode−1, and the Zn‖LiNi0.5Mn1.5O4 cell with PSE delivered a high energy density of up to 416 W h kgcathode−1.
It should be noted that, for a battery, although the energy density can be improved by increasing either the discharge capacity or operating voltage, the voltage enhancement has distinct advantages on improving the energy density of a battery compared with discharge capacity enhancement when the anode is considered. For example, a cathode material with 410 mA h g−1 capacity and 0.8 V voltage output needs at least 0.5 g Zn to match, while a cathode material with 205 mA h g−1 capacity and 1.6 V voltage output only needs 0.25 g Zn to couple. Fig. 4(g) exhibits the energy densities of the reported main cathode materials in zinc batteries (based on the mass of both zinc anode and cathode materials, and N/P is assumed to be 1).24,37,38,53–62 The operating voltages of most of the zinc batteries are located in the green zone (below 2 V). The red zone represents the region that can only be achieved by the organic LIBs previously. Benefiting from the PSE, the zinc/lithium hybrid batteries exhibited high operating voltages comparable to that of LIBs, and the high operating voltages enable the energy density based on the mass of both the anode and cathode to be maximally utilized. As a result, all batteries with the PSE could deliver high energy densities over 250 W h kganode+cathode−1 and an energy density of 362.4 W h kganode+cathode−1 was achieved by the Zn‖LiNi0.5Mn1.5O4 cell.
Rate and cycling performance
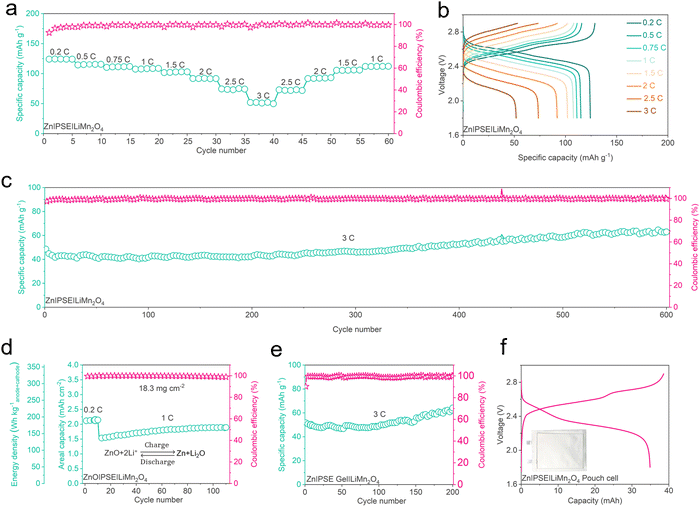
To evaluate the electrochemical stability of zinc/lithium hybrid batteries with the PSE, the developed batteries were tested at 1C for over 50 cycles (ESI,† Fig. S18). Compared with the LiNi0.5Mn1.5O4 and the LiCoMnO4, the commercialized LiFePO4 and LiMn2O4 exhibit better cycling stability. This is because the LiNi0.5Mn1.5O4 and LiCoMnO4 materials usually need coating or additives in the electrolyte to form a stable cathode electrolyte interface (CEI).63–67 The Zn|PSE|LiFePO4 and Zn|PSE|LiMn2O4 batteries delivered high discharge capacities of 151.4 and 100.1 mA h g−1 in over 50 cycles, respectively. SEM images of the LiMn2O4 and LiFePO4 before and after cycling are shown in the ESI,† Fig. S19 and S20. Although LiNi0.5Mn1.5O4 and LiCoMnO4 cathodes did not exhibit a remarkable cycling stability, the morphologies of LiNi0.5Mn1.5O4 and LiCoMnO4 still remained unchanged before and after cycling (ESI,† Fig. S21 and S22). Accordingly, the high voltage LiMn2O4 as a representative cathode was used to further investigate the electrochemical stability of the zinc/lithium hybrid batteries with the PSE. As shown in Fig. 5(a), the rate performance of the Zn|PSE|LiMn2O4 battery was evaluated from 0.2 to 3C. The battery delivered a specific capacity of 124.3, 115, 114.4, 107.4, 102.7, 92.1, 74.5 and 52.1 mA g−1 at 0.2, 0.5, 0.75, 1, 1.5, 2, 2.5 and 3C, respectively. Fig. 5(b) shows the corresponding charge–discharge profiles of the Zn|PSE|LiMn2O4 at different rates. Even at a high rate of 3C, the Zn|PSE|LiMn2O4 battery still exhibits a high working voltage of 2.2 V. In addition, the rate performance of the LiMn2O4 cathode in the single-phase