Open Access Article
Open Access ArticleEmerging concepts in intermediate carbon dioxide emplacement to support carbon dioxide removal†
Hanna Marie
Breunig
 *,
Fabian
Rosner
,
Tae-Hwan
Lim
*,
Fabian
Rosner
,
Tae-Hwan
Lim
 and
Peng
Peng
and
Peng
Peng

Energy Analysis and Environmental Impacts Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA. E-mail: hannabreunig@lbl.gov
First published on 18th April 2023
Abstract
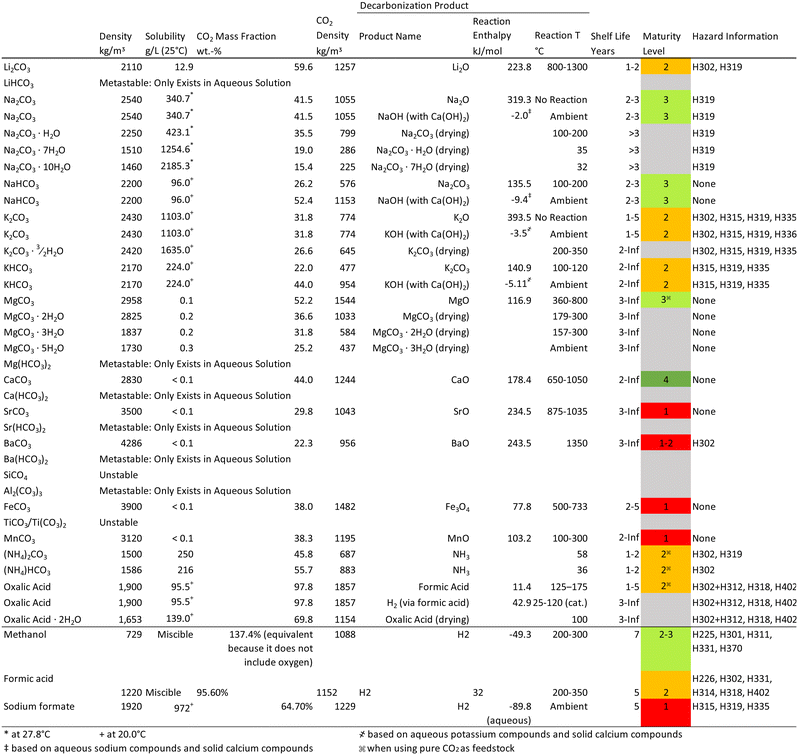
Substantial upscaling of carbon dioxide capture is possible in the coming decades but existing solutions for storage are projected to be limited in annual capacity by as much as 2–10 GtCO2 per year until mid-century. Temporary storage of CO2 in a solid or liquid state could prove useful for filling this gap in capacity, until more permanent and ideally lucrative CO2 sequestration options come online. There are several concepts for reversible solid-state and chemical CO2 storage, but their advantages and limitations have yet to be reviewed in this context. This article focuses on the physical and chemical aspects of CO2 storage via liquid and solid chemical carriers and sorbents, and gives an overview of the energetics around their use, as well as prospects for their future development. Exciting opportunities for coupling capture and medium to high maturity multi-year storage technologies could support carbon removal in the coming decades. Highlights of the analysis are the remarkable storage capacity of oxalic acid and formic acid (CO2-density of 1857 kg m−3 and 1152 kg m−3, compared with condensed liquid CO2 at 993–1096 kg m−3, respectively), the relative scalability and compatibility of carbonate salts for stationary storage with direct air capture, and the potential promise of multiple carriers for CO2 transportation. Solid sorbents do not achieve such ultra-high storage capacities, but could improve storage over compressed gas tanks on a capacity and energetics basis.
