Open Access Article
Open Access ArticleTrimerized small-molecule acceptors enable high-performance organic solar cells with high open-circuit voltage and prolonged life-time†
Jin-Woo
Lee‡
a,
Cheng
Sun‡
bc,
Tan Ngoc-Lan
Phan
a,
Dong Chan
Lee
d,
Zhengping
Tan
a,
Hyesu
Jeon
a,
Shinuk
Cho
 d,
Soon-Ki
Kwon
e,
Yun-Hi
Kim
d,
Soon-Ki
Kwon
e,
Yun-Hi
Kim
 *c and
Bumjoon J.
Kim
*c and
Bumjoon J.
Kim
 *a
*a
aDepartment of Chemical and Biomolecular Engineering, Korea Advanced Institute of Science and Technology (KAIST), Daejeon 34141, Republic of Korea. E-mail: bumjoonkim@kaist.ac.kr
bQingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, Qingdao, 266101, China
cDepartment of Chemistry and RINS, Gyeongsang National University, Jinju 52828, Republic of Korea. E-mail: ykim@gnu.ac.kr
dDepartment of Physics and EHSRC, University of Ulsan, Ulsan 44610, Republic of Korea
eDepartment of Materials Engineering and Convergence Technology and ERI, Gyeongsang National University, Jinju 52828, Republic of Korea
First published on 1st June 2023
Abstract
Although the recent development of Y-series small-molecule acceptors (SMAs) has led to a dramatic increase in the power conversion efficiency (PCE) of organic solar cells (OSCs), the operational stability (device lifetime) of OSCs is inadequate for commercialization. In this study, we develop a new trimer acceptor (TYT), consisting of three Y-based molecules linked by electron-donating spacers, to realize an OSC with high-performance (PCE > 18%) and -stability (t80% lifetime > 8000 h under 1 sun illumination, in which t80% lifetime is the time required for the PCE of the OSC to reach 80% of its initial value). We demonstrate that the trimerization approach affords an acceptor, TYT, with an upshifted lowest unoccupied molecular orbital energy level, which, in turn, affords an efficient OSC with a high open-circuit voltage (0.964 V). In addition, the glass-transition temperature (Tg) of TYT (217 °C) is significantly higher than those of monomer (MYT, Tg = 80 °C) and dimer (DYT, Tg = 127 °C) acceptors, which effectively suppresses the molecular diffusion of TYT in a blend film with a polymer donor. Accordingly, a TYT-based OSC demonstrates a high PCE (18.2%) and long t80% lifetime under 1 sun illumination (8454 h), outperforming MYT- and DYT-based OSCs that exhibit PCEs and t80% lifetimes of 16.4% and 35 h, and 17.3% and 2551 h, respectively. Therefore, this study provides important guidelines for the design of electron acceptors in achieving OSCs with high performance and stability close to a commercial level.
Broader contextHigh power conversion efficiency (PCE) and long-term stability are important requirements for commercialization of organic solar cells (OSCs). Small-molecule acceptors (SMAs) are the core materials that have led to remarkable advances in the PCEs of the OSCs, but the resulting OSCs typically have poor long-term stability. The low glass transition temperatures (Tgs) and rapid diffusion of SMAs owing to their small molecular sizes are recognized as the main causes of the poor stability of OSCs. In addition, the PCEs of OSCs are still lower than those of other types of photovoltaic devices, such as perovskite solar cells, mainly because of their relatively low open-circuit voltage (Voc < 0.90 V). To address these performance limitations, we develop a new trimer acceptor (TYT), consisting of three Y-based molecules linked by electron-donating thiophene spacers. The TYT-based OSCs demonstrate high-performance (Voc = 0.964 V and PCE = 18.15%) and excellent photo-stability (t80% lifetime > 8000 h), which outperform those of the OSCs based on the monomer (MYT, PCE = 16.44% and t80% lifetime = 35 h) and dimer acceptors (DYT, PCE = 17.29% and t80% lifetime = 2551 h). |
Introduction
The rapid increase in the power conversion efficiency (PCE) of organic solar cells (OSCs) to 18–19%, is ascribed to the development of efficient polymer donors and non-fullerene small-molecule acceptors (SMAs).1–10 However, numerous SMA-based OSCs exhibit poor operation stabilities under thermal stress and light-illumination, mainly due to the unstable, kinetically trapped blend morphologies of their active layers, i.e., blends of polymer donors and SMAs.11–15 Due to their small size, SMAs (which typically have low glass-transition temperature (Tg < 100 °C) and high diffusion coefficient (D > 10−20 cm2 s−1 at 85 °C)) in these active layers readily diffuse under thermal stress, resulting in phase separation.16–18 In comparison, polymerized small-molecule-acceptors (PSMAs) typically have higher Tg and lower D than SMAs due to larger molecular sizes. Thus, PSMA-based OSCs often exhibit improved device stabilities and mechanical robustness.12,19–33 However, PSMA-based OSCs often show lower electron mobility and, therefore, lower PCE than SMA-based OSCs as polydisperse chains of the PSMAs suppress the formation of regular and tight packing in films, resulting in comparably less-ordered intermolecular assemblies.Recently, dimerized small-molecule acceptors (DSMAs) have garnered attention as they can leverage the advantages of both SMAs and PSMAs.34–37 DSMAs demonstrate higher electron mobility than PSMAs because their discrete molecular structures can promote regular and tight packing.38–41 In addition, the Tgs and Ds of DSMAs are higher and lower, respectively, than those of SMAs, owing to their comparatively large size; these properties lead to OSCs with enhanced thermal and photo-stability. Wang et al. were the first to develop a DSMA featuring Y-series-based building blocks (dBTICγ-EH) to realize an OSC with a high PCE (16.06%) and operational stability (t80% lifetime; 1020 h).37 Subsequently, researchers developed DSMA-based OSCs with PCEs exceeding 18% and high stability (t80% lifetimes > 1000 h) through the judicious selection of electron-donating spacers to link the SMA building blocks of the DSMAs.34
Despite the demonstrated potential of DSMA systems, the stability of the OSCs remains a challenge; the operational stability of the DSMA-based OSCs falls short of the commercial requirement, i.e., the device lifetime is less than the required minimum of 5 years.42–44 This limitation necessitates the development of novel acceptors using a rational molecular design approach. Recent studies concerning DSMAs imply that to realize stable OSCs with long operational lifetimes, acceptors must have high Tgs (>200 °C) and low D85 (<10−24 cm2 s−1 at 85 °C).16–18 Accordingly, we anticipate that a trimer acceptor will demonstrate such properties, owing to its greater molecular size (relative to that of DSMAs), and afford OSCs with longer operational lifetimes.45–50 The proposed trimerization approach may offer an additional handle for tuning the structures of molecules and expand the library of potential electron acceptors. Moreover, this approach allows for the increased flexibility in the design of the materials with tuned energy levels and enhanced light absorbance.46,49–51 For instance, unlike DSMAs and SMAs, which incorporate one or zero electron-donating linkers, respectively, trimer acceptors incorporate two electron-donating linkers. Thus, the trimer acceptors can have upshifted energy levels of the lowest unoccupied molecular orbital (LUMO) relative to those of corresponding SMAs and DSMAs, which may lead to OSCs with a higher open-circuit voltage (Voc) and PCE. Hence, trimer acceptors can address a critical limitation of OSCs, i.e., low Voc (<0.9 V) relative to that of other types of solar cells (e.g., perovskite solar cells with Voc > 1.0 V).52–57
In this study, we synthesize a new trimer acceptor (TYT) consisting of three SMA units linked by two electron-donating thiophene spacers. Using TYT, we realize OSCs with a high PCE (18.15%) and excellent operational stability under 1 sun illumination (t80% lifetime = 8454 h). To investigate the origin of the high efficiency and stability of the TYT-based OSCs, we also prepare and consider corresponding monomer and dimer acceptors (MYT and DYT, respectively) to establish the molecular structure–acceptor property–device performance relationship. We observe that the LUMO energy levels of the acceptors increase in the order of MYT (−4.04 eV) < DYT (−3.94 eV) < TYT (−3.86 eV), owing to the increase in the number of their electron-donating thiophene linkers. As a result, the Vocs of associated OSCs increase in the same order (in terms of their acceptors), accounting for the corresponding trend in their PCEs; the Vocs and PCEs of the MYT-, DYT-, and TYT-based OSCs are 0.917 V and 16.44%, 0.942 V and 17.29%, and 0.964 V and 18.15%, respectively. In addition, the extended molecular structure of the trimer acceptor accounts for the excellent operational stability of the TYT-based OSCs. TYT is found to have a significantly higher Tg of 217 °C and lower D85 of 1.44 × 10−25 cm2 s−1, respectively, compared to those of MYT (Tg = 80 °C and D85 = 1.21 × 10−16 cm2 s−1) and DYT (Tg = 127 °C and D85 = 1.05 × 10−19 cm2 s−1). The high Tg and low D of TYT afford an OSC with excellent device stability under illumination. For example, the t80% lifetime of the TYT-based OSC (8454 h) is significantly longer than those of the MYT- and DYT-based OSCs (35 and 2551 h, respectively). Thus, our results highlight the great potential of the trimer acceptor system for realizing stable, high-performance OSCs.