Py14TFSI-HFE electrolyte was also evaluated with the lithium anode at the same rates (ESI,† Fig. S23a and b). The Li|Py14TFSI-HFE|LiMn2O4 battery indicates that the rate performance of cathode materials in the PSE mainly depends on the oil phase component. However, the high discharge capacity of the cell at a high rate can be obtained by constant voltage charging (ESI,† Fig. S24). Fig. 5(c) exhibits the long-term cycling performance of the Zn|PSE|LiMn2O4 battery at 3C. The battery delivered an initial discharge capacity of 55 mA g−1 and maintained about 60 mA g−1 capacity after over 600 cycles, indicating the excellent cycling performance of the Zn|PSE|LiMn2O4 battery. The average coulombic efficiency is over 99.6%, which is even higher than most of the zinc batteries with single-phase electrolytes, and the average operating voltage remained unchanged over 600 cycles (ESI,† Fig. S25). The cycling performance of the Zn‖LiMn2O4 with concentrated alkaline PSE was also evaluated and the results are shown in the ESI,† Fig. S26. For the zinc/lithium hybrid battery with the zinc anode in PSE, the capacity is determined by the solubilities of the ZnO in the aqueous electrolyte solutions, which limits its further practical application. For commercial zinc–nickel batteries, due to H+ as the charge carrier, ZnO is employed as the anode to meet the requirements of high areal capacity and practical application. Accordingly, to evaluate the practical potential of the PSE, a ZnO anode and a LiMn2O4 cathode with a high cathode loading mass of 18.3 mg cm−2 were assembled. As shown in Fig. 5(d), the high capacity ZnO|PSE|LiMn2O4 cell was first cycled at 0.2C, and it delivered a high areal capacity of 2.14 mA h cm−2. The electrochemical reaction process is similar to the zinc–nickel batteries. Li+ replaces H+ as charge carriers, and ZnO is reduced to Zn and Li2O during charging, so the discharge capacity of the cell is determined by the mass of the electrode materials. The corresponding charge–discharge profiles at 0.2C is shown in the ESI,† Fig. S27. After 10 cycles, the discharge rate was changed into 1C, and the battery could still deliver a high specific capacity of 84 mA h g−1 and maintained an areal capacity of ∼1.88 mA h cm−2 after over 110 cycles with an average coulombic efficiency over 99.6%. Benefiting from the high operating voltage of the PSE based batteries, the energy density of the full cell could reach a high level of 191.3 W h kganode+cathode−1 (the N/P ratio is 1.52). In addition, we also fabricated a cell with gel electrolyte, as illustrated in the ESI,† Fig. S28. It delivered a specific capacity of 61.6 mA h g−1 after 200 cycles (Fig. 5(e)), and the pouch cell with gel electrolyte delivers a 35 mA h discharge capacity (Fig. 5(f)).Conclusion