Broader contextTo mitigate climate change, we must move an enormous amount of CO2 out of the atmosphere, greater than any mass moved by humanity to date (∼473 Gt to lower CO2 concentration to 350 ppm). Identifying CO2 storage solutions to serve booming CO2 capture developments until permanent geologic storage options come online remains an underappreciated scientific challenge of the highest importance. |
Introduction
Carbon dioxide removal (CDR) technologies can slow the accumulation of CO2 in the atmosphere if deployed in the first half of the century, which is essential for staying below 2 °C warming.1 Direct air capture (DAC) and bioenergy with carbon capture and sequestration (BECCS) are commercially viable CDR technologies that generate gas with high concentrations of CO2. Cost predictions from the IPCC and some of this study's authors suggest that deploying these and other CO2 capture technologies could greatly reduce the cost of meeting emissions reductions targets,2 but would necessitate global capture on the order of 7 GtCO2 per year or more by 2050.3 Substantial upscaling of BECCS is possible based on existing available biomass, while DAC which is only cost limited, can upscale rapidly in areas with cheap renewable energy resources, land, and water. In the last few years, commercial development of capture systems has increased rapidly,4 but supporting the boom in capture will require deploying equal or greater annual CO2 storage capacity where capture is occurring. In this paper, we investigate the possibility of using materials that can store CO2 or carbon from CO2, even temporarily, to supplement geologic storage.Upscaling CO2 storage is nontrivial and has been hampered by the slow arduous regulatory and construction processes involved in bringing permanent geologic sequestration projects online. Geologic storage capacity is plentiful, but geographically limited and commercial capacity has remained relatively stable at approximately 40 MtCO2 per year.4 Ringrose and Meckel suggest that necessary injection well development, on the order of 10–14 thousand wells by 2050, is not outside of what has been historically achieved through petroleum exploitation, but relies heavily on undeveloped offshore regions and subsequent CO2 shipping or pipeline delivery.5,6 Several large projects are now under development, such as the Northern Lights Project in Europe which proposes to use ships to transport liquid CO2 to a terminal for pipeline transport at capacities of up to 1.5 million tonnes of CO2 per year.7 Even as offshore transport options come online, new pipeline or rail connections transporting CO2 to shipping terminals are unlikely to be viable for small- to medium-scale capture facilities (120 tonnes CO2 per day or less).8
Additionally, dozens of new and existing utilization pathways have been identified that use CO2 directly or as a carbon feedstock for building materials, chemicals and fuels,9,10 but most are limited in that: (1) CO2 is released immediately upon use; (2) potential consumption of CO2 is projected to be small and not easily collocated with capture facilities; and (3) resulting products do not yet meet cost parity with incumbent petroleum-based products. For example, CO2 can be sequestered in cement and concrete materials via mineral carbonation. However, it is projected that the near-term implementation of CO2 for cement production in the United States (US) would require transporting less than a kilo-tonne of CO2 per year to over 8000 distributed production sites.11 Carbon dioxide can also be stored semi-irreversibly for very long periods of time through the mineralization of natural or waste alkaline materials. These reactions achieve the ultimate goal of carbon sequestration and thus are excellent storage solutions, but reputable reviews suggest they will be limited by rates of reaction and starting materials in the near future.12,13
Despite the lack of storage options available, large-scale carbon capture and storage (CCS) continues to be included in possible response options to climate change. Shared socio-economic pathways (SSP) are one such example where possible socio-political, economic, and technological changes and their climate implications are modeled.14 As depicted in Fig. 1A, demand for CO2 storage surpasses 2 GtCO2 per year by 2035 in pathways where countries adopt a wide range of aggressive emissions reduction policies and reliance on CCS is minimal (SSP1 and SSP2).15 In comparison, the necessary supply of storage from geologic storage and CO2 utilization with meaningful carbon retention will not occur until after 2045.4,5,16 This simple representaton suggests that the gap in storage supply will be massive, at 2–10 GtCO2 per year, but will only last for a few decades (Fig. 1B). As such, temporary reversible storage solutions that act as “batteries” for CO2 may be of interest. We estimate the total amount of storage capacity and duration required for storage systems based on a physical process or material that releases the CO2 or “self-discharges” on relatively short timescales, such as 3 or 10 years (Fig. 1B). Additional capture and storage would be required, compared with the SSP baselines, to compensate for self-discharge, which would ideally occur in a controlled measurable manner, unlike the gradual loss of carbon stored in biomass, biochar, or construction materials. Similar to the way a battery revolutionized society despite its cost and inevitable self-discharge, we see great value in methods of storage that offer temporary storage of CO2, not for commercial use, but for the sole purpose of meeting near-term target removal rates in the first-half of the century. As such, unlike previous reviews of CO2 conversion and utilization, in the context of CO2 storage, we consider a much broader range of materials, as we are not concerned with future commercial value.
