How alkali cations affect salt precipitation and CO2 electrolysis performance in membrane electrode assembly electrolyzers†
Abstract
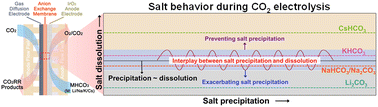
Electrocatalytic CO2 reduction in membrane electrode assembly (MEA) electrolyzers is a promising approach to producing carbon-neutral chemicals and fuels at commercially relevant rates. However, short-duration stability owing to cathode flooding and salt precipitation in MEAs is a significant challenge for commercializing this technology. Using operando wide-angle X-ray scattering (WAXS), we demonstrate how the formation of salt precipitates occurs and varies with alkali cations. We also correlate this formation of precipitates with CO2 reduction reaction (CO2RR) and hydrogen evolution reaction (HER) selectivity by measuring the anode and cathode products using an in-line gas chromatograph. We found that low-solubility salts can quickly precipitate over the catalyst layer and limit the CO2 from accessing the catalyst thereby enhancing the HER. Although salts with marginal solubility demonstrate an oscillatory trend between salt precipitation and dissolution, the use of highly soluble Cs salts prevents salt precipitation and mitigates flooding of the gas diffusion layer. In addition, diluting cation concentration in the anolyte significantly decreases salt precipitation as well as improves the CO2RR product selectivity. This work suggests that the key to circumventing salt precipitation is to use highly soluble alkali cation salts as the anolyte (e.g. CsHCO3) along with an optimal salt concentration between 0.01 and 0.1 M.


 Please wait while we load your content...
Please wait while we load your content...