Results and discussion
To investigate the relationship between the molecular structures of acceptors of different size, their properties, and OSC performance, we synthesized a series of electron acceptors, i.e., monomer, dimer, and trimer acceptors (MYT, DYT, and TYT, respectively) (Fig. 1(a)). We prepared an asymmetric Y-series-based compound with a dichlorinated 1,1-dicyanomethylene-3-indanone (IC) group at one end and a brominated IC group at the other (compound 2, Scheme S1, ESI†); this compound was dimerized by the Stille coupling reaction with bis(trimethyltin)-functionalized thiophene (Th–SnMe3) monomers to produce DYT (Fig. 1(a) and Scheme S3, ESI†). We also prepared a Y-series–based compound with two brominated IC end units (compound 3). The trimer acceptor, TYT, was synthesized by two-step reactions using the three monomers of compound 2, compound 3, and Th–SnMe3 (Fig. 1(a) and Scheme S4, ESI†). Compound 3 and Th–SnMe3 firstly reacted by the Stille coupling for 3 h. Then, compound 2 was sequentially added during the reaction, and the coupling continued for additional 10 h. The synthetic and purification procedures are detailed in the ESI.†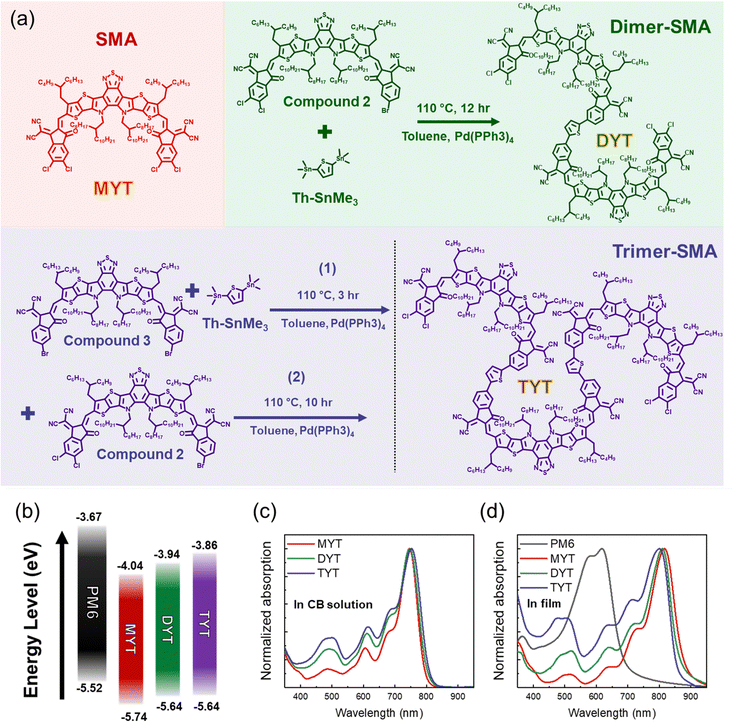 | ||
| Fig. 1 (a) Chemical structures and synthetic procedures, (b) energy levels, and (c), (d) UV-Vis absorption spectra in (c) solution (CB) and (d) film of the materials used in this study. | ||
The chemical structures of the acceptors (MYT, DYT, and TYT) and their intermediates were confirmed by nuclear magnetic resonance (NMR) spectroscopy (Fig. S1–S5, ESI†). The discrete molecular weights of MYT, DYT, and TYT were determined by matrix-assisted laser desorption/ionization time-of-flight (MALDI-ToF) mass spectrometry, which confirm the successful synthesis of pure acceptor materials (Fig. S6, ESI†). The determined molar masses of MYT, DYT, and TYT were 1881, 3712, and 5540 g mol−1, respectively. Based on optimized structures from density functional theory (DFT) calculations, the dihedral angles near the IC units of all the acceptors were found to be less than 16°, which indicates that the molecules adopt relatively planar molecular conformations (Fig. S7, ESI†).
We investigated the electrochemical properties of the synthesized acceptors using cyclic voltammetry (CV) (Fig. S8, ESI† and Table 1). The LUMO energy levels of the acceptors were linearly upshifted with increasing molecular size; MYT (−4.04 eV) < DYT (−3.94 eV) < TYT (−3.86 eV). The upshifted LUMO energy level of TYT leads to an OSC with a comparatively high Voc. The respective LUMO and highest occupied molecular orbital (HOMO) energy levels of the three acceptors align appropriately with those of the polymer donor, poly[(2,6-(4,8-bis(5-(2-ethylhexyl-3-fluoro)thiophene-2-yl)-benzo[1,2-b:4,5-b′]dithiophene))-alt-(5,5-(1′,3′-di-2-thienyl-5′,7′-bis(2-ethylhexyl)benzo[1′,2′-c:4′,5′-c′]dithiophene-4,8-dione)] (PM6), used in this study (Fig. 1(b)). Although the wavelengths at maximum ultraviolet-visible (UV-Vis) absorption (λmaxs) of all the acceptors in chlorobenzene (CB) were similar (Fig. 1(c)), their λmaxs in film slightly blue-shifted (decreased) in the order of MYT (λfilmmax: 816 nm), DYT (λfilmmax: 809 nm), and TYT (λfilmmax: 802 nm; Fig. 1(d)). The maximum absorption coefficients of the three acceptors in film (εfilmmaxs) were similar, i.e., in the range of 1.54–1.62 × 105 cm−1.