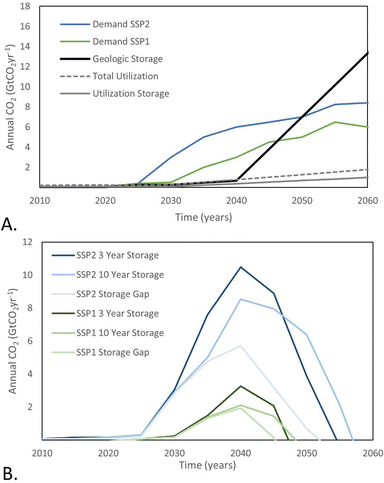 | ||
| Fig. 1 (A) Demand for CO2 storage across scenarios towards limiting global mean temperature rise to 2 degrees warming (SSP1 and SSP2), plotted with historical and projected rates of supply of CO2 storage. The geologic storage projection is based on proposed storage capacity under development out to 2040, and on optimistic but feasible injection well development rates (Scenario F from Ringrose & Meckel).5 The utilization storage projection is based on CO2 consumption for enhanced oil recovery, plastics and building materials; the projection for total utilization is plotted as a reference, and includes these applications as well as chemicals and fuels. (B) Gap in annual CO2 storage capacity for SSP1 and SSP2 plotted with required storage capacity for systems that offer 3 or 10 years of storage. | ||
Currently, storing CO2 in its molecular form without any chemical conversion at reasonable sizes for transportation or stationary storage involves compressing and/or cooling the gas. At standard ambient pressure and temperature, 1 tonne of CO2 has a volume of 506 m3, which can be imagined as a cube with sides as long as a bus. Industries already transport gaseous or liquid CO2 by truck, ship, rail, or pipeline, with conditions presented in Fig. 2. Condensed liquid CO2 is typically stored at 238 to 261 K at 1.2–2.5 MPa with densities between 993–1096 kg m−3. Liquid CO2 transport ships such as the Coral Carbonic offer capacities between 1200–1500 m3 while larger ships proposed by Daewoo Shipbuilding & Marine Engineering offer capacities as large as 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3.17,18 New state of the art ships proposed by Knutsen NYK Carbon Carriers have moved towards ambient temperature and high pressure (4.5 MPa) liquid storage to boost capacity.19 Despite these demonstrations of large-scale liquefaction, it is limited in that it requires expensive specialized tanks, energy intensive liquefaction processes, and offers limited storage durations (days) due to boil-off issues. Compressed CO2 can be achieved at moderate pressures, but storing CO2 for long periods in pressurized tanks presents safety and physical footprint concerns. Furthermore, just drying and compressing CO2 for pipeline transport (7.5 MPa) may require more electricity (256–475 kW h per tCO2) than reported minimum mole-specific work for separating CO2 from air (e.g. Carbon Engineering: 130 kW h per tCO2 or amine capture 380 kW h per tCO2).20–22 Solid CO2, commonly referred to as “dry ice” is used in industry for short duration refrigeration, but it is not used for long duration storage or transportation. We do not consider compressed gas or dry ice storage further in this analysis.