In summary, we developed a novel and unique phase-separation electrolyte consisting of completely immiscible aqueous phase and oil-phase components. The alkaline aqueous electrolyte takes advantage of the lower electrode potential of the Zn anode and the oil phase Py14TFSI-HFE electrolyte remarkably extends the selection of cathode materials. In addition, the interface ion transfer electrochemistry further boosts the operating voltage by ∼0.35 V. Zn2+ ions were confined in the aqueous electrolyte via the strong coordination effect between Zn2+ and OH−, ultimately eliminating the Zn dendrites approaching the cathode and meanwhile avoiding the irreversible Zn2+ intercalation to the cathode. The oil phase electrolyte has excellent compatibility with various cathode materials compared to single phase aqueous electrolytes and the cathodic limitation is extended to 2.9 V. As a result, the zinc/lithium hybrid batteries achieved a record-high average operating voltage comparable to those of LIBs, up to 3.41V, and it delivers a high energy density of 362.4 W h kganode+cathode−1 (N/P is assumed to be 1). The Zn|PSE|LiMn2O4 delivers an excellent cycle performance over 600 cycles with a 99.6% coulombic efficiency. In addition, the full cell with a commercial level cathode loading of 18.3 mg cm−2 delivered a high areal capacity of 2.14 mA h cm−2. The unconventional electrolyte system provides a new approach to fabricate high performance zinc-based batteries and may be used for other battery systems to achieve extraordinary voltage output.Author contributions
Conceptualization: A. C.; methodology: A. C., Y. Q. Z., Q. L., G. J. L., and S. Y.; investigation: A. C., Y. Q. Z., Z. D. H., Q. Y., X. L. L., H. H., Z. C., J. F., and C. Y. Z.; computation: Y. Q. C.; writing – original draft: A. C. and C. Y. Z.; writing review and editing: A. C., Y. Q. Z., Q. L., G. J. J., S. Y., Z. D. H., Q. Y., H. H., X. L. L, Z. C., J. F., and C. Y. Z.; J. F., and C. Y. Z. jointly supervised this work.Conflicts of interest
The authors declare no competing interests.Acknowledgements
This research was supported by the National Key R&D Program of China under Project 2019YFA0705104 and CRF under Project C1002-21G funded by RGC.References
- G. Liang, F. Mo, X. Ji and C. Zhi, Nat. Rev. Mater., 2021, 6, 109–123 CrossRef CAS.
- J. Z. Guo, Y. Yang, D. S. Liu, X. L. Wu, B. H. Hou, W. L. Pang, K. C. Huang, J. P. Zhang and Z. M. Su, Adv. Energy Mater., 2018, 8, 1702504 CrossRef.
- L. Liang, X. Li, F. Zhao, J. Zhang, Y. Liu, L. Hou and C. Yuan, Adv. Energy Mater., 2021, 11, 2100287 CrossRef CAS.
- Q. Zhang, Y. Lu, L. Miao, Q. Zhao, K. Xia, J. Liang, S. L. Chou and J. Chen, Angew. Chem., Int. Ed., 2018, 57, 14796–14800 CrossRef CAS PubMed.
- Q. D. Truong, M. Kempaiah Devaraju, Y. Nakayasu, N. Tamura, Y. Sasaki, T. Tomai and I. Honma, ACS Omega, 2017, 2, 2360–2367 CrossRef CAS PubMed.
- T. Gao, F. Han, Y. Zhu, L. Suo, C. Luo, K. Xu and C. Wang, Adv. Energy Mater., 2015, 5, 1401507 CrossRef.
- X. Sun, V. Duffort and L. F. Nazar, Adv. Sci., 2016, 3, 1600044 CrossRef PubMed.
- Y. Wang, C. Wang, X. Yi, Y. Hu, L. Wang, L. Ma, G. Zhu, T. Chen and Z. Jin, Energy Storage Mater., 2019, 23, 741–748 CrossRef.
- J. Lang, C. Jiang, Y. Fang, L. Shi, S. Miao and Y. Tang, Adv. Energy Mater., 2019, 9, 1901099 CrossRef.
- N. Wu, W. Yao, X. Song, G. Zhang, B. Chen, J. Yang and Y. Tang, Adv. Energy Mater., 2019, 9, 1803865 CrossRef.
- Y. Tang, X. Li, H. Lv, W. Wang, Q. Yang, C. Zhi and H. Li, Angew. Chem., Int. Ed., 2021, 133, 5503–5512 CrossRef.
- M. Rashad, M. Asif, Y. Wang, Z. He and I. Ahmed, Energy Storage Mater., 2020, 25, 342–375 CrossRef.