| Material |
λ
solmax
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (nm)
(nm) |
λ
filmmax
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (nm)
(nm) |
ε
filmmax
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (× 105 cm−1)
(× 105 cm−1) |
E LUMO (eV) | E HOMO (eV) |
|---|---|---|---|---|---|
| a Wavelength of maximum UV-Vis absorbance (λmax) in solution. b Wavelength of maximum UV-Vis absorbance (λmax) in film. c Maximum absorption coefficients in film UV-Vis absorbances. d Measured by cyclic voltammetry. | |||||
| PM6 | — | 621 | 0.79 | −3.67 | −5.52 |
| MYT | 745 | 816 | 1.54 | −4.04 | −5.74 |
| DYT | 745 | 809 | 1.62 | −3.94 | −5.64 |
| TYT | 745 | 802 | 1.60 | −3.86 | −5.64 |
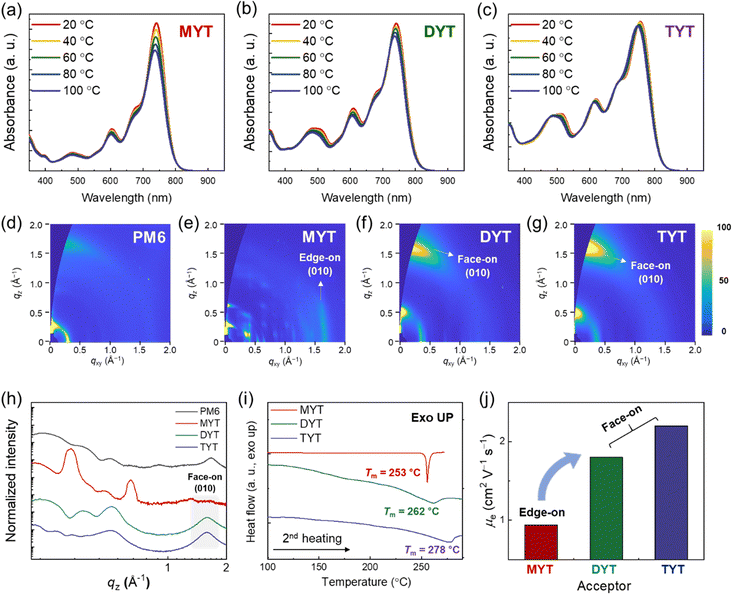
The aggregation behavior of the acceptors in solution was analyzed by investigating their temperature-dependent UV-Vis absorbance in CB in the temperature range of 20–100 °C (Fig. 2(a)–(c)). The UV-Vis absorbance of MYT and DYT decreased with increasing solution temperature (Fig. 2(a) and (b)), suggesting that the tendency of MYT and DYT molecules to aggregate decreases with increasing temperature. In contrast, the UV-Vis absorbance of TYT did not decrease with increasing temperature to 100 °C (Fig. 2(c)), suggesting that the TYT molecules aggregate more readily at high temperatures than the MYT and DYT molecules.
The crystalline properties of the acceptors were investigated by grazing incidence wide angle X-ray scattering (GIWAXS) (Fig. 2(d)–(h), Fig. S9 and Table S1, ESI†). MYT, in film, preferred an edge-on molecular packing orientation as indicated by the 2D GIWAXS patterns showing distinct (100) and (010) peaks in the out-of-plane (OOP) and in-plane (IP) directions, respectively (Fig. 2(e)). In contrast, PM6, DYT, and TYT preferred a face-on molecular packing orientation as indicated by (100) and (010) peaks in the IP and OOP directions, respectively (Fig. 2(d), (f), and (g)). Pole figures of the MYT, DYT, and TYT acceptors were obtained from the (010) peaks in the GIWAXS results to assess the molecular packing orientation (Fig. S10, ESI†). The thermal properties of the acceptors were analyzed by differential scanning calorimetry (DSC). The DSC curves (2nd heating cycles) of MYT, DYT, and TYT showed transition peaks corresponding to melting, at temperatures exceeding 200 °C (Fig. 2(i)). The melting temperatures (Tms) of the acceptors increased with increasing molecule size; MYT (Tm = 253 °C) < DYT (Tm = 262 °C) < TYT (Tm = 277 °C). The electrical properties of the acceptors in film were investigated by performing space-charge limited current (SCLC) mobility measurements (Fig. 2(j) and Table S2, ESI†).58 The determined electron mobilities (μes) of DYT and TYT were significantly higher than that of MYT. This is attributed to the face-on oriented packing structures of DYT and TYT which, unlike the edge-on oriented packing structures of MYT, facilitate charge transport in the vertical direction.59,60 Overall, the TYT showed the highest degree of aggregation and highest μe among the three acceptors.
We then investigated the photovoltaic performance of the acceptors by fabricating OSCs with a normal-type device structure; the OSC fabrication procedures and conditions are detailed in the ESI.† The J–V curves of the OSCs are shown in Fig. 3(a). The PCEs of the OSCs increased, in terms of the blend films, in the order of PM6:MYT (PCE = 16.44%), PM6:DYT (PCE = 17.29%), and PM6:TYT (PCE = 18.15%). The parameter mainly accounting for the difference in PCE was Voc, which increased in the order of PM6:MYT (Voc = 0.917 V), PM6:DYT (Voc = 0.942 V), and PM6:TYT (Voc = 0.964 V) (Table 2). The high Vocs of the PM6:DYT and PM6:TYT OSCs are attributed to their relatively high LUMO energy levels. We note that the PM6:TYT OSCs demonstrate the highest Voc (0.964 V) among reported high-performance (PCE > 18%) OSCs (Fig. S11 and Table S3, ESI†). In addition, we fabricated OSCs using three acceptors (MYT, DYT, and TYT) in combination with different donor pairs (D18, D18-Cl, and PBQx-TF). Our findings showed that the Voc increased sequentially for the devices using MYT, DYT, and TYT acceptors, as listed in Table S4 (ESI†). The short-circuit current densities (Jscs) of the OSCs increased in the same order as their PCEs and Vocs. The fill factor (FF) of the PM6:TYT OSCs (0.75) was slightly higher than those of the PM6:MYT and PM6:DYT OSCs (0.74). PCEs of the OSCs show Gaussian distributions with small PCE deviations, indicating that the OSCs are reproducible (Fig. 3(b)). The external quantum efficiency (EQE) spectra of the OSCs are shown in Fig. 3(c), and the Jscs calculated from the EQE spectra are consistent with the measured device Jscs (Table 2).
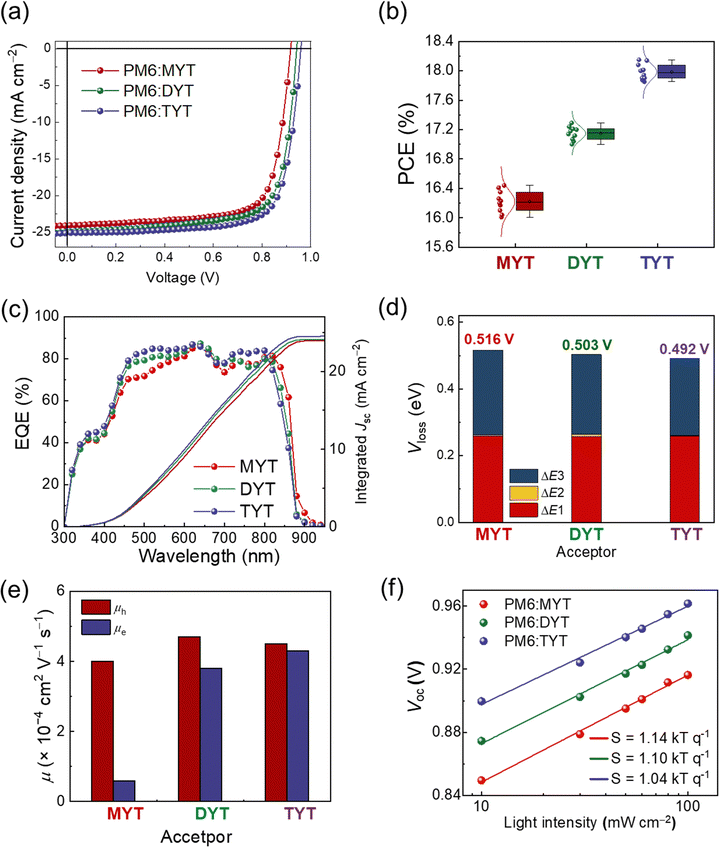 | ||
| Fig. 3 (a) J–V curves, (b) PCE distributions, (c) EQE spectra, (d) Vloss parameters, (e) SCLC mobilities, and (f) light-intensity dependent Voc plots of PM6:acceptor-based OSCs. | ||
We characterized the electrical properties of the OSCs to elucidate the trends in their photovoltaic performance. The voltage loss (Vloss) of the OSCs was measured using Fourier-transform photocurrent spectroscopy (FTPS)-EQE and electroluminescence (EL) spectroscopy to analyze the high Voc of the PM6:TYT OSCs (Fig. 3(d) and Fig. S12–S14, ESI†). The Vloss of OSCs can be expressed in terms of ΔE1, ΔE2, and ΔE3; Vtotalloss = (EPVg/q − VSQoc) + (VSQoc − Vradoc) + (Vradoc − VPVoc) = ΔE1 + ΔE2 + ΔE3, where q, EPVg, VSQoc, Vradoc, and VPVoc are the elementary charge, photovoltaic bandgap, maximum voltage in the Shockley–Queisser limit, voltage in radiative limit, and photovoltaic Voc, respectively (Fig. S12, ESI†).41,61,62 ΔE1 is the intrinsic Vloss of the OSCs within a range of 0.20–0.30 eV. ΔE2 is the radiative recombination loss from the tail energy states below the bandgap. ΔE3 is the non-radiative Vloss, caused by non-radiative recombination in the OSCs. Detailed procedures for the calculation of Vloss are provided in the Experimental Section. The Vtotalloss of the OSCs decreased in the order of PM6:MYT (0.516 V), PM6:DYT (0.503 V), and PM6:TYT (0.492 V) (Fig. 3(d)). The main parameter accounting for the Vtotalloss trend is ΔE3; PM6:MYT (0.253 V), PM6:DYT (0.239 V), and PM6:TYT (0.231 V) (Table 3). The lowest Vtotalloss of the PM6:TYT OSCs contributes to their highest Voc (0.964 V) among the series.