000 m3.17,18 New state of the art ships proposed by Knutsen NYK Carbon Carriers have moved towards ambient temperature and high pressure (4.5 MPa) liquid storage to boost capacity.19 Despite these demonstrations of large-scale liquefaction, it is limited in that it requires expensive specialized tanks, energy intensive liquefaction processes, and offers limited storage durations (days) due to boil-off issues. Compressed CO2 can be achieved at moderate pressures, but storing CO2 for long periods in pressurized tanks presents safety and physical footprint concerns. Furthermore, just drying and compressing CO2 for pipeline transport (7.5 MPa) may require more electricity (256–475 kW h per tCO2) than reported minimum mole-specific work for separating CO2 from air (e.g. Carbon Engineering: 130 kW h per tCO2 or amine capture 380 kW h per tCO2).20–22 Solid CO2, commonly referred to as “dry ice” is used in industry for short duration refrigeration, but it is not used for long duration storage or transportation. We do not consider compressed gas or dry ice storage further in this analysis.
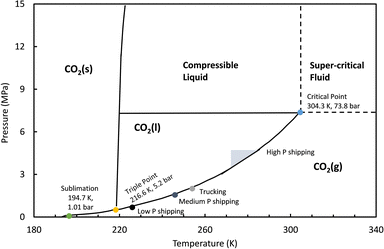 | ||
| Fig. 2 Carbon dioxide phase diagram with pressure and temperature conditions for commercial and state of the art storage and transport modes. | ||
Storage of CO2 using a material, however briefly, is already the motivation of DAC and other point-source CO2 capture technologies that rely on a material to separate CO2 from other gases. Liquid and solid materials in these systems have been extensively studied for carbon capture, but not for temporary CO2 storage applications. A class of solids well known for their ability to store CO2 are carbonate salts, including alkali metal and alkaline earth metal carbonates. If stored properly, carbonate salts can retain CO2 at ambient conditions for months or years. In theory, carbonates could offer permanent storage, but in practice storage duration is affected by material morphology, and not just chemical stability. For example, solids need to maintain their powdery character in order to enable loading and transport to a final storage or utilization end-use. Carbonate salts are widely utilized in cement,23 paper and pulp,24 and iron and steel industry25 as a chemical feedstock, and more recently as chemical intermediates in proposed aqueous and mineral carbonation-based DAC processes by companies such as Carbon Engineering20 and Heirloom.26 In these cases, CO2 storage via carbonate salts may be leveraged in these existing and forthcoming processes with minor modifications.
Advanced porous materials such as zeolites, mesoporous silica, polymeric resins, activated carbon, and rigid and flexible metal organic frameworks (MOF) are another material class that have been investigated in the separation of CO2 from ambient air and flue gases.27 Porous materials offer large internal surface areas with amine functionalized groups to which CO2 can selectively and reversibly bind.28 These materials are being tuned to minimize the energy required to take CO2 off (i.e. regeneration), while remaining selective to CO2 over other compounds such as water. This challenge is key to outcompeting energy intensive absorptive liquids such as monoethanolamine, and achieving this balance while ensuring the material can take up CO2 at high densities has yet to be achieved for DAC. Still, existing materials today have demonstrated higher densities than what is achieved by compressed gas tanks at the same pressure.27 This is an active area of research, and new materials and design considerations may lead to breakthroughs in performance.
A third class of materials that could be used to store CO2 is liquid organic hydrogen carriers (LOHC) with high carbon contents, include ethanol, methanol, formic acid (FA), formate salts (FS), and other hydrocarbons. These carriers can be dehydrogenated at elevated temperatures with a catalyst to deliver high purity H2, the status of which is thoroughly discussed by Southall and Lukashuk.29 While well established, generating these materials directly from CO2 requires new approaches, such as electrochemical processes. Furthermore, the use of CO2 as a material to store H2 has been well studied, but never from the perspective of CO2 storage. This might change if the value of mitigating or capturing a tonne of CO2 is higher than the value of delivering the stored hydrogen. The availability of affordable H2 is considered a key assumption in the projected growth of CO2 utilization pathways for fuel and chemical development, and is reflected in our projections of future demand for CO2 storage.