- P. Wang and X. Yan, Energy Storage Mater., 2022, 45, 142–181 CrossRef.
- Y. Li, Q. An, Y. Cheng, Y. Liang, Y. Ren, C.-J. Sun, H. Dong, Z. Tang, G. Li and Y. Yao, Nano Energy, 2017, 34, 188–194 CrossRef CAS.
- Y. Xu, W. Cao, Y. Yin, J. Sheng, Q. An, Q. Wei, W. Yang and L. Mai, Nano Energy, 2019, 55, 526–533 CrossRef CAS.
- Y. Yang, S. Guo, Y. Pan, B. Lu, S. Liang and J. Zhou, Energy Environ. Sci., 2023, 16, 2358–2367 RSC.
- J. Zhang, W. Li, J. Wang, X. Pu, G. Zhang, S. Wang, N. Wang and X. Li, Angew. Chem., Int. Ed., 2023, 62, e202215654 CrossRef CAS PubMed.
- Y. Yuan, R. Sharpe, K. He, C. Li, M. T. Saray, T. Liu, W. Yao, M. Cheng, H. Jin and S. Wang, Nat. Sustain., 2022, 5, 890–898 CrossRef.
- X. Xie, J. Li, Z. Xing, B. Lu, S. Liang and J. Zhou, Natl. Sci. Rev., 2023, 10, nwac281 CrossRef PubMed.
- V. Mathew, B. Sambandam, S. Kim, S. Kim, S. Park, S. Lee, M. H. Alfaruqi, V. Soundharrajan, S. Islam and D. Y. Putro, ACS Energy Lett., 2020, 5, 2376–2400 CrossRef CAS.
- T. Lv, G. Zhu, S. Dong, Q. Kong, Y. Peng, S. Jiang, G. Zhang, Z. Yang, S. Yang and X. Dong, Angew. Chem., Int. Ed., 2023, 62, e202216089 CrossRef CAS PubMed.
- Y. Liu, S. Liu, X. Xie, Z. Li, P. Wang, B. Lu, S. Liang, Y. Tang and J. Zhou, InfoMat, 2023, 5, e12374 CAS.
- F. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu and C. Wang, Nat. Mater., 2018, 17, 543–549 CrossRef CAS PubMed.
- Z. Liu, Q. Yang, D. Wang, G. Liang, Y. Zhu, F. Mo, Z. Huang, X. Li, L. Ma and T. Tang, Adv. Energy Mater., 2019, 9, 1902473 CrossRef CAS.
- W. Pan, Y. Wang, X. Zhao, Y. Zhao, X. Liu, J. Xuan, H. Wang and D. Y. C. Leung, Adv. Funct. Mater., 2021, 31, 2008783 CrossRef CAS.
- C. Zhong, B. Liu, J. Ding, X. Liu, Y. Zhong, Y. Li, C. Sun, X. Han, Y. Deng and N. Zhao, Nat. Energy, 2020, 5, 440–449 CrossRef CAS.
- D. Chao, C. Ye, F. Xie, W. Zhou, Q. Zhang, Q. Gu, K. Davey, L. Gu and S. Z. Qiao, Adv. Mater., 2020, 32, 2001894 CrossRef CAS PubMed.
- Y.-f Cui, Y.-h Zhu, J.-y Du, Y.-l Zhang, K. Li, W.-q Liu, G. Huang and X.-b Zhang, Joule, 2022, 6, 1617–1631 CrossRef CAS.
- W. Shang, W. Yu, Y. Liu, R. Li, Y. Dai, C. Cheng, P. Tan and M. Ni, Energy Storage Mater., 2020, 31, 44–57 CrossRef.
- J. Wang, L. Zhang, C. Zhang and J. Zhang, J. Power Sources, 2001, 102, 139–143 CrossRef CAS.
- Z. Zhao, X. Fan, J. Ding, W. Hu, C. Zhong and J. Lu, ACS Energy Lett., 2019, 4, 2259–2270 CrossRef CAS.