| Acceptor | E g | qV SQoc | ΔE1 | qV radoc | ΔE2 | qV oc | ΔE3 | V totalloss |
|---|---|---|---|---|---|---|---|---|
| (eV) | ||||||||
| MYT | 1.433 | 1.174 | 0.259 | 1.170 | 0.004 | 0.917 | 0.253 | 0.516 |
| DYT | 1.447 | 1.187 | 0.258 | 1.181 | 0.006 | 0.942 | 0.239 | 0.503 |
| TYT | 1.458 | 1.197 | 0.259 | 1.195 | 0.002 | 0.964 | 0.231 | 0.492 |
The charge generation of each OSC was investigated by measuring its photocurrent density (Jph) as a function of effective voltage (Veff) (Fig. S15, ESI†).63 The exciton dissociation probability (P(E,T)) of each OSC was calculated by dividing the Jsc by the saturated current density (Jsat) at Veff = 2 V. The P(E,T)s of the OSCs were similarly high (∼94%), indicating that the excitons dissociate well in all the blend systems. The charge transport of the OSCs was assessed by measuring the SCLC mobility of corresponding blend films (Fig. 3(e) and Table S5, ESI†). As the same PM6 donor was used, the hole mobilities (μhs) were comparable across all blend films ranging from 4.0 to 4.7× 10−4 cm2 V−1 s−1. Interestingly, the trend in the μes of the blend films was consistent with the trend in the μes of their pristine acceptor constituents; i.e., the μes of the blend films increased in the order of PM6:MYT (5.8 × 10−5 cm2 V−1 s−1) < PM6:DYT (3.8 × 10−4 cm2 V−1 s−1) < PM6:TYT (4.2 × 10−4 cm2 V−1 s−1) (Table S5, ESI†). The trend in the μes of the blend films is consistent with the trend in the Jscs of the corresponding OSCs. Finally, the charge recombination of each OSC was investigated by measuring its light intensity (P)-dependent Jsc and Voc (Fig. 3(f) and Fig. S16, Fig. ESI†). The slope (α) of the P vs. Jsc plots of the OSCs was similar (0.81–0.82), indicating that the OSCs experience similar degrees of bimolecular recombination (Fig. S16, ESI†).64 However, the slope (S) of the P vs. Voc plots of the OSCs differed; the S value associated with the PM6:TYT OSC (1.04 kT q−1) was closer to 1 than those associated with the PM6:MYT and PM6:DYT OSCs (1.14 and 1.10 kT q−1, respectively), indicating that monomolecular/trap-assisted recombination is more suppressed in the PM6:TYT OSC (Fig. 3(f)).64 The suppressed monomolecular/trap-assisted recombination observed in the PM6:TYT OSC supports its comparatively high FF and low Vtotalloss compared to the other OSCs.
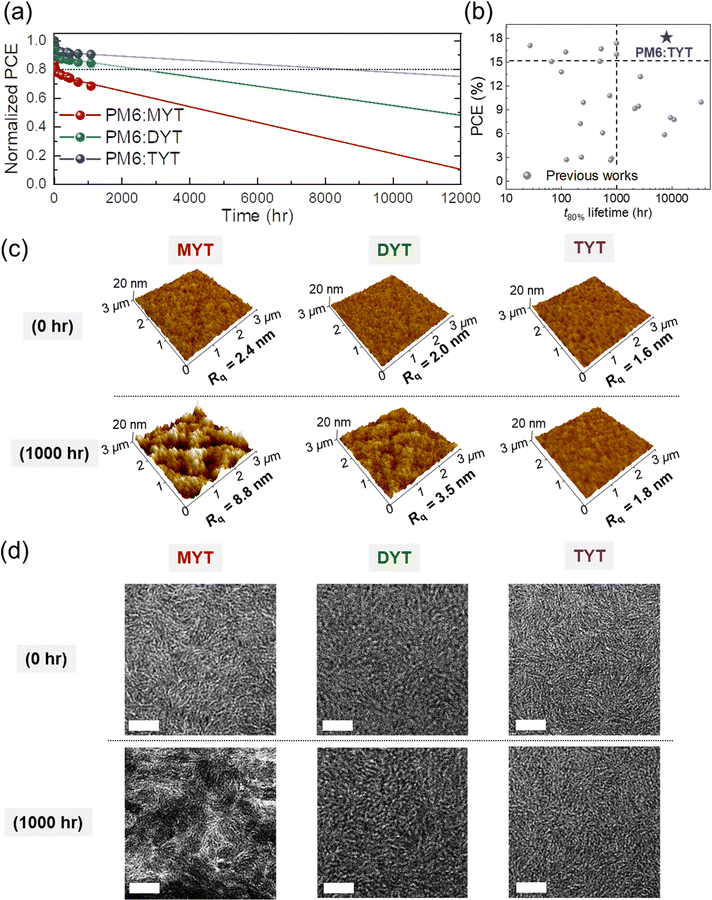
Operational stability is another key requirement for the commercialization of OSCs. To investigate the long-term stability of the PM6:MYT, PM6:DYT, and PM6:TYT OSCs, we measured their PCE values under continuous 1 sun illumination (100 mW cm−2) for 1080 h. Then, we estimated the t80% lifetime of each device (i.e., the time required for the PCE of the device to reach 80% of its initial PCE) by extrapolating the PCE of the device to that in longer illumination time (i.e., 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h) (Fig. 4(a)).65–67 We note that the datapoints in Fig. 4(a) are the averaged values collected from three independent experiments for reliability. The stability test as well as the extrapolation procedures are described in the ESI.† The stability of the OSCs increased dramatically with increasing acceptor chain length (molecular size), based on the t80% lifetimes of the PM6:MYT, PM6:DYT, and PM6:TYT OSCs which were 35, 2551, and 8454 h, respectively. It should be noted that the PM6:TYT demonstrates both high PCE (>18%) and high stability (t80% lifetime > 8000 h) (Fig. 4(b) and Table S6, ESI†). To emphasize the excellent operational stability of the PM6:TYT OSCs, we estimated its device lifetime when operated in different locations (countries) considering the daily solar irradiance in each location.66,68 The expected device lifetime of the PM6:TYT OSC was several years; 4.8 (Seoul), 7.2 (Paris), and 7.7 years (Berlin) (Table S7, ESI†). Additionally, we evaluated the thermal stability of the active layers at 80 °C (Fig. S17, ESI†). Each active layer was annealed before depositing the electron transporting layer, and the photovoltaic performance of the fully assembled devices was examined. The thermal stability test exhibited a similar trend to that of the photo-stability test. For example, after 480 h of annealing, the normalized PCEs for the MYT-, DYT-, and TYT-based OSCs reached 76.2, 89.2, and 93.3% of their initial values, respectively.