We believe reversibility of storage is an acceptable and even beneficial aspect of the materials evaluated for several reasons. As we envision these storage systems to be only temporary “last resort” solutions that may take up large amounts of land, reversibility offers the possibility of decommissioning and later use of the CO2 and storage material. Ideally reversibility is a controlled process, but even a material that exhibits gradual release of CO2 could be of value if it is ready to be deployed and helps us achieve significant near-term capture. Finally, reversibility allows for materials to double as carriers of CO2 for transportation.
In this study, we screen materials based on gravimetric (i.e. CO2 mass fraction, CO2 wt%) and volumetric CO2 capacities (i.e. CO2-density), stability, and the energetics of getting CO2 on and off materials. We focus on materials that can offer high densities of CO2 to minimize material requirements. Overcoming the low volumetric density of CO2 at ambient conditions (1.98 kg m−3) will be necessary to reduce the number of tanks and land required for storage. The storage methods included in this study are: carbonate salts, sorbents, liquid organic carriers, and chemical solids that allow for reversible CO2 storage. We estimate the material required and land footprint of multi-year storage. While the focus of this paper is on the storage of CO2, we include a perspective on the use of materials for truck transportation of CO2.
Methods and materials
The feasibility of CO2 storage was estimated for materials in each class. Upon down selection, candidates were evaluated for CO2 capture facilities with capacities of 10 tonnes per year (tpy) and 1 million tonnes per year (Mtpy), which are scales typically used to represent pilot and large DAC facilities. The source could be a facility that generates CO2 as a by-product or captures it from a waste stream, or a dedicated CO2 removal facility such as DAC. We note that some materials evaluated capture CO2 directly in the process of storage, which obviates the need for a CO2 source.Screening method
We start by conducting a broad screening of material classes that can store CO2 or carbon from CO2 through adsorption or chemical bonds. As gleaned from research on H2 carriers, there are a number of properties to consider when selecting a storage material.30 These include properties such as safety, toxicity, compatibility with existing infrastructure, total cost of ownership, material availability, storage duration, storage capacity, reversibility, material durability/cyclability, energy efficiency, and life cycle impacts. As many of the materials evaluated in this analysis have yet to be properly explored for CO2 storage and transport, there are a number of data gaps limiting the ability to estimate these properties. As such we kept our initial screening to general assessments of technology maturity, stability, reversibility, energetics, and CO2 capacity, which we define as: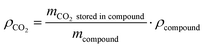 | (1) |
Gravimetric capacity is the quantity of CO2 stored within a unit mass of material and is of major relevance to transportation applications which in many cases are weight limited. It is important to note that a fair comparison of material storage options with compressed gas or liquid CO2 storage will include the additional weight and volume of the containers used, as is done in this study.
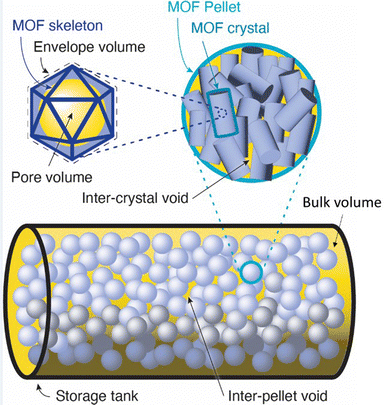
For sorbents, we modelled storage tanks filled with porous packing material (pellets made of crystal sorbent material) as packed beds. The mass of sorbent material within the container is estimated using (eqn (2)) where ρs is the single crystal density in kg m−3 and ε is the porosity of the pellet or tank bed. Unlike skeletal density, which is the ratio of the mass of crystal solid to the volume of the solid excluding pore volume, crystal density divides the same mass of crystal solid by the envelope volume of the crystal, giving a much lower density (Fig. 3). In this analysis, the porosity of the pellet and bed are used to derive the inter-crystal and inter-pellet pore volumes, respectively, and exclude crystal pore volume which is evaluated separately.
