An abnormal size effect enables ampere-level O2 electroreduction to hydrogen peroxide in neutral electrolytes†
Abstract
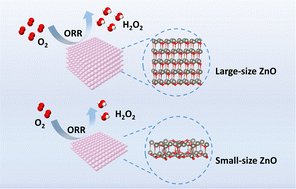
The principle of size effect was noticed a century ago, and is now widely used to express the activity enhancement of a catalyst as a result of size decrease. However, there are exceptions. In this study we observe an abnormal size effect in oxygen electroreduction comprised of two- (to generate H2O2) and four-electron-transfer pathways (to generate H2O), where hydrogen peroxide (H2O2) is the target product. We have synthesized three zinc oxide nanoplate catalysts with different thicknesses (i.e., 87.7, 26.3 and 1.70 nm). In contrast to common expectation, the large-thickness zinc oxide plate (i.e., 87.7 nm) showed superior oxygen electroreduction activities with nearly 100% selectivity to H2O2 in the current density range of 0.05–1.3 A cm−2 in neutral electrolytes, while other size counterparts only demonstrated moderate activities. A mechanism study through theoretical calculations and in situ Raman spectra highlight the critical role of abnormal size effects in enabling oxygen electroreduction at elevated current densities, which can not only activate O2 but also stabilize the key reaction intermediate (*OOH) by shifting the d-band center toward the Femi level. Furthermore, the feasibility of the present technology is evaluated by following a rigorous techno-economic assessment protocol, showing a threshold current density of 0.2 A cm−2 for economically profitable manufacture, and the lowest H2O2 production cost (0.4 $ kg−1) achieved at 1.0 A cm−2.


 Please wait while we load your content...
Please wait while we load your content...