- Y. Song, P. Ruan, C. Mao, Y. Chang, L. Wang, L. Dai, P. Zhou, B. Lu, J. Zhou and Z. He, Nano-Micro Lett., 2022, 14, 218 CrossRef CAS PubMed.
- C. Bai, F. Cai, L. Wang, S. Guo, X. Liu and Z. Yuan, Nano Res., 2018, 11, 3548–3554 CrossRef CAS.
- K. Amine, H. Yasuda and M. Yamachi, Electrochem. Solid-State Lett., 2000, 3, 178 CrossRef CAS.
- H. Lim, A. Lackner and R. Knechtli, J. Electrochem. Soc., 1977, 124, 1154 CrossRef CAS.
- C. Yang, X. Ji, X. Fan, T. Gao, L. Suo, F. Wang, W. Sun, J. Chen, L. Chen and F. Han, Adv. Mater., 2017, 29, 1701972 CrossRef PubMed.
- H. Y. Shi, Y. Song, Z. Qin, C. Li, D. Guo, X. X. Liu and X. Sun, Angew. Chem., Int. Ed., 2019, 58, 16057–16061 CrossRef CAS PubMed.
- H. Pan, Y. Shao, P. Yan, Y. Cheng, K. S. Han, Z. Nie, C. Wang, J. Yang, X. Li and P. Bhattacharya, Nat. Energy, 2016, 1, 1–7 Search PubMed.
- P. Senguttuvan, S. D. Han, S. Kim, A. L. Lipson, S. Tepavcevic, T. T. Fister, I. D. Bloom, A. K. Burrell and C. S. Johnson, Adv. Energy Mater., 2016, 6, 1600826 CrossRef.
- W. Zhang, C. Zuo, C. Tang, W. Tang, B. Lan, X. Fu, S. Dong and P. Luo, Energy Technol., 2021, 9, 2000789 CrossRef CAS.
- Y. Lyu, X. Wu, K. Wang, Z. Feng, T. Cheng, Y. Liu, M. Wang, R. Chen, L. Xu and J. Zhou, Adv. Energy Mater., 2021, 11, 2000982 CrossRef CAS.
- G. Zampardi and F. La Mantia, Curr. Opin. Electrochem., 2020, 21, 84–92 CrossRef CAS.
- A. K. Padhi, K. S. Nanjundaswamy and J. B. Goodenough, J. Electrochem. Soc., 1997, 144, 1188 CrossRef CAS.
- T.-F. Yi, C.-L. Hao, C.-B. Yue, R.-S. Zhu and J. Shu, Synth. Met., 2009, 159, 1255–1260 CrossRef CAS.
- R. Santhanam and B. Rambabu, J. Power Sources, 2010, 195, 5442–5451 CrossRef CAS.
- L. Chen, X. Fan, E. Hu, X. Ji, J. Chen, S. Hou, T. Deng, J. Li, D. Su and X. Yang, Chem, 2019, 5, 896–912 CAS.
- J. Hao, J. Long, B. Li, X. Li, S. Zhang, F. Yang, X. Zeng, Z. Yang, W. K. Pang and Z. Guo, Adv. Funct. Mater., 2019, 29, 1903605 CrossRef.
- W. Xiong, D. Yang, T. K. Hoang, M. Ahmed, J. Zhi, X. Qiu and P. Chen, Energy Storage Mater., 2018, 15, 131–138 CrossRef.
- Y. Zhang and M. Muhammed, Hydrometallurgy, 2001, 60, 215–236 CrossRef CAS.
- K.-W. Nam, W.-S. Yoon, H. Shin, K. Y. Chung, S. Choi and X.-Q. Yang, J. Power Sources, 2009, 192, 652–659 CrossRef CAS.
- T. Liu, A. Dai, J. Lu, Y. Yuan, Y. Xiao, L. Yu, M. Li, J. Gim, L. Ma and J. Liu, Nat. Commun., 2019, 10, 1–11 CrossRef PubMed.
- X. Tang, J. Zhou, M. Bai, W. Wu, S. Li and Y. Ma, J. Mater. Chem. A, 2019, 7, 13364–13371 RSC.