The CO2 stored inside the crystal envelope volume of the sorbent 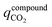 in kg-CO2 per kg-material includes CO2 adsorbed on the material surface and CO2 stored in the pore volume (eqn (3)) where V is the pore volume in m3 kg−1 and ρgasCO2 is the CO2 gas density in kg m−3. The CO2 stored in the tank is the combination of
in kg-CO2 per kg-material includes CO2 adsorbed on the material surface and CO2 stored in the pore volume (eqn (3)) where V is the pore volume in m3 kg−1 and ρgasCO2 is the CO2 gas density in kg m−3. The CO2 stored in the tank is the combination of 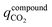 , the CO2 in the inter-crystal void inside the pellets, and the CO2 in the inter-pellet void space at a specific temperature and pressure (eqn (4)). The amount adsorbed and in the pore volume at a given temperature and pressure is derived from experimental isotherm data, which tend to be reported for crystals at conditions relevant to CO2 capture (0.1 MPa or lower). The absolute amount of CO2 storage with adsorbents in a tank can be maximized by increasing the pressure, which is limited to around 6.4 MPa at 298 K due to liquefaction.
, the CO2 in the inter-crystal void inside the pellets, and the CO2 in the inter-pellet void space at a specific temperature and pressure (eqn (4)). The amount adsorbed and in the pore volume at a given temperature and pressure is derived from experimental isotherm data, which tend to be reported for crystals at conditions relevant to CO2 capture (0.1 MPa or lower). The absolute amount of CO2 storage with adsorbents in a tank can be maximized by increasing the pressure, which is limited to around 6.4 MPa at 298 K due to liquefaction.
| ρbulk = ρs(1 − εpellet)(1 − εbed) | (2) |
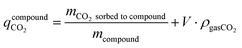 | (3) |
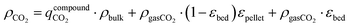 | (4) |
A number of materials identified were too early stage or do not meet our screening criteria. We provide additional details on these materials in the ESI† to guide future work in this space. For solid and liquid carriers, we define four levels of technology maturity: L1: neither CO2 fixation nor regeneration are commercial; L2: either fixation or regeneration is commercial; L3: fixation and regeneration are commercial but require validation for storage; and L4: commercially used. For sorbents, as the reactor design for CO2 storage may be similar to existing concepts for compressed gas storage, the biggest bottleneck is likely to be materials synthesis. As such, we define four levels of technology maturity as: L1: a theoretical concept; L2: lab-scale synthesis of material; L3: demonstration for DAC; and L4: industrial-scale synthesis.
Truck transport model
We estimated the energy associated with transporting CO2 by road for each candidate material and for liquified CO2 assuming long haul commercial trucks with B-train configuration that comprises a tractor and two trailers. We assume liquid CO2 is transported at 2.2 MPa and 255 K, giving a bulk density of 1022 kg m−3. Tare weight, or empty weight of the truck without material is the sum of the tractor and trailer weights, which is 21.0, 18.0, and 19.3 tonnes for trucks designed for compressed gas (including cryogenic liquid CO2), liquids, and solids, respectively. We assumed trucks are filled until either the United States federal limit on gross weight rating of the truck (70.0 tonnes) or the volume limit of the trailer (84.7 m3) is met. For perspective, commercial trucks transport CO2 in small quantities of 2–30 tonnes, while rail cars can transport larger quantities ∼60 tonnes but are limited to rail networks.Upon delivery of the CO2, we assumed trucks will return to the CO2 capture facility either empty (in the case of liquid CO2, liquid organic carriers or other one-way carriers), or with the regenerated material. Trucks were assumed to travel on ungraded highway networks at a fixed speed of 100 km h−1, which is reflective of speed limits for freight trucks in the United States and in the European Union (80–130 km h−1). The energy needed for trucking was calculated based on the energy needed to overcome the rolling resistance and aerodynamic drag of a typical heavy-duty truck.31 We then assumed an engine and transmission efficiency of 40% and 90%, respectively. Total energy required takes into account the mass of the transported CO2, the material, and the tare weight of the B-Train on the outbound and inbound trips.