000 h) (Fig. 4(a)).65–67 We note that the datapoints in Fig. 4(a) are the averaged values collected from three independent experiments for reliability. The stability test as well as the extrapolation procedures are described in the ESI.† The stability of the OSCs increased dramatically with increasing acceptor chain length (molecular size), based on the t80% lifetimes of the PM6:MYT, PM6:DYT, and PM6:TYT OSCs which were 35, 2551, and 8454 h, respectively. It should be noted that the PM6:TYT demonstrates both high PCE (>18%) and high stability (t80% lifetime > 8000 h) (Fig. 4(b) and Table S6, ESI†). To emphasize the excellent operational stability of the PM6:TYT OSCs, we estimated its device lifetime when operated in different locations (countries) considering the daily solar irradiance in each location.66,68 The expected device lifetime of the PM6:TYT OSC was several years; 4.8 (Seoul), 7.2 (Paris), and 7.7 years (Berlin) (Table S7, ESI†). Additionally, we evaluated the thermal stability of the active layers at 80 °C (Fig. S17, ESI†). Each active layer was annealed before depositing the electron transporting layer, and the photovoltaic performance of the fully assembled devices was examined. The thermal stability test exhibited a similar trend to that of the photo-stability test. For example, after 480 h of annealing, the normalized PCEs for the MYT-, DYT-, and TYT-based OSCs reached 76.2, 89.2, and 93.3% of their initial values, respectively.
The photo-stability of the OSCs can be affected by various intrinsic and extrinsic factors including metastable blend morphology, oxidation, diffusion of electrodes/interlayers, chemical degradation between the consisting layers, and trap-states of active components.39,40,69 Particularly, change of the blend morphology under the light illumination is one of the most crucial factors that determine the operational stability of the OSCs.16,69 To better understand the different operational stability of the OSCs, we examined the morphological stability of the blend films under 1 sun illumination. As the elevated temperature caused by the light illumination can provide strong thermal energy and accelerate the molecular motion and diffusion of the acceptor molecules, the photo-stability of OSCs is strongly related to the morphological stability of the active layer.18,32,33 Therefore, we compared the blend films before and after 1000 h of illumination by atomic force microscopy (AFM) and transmission electron microscopy (TEM) (Fig. 4(c) and (d)). AFM revealed that the surface roughness of a PM6:MYT blend film increased over the course of 1000 h of illumination. In contrast, the surface roughness of a PM6:TYT blend film was almost unchanged. In detail, the root mean square roughness (Rq) of the PM6:MYT blend film increased from 2.4 to 8.8 nm after 1000 h of illumination, whereas that of the PM6:TYT blend film increased from 1.6 to 1.8 nm. The Rq of a PM6:DYT blend film increased from 2.0 to 3.5 nm after 1000 h of illumination (Fig. 4(c)). TEM revealed a similar trend; the PM6:MYT blend film experienced significantly greater phase separation during 1000 h illumination than the PM6:DYT and PM6:TYT blend films (Fig. 4(d)).
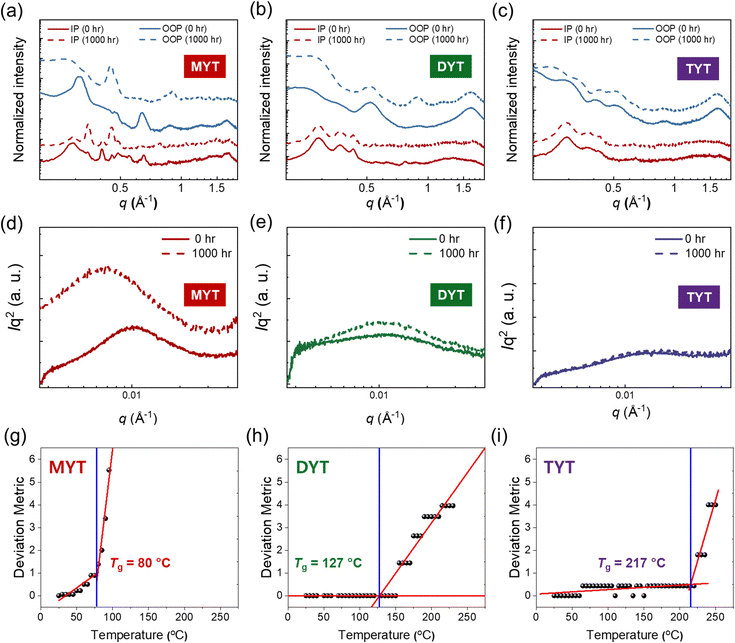
We analyzed the crystal structures in blend films before and after 1000 h of illumination by GIWAXS (Fig. 5(a)–(c) and Fig. S18, ESI†). The crystalline structures of a PM6:MYT blend film were significantly altered by illumination, as indicated by the appearance of many sharp scattering peaks after 1000 h in its GIWAXS linecut profiles in the IP (qxy: ∼0.35 and ∼0.45 Å−1) and OOP directions (qxy: ∼0.45 and ∼0.90 Å−1) (Fig. 5(a)). In contrast, the crystal structures of PM6:DYT and PM6:TYT blend films were almost unaffected by illumination (Fig. 5(b)–(c)). The domain size and purity of the blend films were analyzed by resonant soft X-ray scattering (RSoXS) (Fig. 5(d)–(f) and Table S8, ESI†). A beam energy of 284.4 eV was used to maximize material contrast.69 The RSoXS patterns of a PM6:MYT blend film before and after illumination showed that its domain size and relative domain purity (r-DP) significantly increased by irradiation, from 29 to 46 nm and 0.59 to 1.00, respectively. In contrast, the changes in the blend morphology of the PM6:DYT and PM6:TYT blends were not noticeable. The domain size and r-DP for the PM6:DYT blend increased from 30 to 31 nm and 0.47 to 0.52, while the domain size (25 nm) and r-DP (0.45) for the PM6:TYT blend were the same (Table S6, ESI†). We then conducted photoluminescence (PL) emission spectra measurements on both pristine acceptors and PM6:acceptor blend films to further examine the impact of illumination on blend morphology (Fig. S19, ESI†). Under continuous illumination, the PL quenching efficiencies (ηs) of the blend films rapidly decreased in the PM6:MYT blend film, whereas the η values remained nearly constant in the PM6:TYT blend film. For instance, after 24 h of illumination, the decrease in η values for the blend films is in the order of PM6:TYT blend films (93.8 → 93.3%), PM6:DYT (93.4 → 85.6%), and PM6:MYT (94.7 → 60.8%). This finding is consistent with the above results, indicating that the photo-stability of the blend films significantly increased in the order of PM6:MYT, PM6:DYT, and PM6:TYT.
The photo-stable blend morphology and device performance of the PM6:TYT OSC are mainly attributed to the relatively large molecular size of TYT and the related physical properties including the Tg and molecular diffusivity. To determine the correlation between the molecular size of the acceptor and its diffusivity, we estimated the Tgs of the acceptors by measuring their UV-Vis absorbance in film at various annealing temperatures (20–240 °C) (Fig. S20, ESI†). The Tg of SMAs corresponds to an onset temperature for thermal-induced molecular motions and diffusions, at which the SMAs start to be significantly mobile and imperfect SMA crystals are reorganized.16,69 Thus, the estimated Tgs of the SMAs can be also affected by their kinetically trapped morphologies in film in addition to the intrinsic molecular properties. To precisely correlate the estimated Tgs of the acceptors with those in the OSC device, we applied the same processing conditions used in the OSC fabrication to prepare the films for Tg measurements. A deviation metric (DMT), quantifying the change in the UV-Vis absorbance of each film during annealing, was used to estimate the Tgs of the acceptor films (Fig. 5(g)–(i)).16,70,71 Details of the procedure are provided in the ESI.† The Tgs of the acceptors increased with increasing molecular size; the Tgs of MYT, DYT, and TYT were 80, 127, and 217 °C, respectively. Based on the Tgs of the acceptors, we estimated their D values in the blend films with the PM6 donor at 85 °C using a previously reported approach.72 The D85s of the acceptors decreased with increasing Tgs (and molecular size); i.e., in the order of MYT (D85: 1.2 × 10−16 cm2 s−1), DYT (D85: 1.1 × 10−19 cm2 s−1), and TYT (D85: 1.4 × 10−25 cm2 s−1). In addition, the D85 of TYT was 106–109 of magnitude lower than those of the other reported high-performance acceptor materials (D85 = 10−19–10−16 cm2 s−1).16,72 As a result, the diffusion time of the acceptor molecules to move 10 nm (t10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nmD) at 85 °C in the PM6:acceptor blends increased in the order of MYT (t10
nmD) at 85 °C in the PM6:acceptor blends increased in the order of MYT (t10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nmD: 2.3 × 100 h), DYT (t10
nmD: 2.3 × 100 h), DYT (t10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nmD: 2.7 × 103 h), and TYT (t10
nmD: 2.7 × 103 h), and TYT (t10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nmD: 1.9 × 109 h) (Table 4).73,74 Moreover, D120 values measured by time-of-flight secondary ion mass spectrometry (ToF-SIMS) profiles of PM6:acceptors bilayers demonstrated the same trend, exhibiting a progressive decrease in the diffusion coefficient within the donor matrix as molecular size increases (Fig. S21 and Table S9, ESI†). Therefore, the high Tg (217 °C) and lower D85 (1.4 × 10−25 cm2 s−1) of TYT, relative to those of MYT (Tg = 80 °C and D85 = 1.2 × 10−16 cm2 s−1) account for the excellent operational stability of the PM6:TYT OSCs.