- L. Ma, N. Li, C. Long, B. Dong, D. Fang, Z. Liu, Y. Zhao, X. Li, J. Fan and S. Chen, Adv. Funct. Mater., 2019, 29, 1906142 CrossRef CAS.
- J. Hao, X. Li, S. Zhang, F. Yang, X. Zeng, S. Zhang, G. Bo, C. Wang and Z. Guo, Adv. Funct. Mater., 2020, 30, 2001263 CrossRef CAS.
- Y. Luo, In situ and ex situ studies of materials with relevance to electrochemical energy storage and energy generation, Case Western Reserve University, 2002 Search PubMed.
- T. Gao, Y. Sun, L. Gong, C. Ma, J. Wang and L. Su, J. Electrochem. Soc., 2020, 167, 020552 CrossRef CAS.
- N. Zhang, Y. Dong, M. Jia, X. Bian, Y. Wang, M. Qiu, J. Xu, Y. Liu, L. Jiao and F. Cheng, ACS Energy Lett., 2018, 3, 1366–1372 CrossRef CAS.
- F. Wan, Y. Zhang, L. Zhang, D. Liu, C. Wang, L. Song, Z. Niu and J. Chen, Angew. Chem., Int. Ed., 2019, 58, 7062–7067 CrossRef CAS PubMed.
- I. A. Rodríguez-Pérez, L. Zhang, J. M. Wrogemann, D. M. Driscoll, M. L. Sushko, K. S. Han, J. L. Fulton, M. H. Engelhard, M. Balasubramanian and V. V. Viswanathan, Adv. Energy Mater., 2020, 10, 2001256 CrossRef.
- L. Zhang, L. Chen, X. Zhou and Z. Liu, Adv. Energy Mater., 2015, 5, 1400930 CrossRef.
- N. Zhang, F. Cheng, Y. Liu, Q. Zhao, K. Lei, C. Chen, X. Liu and J. Chen, J. Am. Chem. Soc., 2016, 138, 12894–12901 CrossRef CAS PubMed.
- M. Gong, Y. Li, H. Zhang, B. Zhang, W. Zhou, J. Feng, H. Wang, Y. Liang, Z. Fan and J. Liu, Energy Environ. Sci., 2014, 7, 2025–2032 RSC.
- X. Xu, S. Deng, H. Wang, J. Liu and H. Yan, Nano-Micro Lett., 2017, 9, 1–19 CrossRef PubMed.
- H. J. Lee, Z. Brown, Y. Zhao, J. Fawdon, W. Song, J. H. Lee, J. Ihli and M. Pasta, Chem. Mater., 2021, 33, 1238–1248 CrossRef CAS.
- L. Chen, X. Fan, E. Hu, X. Ji, J. Chen, S. Hou, T. Deng, J. Li, D. Su and X. Yang, Chem, 2019, 5, 896–912 CAS.
- Z. Chang, Y. Qiao, H. Yang, H. Deng, X. Zhu, P. He and H. Zhou, Energy Environ. Sci., 2020, 13, 4122–4131 RSC.
- M. Hu, Y. Tian, L. Su, J. Wei and Z. Zhou, ACS Appl. Mater. Interfaces, 2013, 5, 12185–12189 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ee01362f |
| This journal is © The Royal Society of Chemistry 2023 |


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) s−1. (c) Verification of the ESW of the PSE. Anodic and cathodic windows were obtained from LSV analysis at a scan rate of 5 mV s−1 against a Hg/HgO and Ag/AgCl reference electrode, respectively, wherein the potential has been converted to the SHE references for convenience. (d) Comparison of the ESW of various electrolytes and potentials of materials.
s−1. (c) Verification of the ESW of the PSE. Anodic and cathodic windows were obtained from LSV analysis at a scan rate of 5 mV s−1 against a Hg/HgO and Ag/AgCl reference electrode, respectively, wherein the potential has been converted to the SHE references for convenience. (d) Comparison of the ESW of various electrolytes and potentials of materials.