nmD: 1.9 × 109 h) (Table 4).73,74 Moreover, D120 values measured by time-of-flight secondary ion mass spectrometry (ToF-SIMS) profiles of PM6:acceptors bilayers demonstrated the same trend, exhibiting a progressive decrease in the diffusion coefficient within the donor matrix as molecular size increases (Fig. S21 and Table S9, ESI†). Therefore, the high Tg (217 °C) and lower D85 (1.4 × 10−25 cm2 s−1) of TYT, relative to those of MYT (Tg = 80 °C and D85 = 1.2 × 10−16 cm2 s−1) account for the excellent operational stability of the PM6:TYT OSCs.
| Acceptor | t 80% lifetimea (h) | T g (°C) | D 85 (cm2 s−1) |
t
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nmD
(h) nmD
(h) |
|---|---|---|---|---|
| a Estimated from OSC stability test results under 1 sun illumination. b Estimated from DMTvs. temperature plots. c D of the acceptor in a blend with PM6 at 85 °C, D85 = 1.2 × 107 × exp(−0.15 × Tg). d Diffusion time to move a 10 nm distance at 85 °C in a blend with PM6; tD = L2 × D85−1; L = diffusion length. | ||||
| MYT | 35 | 80 | 1.2 × 10−16 | 2.3 × 100 |
| DYT | 2551 | 127 | 1.1 × 10−19 | 2.7 × 103 |
| TYT | 8454 | 217 | 1.4 × 10−25 | 1.9 × 109 |
Conclusions
We developed an efficient (PCE = 18.15%) and highly stable (t80% lifetime = 8454 h) OSC by designing a novel non-fullerene trimer acceptor, TYT, blended with PM6 to form the active layer. TYT consists of three Y-series-based molecules linked by two thiophene spacers and it was found to have a higher LUMO energy level than the corresponding monomer and dimer acceptors (MYT and DYT, respectively) owing to the electron-donating nature of the two thiophene spacers. As a result, the Voc and PCE of a PM6:TYT OSC (0.964 V and 18.15%, respectively) exceeded those of a PM6:MYT OSC (0.917 V and 16.44%, respectively) as well as a PM6:DYT OSC (0.942 V and 17.29%, respectively). Importantly, TYT had an extremely high Tg (217 °C) and low D85 (1.4 × 10−25 cm2 s−1) owing to its relatively large molecular size, which, in turn, imparted greater operational stability to a resulting OSC. Accordingly, the operational stability of a PM6:TYT OSC (t80% lifetime = 8454 h) significantly exceeded that of PM6:MYT (t80% lifetime = 35 h) and PM6:DYT OSCs (t80% = 2551 h). We expect that the molecular structure–acceptor property–device performance relationship established in this study provides important design guidelines for the acceptor materials for producing OSCs with high Voc and excellent device stability.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF-2017M3A7B8065584, 2022R1A2B5B03001761, 2022M3J7A1062940, 2019R1A6A1A11053838, and 2022M3C1A3081211). This research used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231.References
- C. Li, J. D. Zhou, J. L. Song, J. Q. Xu, H. T. Zhang, X. N. Zhang, J. Guo, L. Zhu, D. H. Wei, G. C. Han, J. Min, Y. Zhang, Z. Q. Xie, Y. P. Yi, H. Yan, F. Gao, F. Liu and Y. M. Sun, Nat. Energy, 2021, 6, 605–613 CrossRef CAS.
- J. Yuan, Y. Q. Zhang, L. Y. Zhou, G. C. Zhang, H. L. Yip, T. K. Lau, X. H. Lu, C. Zhu, H. J. Peng, P. A. Johnson, M. Leclerc, Y. Cao, J. Ulanski, Y. F. Li and Y. P. Zou, Joule, 2019, 3, 1140–1151 CrossRef CAS.
- J.-W. Lee, C. Lim, S. W. Lee, Y. Jeon, S. Lee, T. S. Kim, J. Y. Lee and B. J. Kim, Adv. Energy Mater., 2022, 12, 2202224 CrossRef CAS.
- H. Y. Chen, R. Zhang, X. B. Chen, G. Zeng, L. Kobera, S. Abbrent, B. Zhang, W. J. Chen, G. Y. Xu, J. Oh, S. H. Kang, S. S. Chen, C. Yang, J. Brus, J. H. Hou, F. Gao, Y. W. Li and Y. F. Li, Nat. Energy, 2021, 6, 1045–1053 CrossRef CAS.
- Y. H. Cai, Y. Li, R. Wang, H. B. Wu, Z. H. Chen, J. Zhang, Z. F. Ma, X. T. Hao, Y. Zhao, C. F. Zhang, F. Huang and Y. M. Sun, Adv. Mater., 2021, 33, 2101733 CrossRef CAS PubMed.
- G. U. Kim, C. Sun, J. S. Park, H. G. Lee, D. Lee, J.-W. Lee, H. J. Kim, S. Cho, Y. H. Kim, S. K. Kwon and B. J. Kim, Adv. Funct. Mater., 2021, 31, 2100870 CrossRef CAS.
- J.-W. Lee, S. Seo, S.-W. Lee, G.-U. Kim, S. Han, T. N.-L. Phan, S. Lee, S. Li, T.-S. Kim, J.-Y. Lee and B. J. Kim, Adv. Mater., 2022, 34, 2207544 CrossRef CAS PubMed.
- J. H. Hou, O. Inganas, R. H. Friend and F. Gao, Nat. Mater., 2018, 17, 119–128 CrossRef CAS PubMed.
- S. X. Li, L. L. Zhan, Y. Z. Jin, G. Q. Zhou, T. K. Lau, R. Qin, M. M. Shi, C. Z. Li, H. M. Zhu, X. H. Lu, F. L. Zhang and H. Z. Chen, Adv. Mater., 2020, 32, 2001160 CrossRef CAS PubMed.
- C. L. He, Y. W. Pan, Y. N. Ouyang, Q. Shen, Y. Gao, K. R. Yan, J. Fang, Y. Y. Chen, C. Q. Ma, J. Min, C. F. Zhang, L. J. Zuo and H. Z. Chen, Energy Environ. Sci., 2022, 15, 2537–2544 RSC.
- J.-W. Lee, D. Jeong, D. J. Kim, T. N.-L. Phan, J. S. Park, T.-S. Kim and B. Kim, Energy Environ. Sci., 2021, 14, 4067–4076 RSC.
- Q. P. Fan, W. Y. Su, S. S. Chen, W. Kim, X. B. Chen, B. Lee, T. Liu, U. A. Mendez-Romero, R. J. Ma, T. Yang, W. L. Zhuang, Y. Li, Y. W. Li, T. S. Kim, L. T. Hou, C. Yang, H. Yan, D. H. Yu and E. G. Wang, Joule, 2020, 4, 658–672 CrossRef CAS.
- J.-W. Lee, B. S. Ma, H. J. Kim, T.-S. Kim and B. J. Kim, JACS Au, 2021, 1, 612–622 CrossRef CAS PubMed.
- K. E. Hung, Y. S. Lin, Y. J. Xue, H. R. Yang, Y. Y. Lai, J. W. Chang, C. J. Su, A. C. Su, C. S. Hsu, U. S. Jeng and Y. J. Cheng, Adv. Energy Mater., 2022, 12, 2103702 CrossRef CAS.
- J.-W. Lee, B. S. Ma, J. Choi, J. Lee, S. Lee, K. Liao, W. Lee, T. S. Kim and B. J. Kim, Chem. Mater., 2020, 32, 582–594 CrossRef CAS.
- Y. P. Qin, N. Balar, Z. X. Peng, A. Gadisa, I. Angunawela, A. Bagui, S. Kashani, J. H. Hou and H. Ade, Joule, 2021, 5, 2129–2147 CrossRef CAS.
- J.-W. Lee, C. Sun, D. J. Kim, M. Y. Ha, D. Han, J. S. Park, C. Wang, W. B. Lee, S.-K. Kwon, T.-S. Kim, Y.-H. Kim and B. J. Kim, ACS Nano, 2021, 15, 19970–19980 CrossRef CAS PubMed.
- M. Ghasemi, H. W. Hu, Z. X. Peng, J. J. Rech, I. Angunawela, J. H. Carpenter, S. J. Stuard, A. Wadsworth, I. McCulloch, W. You and H. Ade, Joule, 2019, 3, 1328–1348 CrossRef CAS.
- J. B. Zhang, C. H. Tan, K. Zhang, T. Jia, Y. J. Cui, W. Y. Deng, X. F. Liao, H. B. Wu, Q. H. Xu, F. Huang and Y. Cao, Adv. Energy Mater., 2021, 11, 2102559 CrossRef CAS.
- J.-W. Lee, C. Sun, B. S. Ma, H. J. Kim, C. Wang, J. M. Ryu, C. Lim, T. S. Kim, Y. H. Kim, S. K. Kwon and B. J. Kim, Adv. Energy Mater., 2021, 11, 2003367 CrossRef CAS.
- Q. P. Fan, W. Y. Su, S. S. Chen, T. Liu, W. L. Zhuang, R. J. Ma, X. Wen, Z. H. Yin, Z. H. Luo, X. Guo, L. T. Hou, K. Moth-Poulsen, Y. Li, Z. G. Zhang, C. Yang, D. H. Yu, H. Yan, M. J. Zhang and E. G. Wang, Angew. Chem., Int. Ed., 2020, 59, 19835–19840 CrossRef CAS PubMed.
- Y. Li, J. L. Song, Y. C. Dong, H. Jin, J. M. Xin, S. J. Wang, Y. H. Ca, L. Jiang, W. Ma, Z. Tang and Y. M. Sun, Adv. Mater., 2022, 34, 2110155 CrossRef CAS PubMed.
- J.-W. Lee, S. W. Lee, J. Kim, Y. H. Ha, C. Sun, T. N. L. Phan, S. Lee, C. Wang, T. S. Kim, Y. H. Kim and B. J. Kim, J. Mater. Chem. A, 2022, 10, 20312–20322 RSC.
- Z. Genene, J.-W. Lee, S.-W. Lee, Q. Chen, Z. Tan, B. A. Abdulahi, D. Yu, T.-S. Kim, B. J. Kim and E. Wang, Adv. Mater., 2022, 34, 2107361 CrossRef CAS PubMed.
- B. Liu, H. L. Sun, J.-W. Lee, J. Yang, J. W. Wang, Y. C. Li, B. B. Li, M. Xu, Q. G. Liao, W. Zhang, D. X. Han, L. Niu, H. Meng, B. J. Kim and X. G. Guo, Energy Environ. Sci., 2021, 14, 4499–4507 RSC.
- J.-W. Lee, C. Sun, S. W. Lee, G. U. Kim, S. Li, C. Wang, T. S. Kim, Y. H. Kim and B. J. Kim, Energy Environ. Sci., 2022, 15, 4672–4685 RSC.
- H. Yu, Y. Wang, H. Kim, X. Wu, Y. H. Li, Z. F. Yao, M. G. Pan, X. H. Zou, J. Q. Zhang, S. S. Chen, D. H. Zhao, F. Huang, X. H. Lu, Z. L. Zhu and H. Yan, Adv. Mater., 2022, 34, 2200361 CrossRef CAS PubMed.
- C. Sun, J.-W. Lee, S. Seo, S. Lee, C. Wang, H. Li, Z. P. Tan, S. K. Kwon, B. J. Kim and Y. H. Kim, Adv. Energy Mater., 2022, 12, 2103239 CrossRef CAS.
- H. L. Sun, B. Liu, Y. L. Ma, J.-W. Lee, J. Yang, J. W. Wang, Y. C. Li, B. B. Li, K. Feng, Y. Q. Shi, B. H. Zhang, D. X. Han, H. Meng, L. Niu, B. J. Kim, Q. D. Zheng and X. G. Guo, Adv. Mater., 2021, 33, 2102635 CrossRef CAS PubMed.
- S. Seo, C. Sun, J.-W. Lee, S. Lee, D. Lee, C. Wang, T. N. L. Phan, G.-U. Kim, S. Cho, Y. H. Kim and B. J. Kim, Adv. Funct. Mater., 2022, 32, 2108508 CrossRef CAS.
- G. Zeng, W. J. Chen, X. B. Chen, Y. Hu, Y. Chen, B. Zhang, H. Y. Chen, W. W. Sun, Y. X. Shen, Y. W. Li, F. Yan and Y. F. Li, J. Am. Chem. Soc., 2022, 144, 8658–8668 CrossRef CAS PubMed.
- Y. Chen, J. Y. Wan, G. Y. Xu, X. X. Wu, X. Q. Li, Y. X. Shen, F. Yang, X. M. Ou, Y. W. Li and Y. F. Li, Sci. China: Chem., 2022, 65, 1164–1172 CrossRef CAS.
- B. Zhang, F. Yang, S. S. Chen, H. Y. Chen, G. Zeng, Y. X. Shen, Y. W. Li and Y. F. Li, Adv. Funct. Mater., 2022, 32, 2202011 CrossRef CAS.
- L. L. Zhang, Z. Q. Zhang, D. Deng, H. Q. Zhou, J. Q. Zhang and Z. X. Wei, Adv. Sci., 2022, 2202513 CrossRef PubMed.
- Y. C. Liang, D. F. Zhang, Z. R. Wu, T. Jia, L. Luer, H. R. Tang, L. Hong, J. B. Zhang, K. Zhang, C. J. Brabec, N. Li and F. Huang, Nat. Energy, 2022, 7, 1180–1190 CrossRef CAS.
- S. Li, R. Zhang, M. Zhang, J. Yao, Z. Peng, Q. Chen, C. Zhang, B. Chang, Y. Bai, H. Fu, Y. Ouyang, C. Zhang, J. A. Steele, T. Alshahrani, M. B. J. Roeffaers, E. Solano, L. Meng, F. Gao, Y. Li and Z.-G. Zhang, Adv. Mater., 2022, 2206563, DOI:10.1002/adma.202206563,.
- H. T. Wang, C. C. Cao, H. Chen, H. J. Lai, C. X. Ke, Y. L. Zhu, H. Li and F. He, Angew. Chem., Int. Ed., 2022, 61, e202201844 CAS.
- J. Lee, R. Singh, D. H. Sin, H. G. Kim, K. C. Song and K. Cho, Adv. Mater., 2016, 28, 69–76 CrossRef CAS PubMed.
- Y. Z. Lin, Y. F. Wang, J. Y. Wang, J. H. Hou, Y. F. Li, D. B. Zhu and X. W. Zhan, Adv. Mater., 2014, 26, 5137–5142 CrossRef CAS PubMed.
- Y. Z. Lin, J. Y. Wang, S. X. Dai, Y. F. Li, D. B. Zhu and X. W. Zhan, Adv. Energy Mater., 2014, 4, 1400420 CrossRef.
- J. Liu, S. S. Chen, D. P. Qian, B. Gautam, G. F. Yang, J. B. Zhao, J. Bergqvist, F. L. Zhang, W. Ma, H. Ade, O. Inganas, K. Gundogdu, F. Gao and H. Yan, Nat. Energy, 2016, 1, 16089 CrossRef CAS.
- Q. Burlingame, M. Ball and Y. L. Loo, Nat. Energy, 2020, 5, 947–949 CrossRef.
- P. Cheng and X. W. Zhan, Chem. Soc. Rev., 2016, 45, 2544–2582 RSC.
- W. Y. Yang, W. Wang, Y. H. Wang, R. Sun, J. Guo, H. N. Li, M. M. Shi, J. Guo, Y. Wu, T. Wang, G. H. Lu, C. J. Brabec, Y. F. Li and J. Min, Joule, 2021, 5, 1209–1230 CrossRef.
- Y. Zhong, B. Kumar, S. Oh, M. T. Trinh, Y. Wu, K. Elbert, P. P. Li, X. Y. Zhu, S. X. Xiao, F. Ng, M. L. Steigerwald and C. Nuckolls, J. Am. Chem. Soc., 2014, 136, 8122–8130 CrossRef CAS PubMed.
- G. Y. Zhang, J. B. Zhao, P. C. Y. Chow, K. Jiang, J. Q. Zhang, Z. L. Zhu, J. Zhang, F. Huang and H. Yan, Chem. Rev., 2018, 118, 3447–3507 CrossRef CAS PubMed.
- N. N. Liang, K. Sun, Z. Zheng, H. F. Yao, G. P. Gao, X. Y. Meng, Z. H. Wang, W. Ma and J. H. Hou, Adv. Energy Mater., 2016, 6, 1600060 CrossRef.
- Y. W. Duan, X. P. Xu, H. Yan, W. L. Wu, Z. J. Li and Q. Peng, Adv. Mater., 2017, 29, 1605115 CrossRef PubMed.
- P. Cheng, X. G. Zhao and X. W. Zhan, Acc. Mater. Res., 2022, 3, 309–318 CrossRef CAS.
- N. N. Liang, D. Meng and Z. H. Wang, Acc. Chem. Res., 2021, 54, 961–975 CrossRef CAS PubMed.
- Y. Z. Lin and X. W. Zhan, Acc. Chem. Res., 2016, 49, 175–183 CrossRef CAS PubMed.
- N. K. Elumalai and A. Uddin, Energy Environ. Sci., 2016, 9, 391–410 RSC.
- D. P. Qian, Z. L. Zheng, H. F. Yao, W. Tress, T. R. Hopper, S. L. Chen, S. S. Li, J. Liu, S. S. Chen, J. B. Zhang, X. K. Liu, B. W. Gao, L. Q. Ouyang, Y. Z. Jin, G. Pozina, I. A. Buyanova, W. M. Chen, O. Inganas, V. Coropceanu, J. L. Bredas, H. Yan, J. H. Hou, F. L. Zhang, A. A. Bakulin and F. Gao, Nat. Mater., 2018, 17, 703–709 CrossRef CAS PubMed.
- L. L. Zhan, S. X. Li, Y. K. Li, R. Sun, J. Min, Z. Z. Bi, W. Ma, Z. Chen, G. Q. Zhou, H. M. Zhu, M. M. Shi, L. J. Zuo and H. Z. Chen, Joule, 2022, 6, 662–675 CrossRef CAS.
- T. L. Nguyen, T. H. Lee, B. Gautam, S. Y. Park, K. Gundogdu, J. Y. Kim and H. Y. Woo, Adv. Funct. Mater., 2017, 27, 1702474 CrossRef.
- K. Kawashima, Y. Tamai, H. Ohkita, I. Osaka and K. Takimiya, Nat. Commun., 2015, 6, 10085 CrossRef CAS PubMed.
- S. Natsuda, T. Saito, R. Shirouchi, Y. Sakamoto, T. Takeyama, Y. Tamai and H. Ohkita, Energy Environ. Sci., 2022, 15, 1545–1555 RSC.
- Z. Chiguvare and V. Dyakonov, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 235207 CrossRef.
- Y. L. Ma, M. Zhang, S. Wan, P. Yin, P. S. Wang, D. D. Cai, F. Liu and Q. D. Zheng, Joule, 2021, 5, 197–209 CrossRef CAS.
- H. B. Naveed, K. Zhou and W. Ma, Acc. Chem. Res., 2019, 52, 2904–2915 CrossRef CAS PubMed.
- X. K. Chen, D. P. Qian, Y. M. Wang, T. Kirchartz, W. Tress, H. F. Yao, J. Yuan, M. Hulsbeck, M. J. Zhang, Y. P. Zou, Y. M. Sun, Y. F. Li, J. H. Hou, O. Inganas, V. Coropceanu, J. L. Bredas and F. Gao, Nat. Energy, 2021, 6, 799–806 CrossRef CAS.
- T. M. Clarke and J. R. Durrant, Chem. Rev., 2010, 110, 6736–6767 CrossRef CAS PubMed.
- P. W. M. Blom, V. D. Mihailetchi, L. J. A. Koster and D. E. Markov, Adv. Mater., 2007, 19, 1551–1566 CrossRef CAS.
- S. R. Cowan, A. Roy and A. J. Heeger, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 245207 CrossRef.
- Q. Burlingame, X. H. Huang, X. Liu, C. Jeong, C. Coburn and S. R. Forrest, Nature, 2019, 573, 394–397 CrossRef CAS PubMed.
- X. Y. Du, T. Heumueller, W. Gruber, A. Classen, T. Unruh, N. Li and C. J. Brabec, Joule, 2019, 3, 215–226 CrossRef CAS.
- X. Xu, J. Y. Xiao, G. C. Zhang, L. Wei, X. C. Jiao, H. L. Yip and Y. Cao, Sci. Bull., 2020, 65, 208–216 CrossRef CAS PubMed.
- PVWatts Calculator. Solar Resource Data, https://pvwatts.nrel.gov/pvwatts.php, National Renewable Energy Laboratory, 2022, (accessed November 2022).
- J.-W. Lee, G.-U. Kim, D. J. Kim, Y. Jeon, S. Li, T. S. Kim, J. Y. Lee and B. J. Kim, Adv. Energy Mater., 2022, 12, 2200887 CrossRef CAS.
- S. E. Root, M. A. Alkhadra, D. Rodriquez, A. D. Printz and D. J. Lipomi, Chem. Mater., 2017, 29, 2646–2654 CrossRef CAS.
- D. Han, Y. Han, Y. Kim, J.-W. Lee, D. Jeong, H. Park, G.-U. Kim, F. S. Kim and B. J. Kim, J. Mater. Chem. A, 2022, 10, 640–650 RSC.
- M. Ghasemi, N. Balar, Z. X. Peng, H. W. Hu, Y. P. Qin, T. Kim, J. J. Rech, M. Bidwell, W. Mask, I. McCulloch, W. You, A. Amassian, C. Risko, B. T. O'Connor and H. Ade, Nat. Mater., 2021, 20, 525–532 CrossRef CAS PubMed.
- M. T. Sajjad, A. Ruseckas and I. D. W. Samuel, Matter, 2020, 3, 341–354 CrossRef.
- S. M. Menke and R. J. Holmes, Energy Environ. Sci., 2014, 7, 499–512 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Cyclic voltammogram, UV-Vis spectra, additional GIWAXS analysis, and DMT analysis) and experimental procedures. See DOI: https://doi.org/10.1039/d3ee00272a |
| ‡ J.-W. Lee and C. Sun contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2023 |