For the MOF-filled trailers, we assumed CO2 is transported at 5.6 MPa and 98–99% of CO2 is discharged upon arrival. This reflects a scenario where pressure is decreased to 0.03 MPa. Thermophysical properties of CO2 are retrieved from National Institute of Standards and Technology (NIST) Chemistry WebBook.32 Loading trucks with solid powders or stable liquids, as would be the case for solid and liquid carriers, at ambient conditions will not require significant energy, but the chemical conversion of CO2 to a carrier likely will. As significant uncertainty remains regarding the supply chain logistics of chemical solid and liquid carriers, we believe it would be too preliminary to present results for charging and discharging across material classes.
Pipeline model
Today, there are over 6500 km of CO2 pipelines worldwide, transporting approximately 68 million tonnes of CO2 per year.33 Pipelines are designed to keep CO2 in either a gaseous, dense liquid, or supercritical phase, and CO2 is typically compressed to pressures above 7.5 MPa to avoid two-phase flow regimes and changes in compressibility at temperatures of the surrounding soil. Because CO2 cannot exist as a liquid at atmospheric conditions, it takes pressurization and cooling to achieve a liquid state. Supercritical CO2 storage is based on compression to 10.0–15.2 MPa at 313 K. Supercritical pipelines are used when moving large quantities of CO2 over a long distance. The high density of the supercritical CO2 (629–783 kg m−3), which is comparable to liquid phase, allows for a high throughput transport while its low viscosity (48–69 μPa s), similar to vapor state, helps reduces friction loss during transport.Numerous models for estimating pipeline pressure drop and necessary pipeline diameters exist, but real-world examples of CO2 pipelines suggest design and operation is highly location dependent and requires proper engineering especially for dense state CO2.34,35 As such, we used values from a similar-scale (12 inch diameter) hypothetical pipeline and assumed recompression from 10.3 to 15.2 MPa at 313 K is required every 100 km (49 Pa m−1 pressure drop), to avoid pressure falling below 10.3 MPa.36 From this, we derived a single-stage recompression energy (14.7 kJ per kg-CO2) using the Peng–Robinson equation of state in ProSim software, assuming a 98% mechanical efficiency, 97% electrical efficiency, 65% isentropic efficiency, and 10% additional power consumption for auxiliaries. This is much lower than the initial compression required to take gaseous CO2 from 0.1 MPa to a supercritical state. For example, Climeworks derives a 363.3 kJ per kg-CO2 compression energy assuming an 8 stage compression and intercooling system is used to take CO2 from 0.1 to 15.0 MPa at 313 K.37 Carbon Engineering derives a 475.2 kJ per kg-CO2 compression energy assuming a 4 stage compression system is used to take CO2 to 15.2 MPa at 313 K.20 Neither report recompression energy for pipelines. These compression energies match well with that reported by the Carbon Capture Simulation Initiative Toolset implemented in Aspen Plus for the conditioning of ambient pressure CO2 to 15.2 MPa (370–441 kJ per kg-CO2).38 We find our approach for estimating recompression energy to be reasonable as we were able to derive similar compression energies of 392 to 420 kJ per kg-CO2 assuming 4 and 3 stage compression, respectively.
CO2 emissions from transport
Emissions during transport are estimated by accounting for the emission factor of diesel fuel (0.07 kgCO2 per MJ) for trucking39 and the national grid carbon intensity in the US in 2020 (0.38 kg kW−1 h−1 or 0.107 kgCO2 per MJ)40 for pipeline recompression. Based on this, we estimated a breakeven distance for all options where CO2 emissions generated during transport are equal to the quantity of CO2 being transported. Note that these estimations are based on first-order calculations to compare different CO2 carrier options and the more accurate emissions estimations would require dedicated life-cycle assessment for each option.Stationary storage analysis
Gravimetric and volumetric capacities were used to estimate the necessary storage material, total weight, and total volume required for the pilot facility and regional hub scenarios, assuming perfect conversion for chemical solid and liquid storage. As MOFs require temperature and pressure maintenance, we excluded them from long-duration stationary storage. Typical storage equipment used in the fossil fuel industry for powder solids, flammable liquids, and liquid CO2 were assumed. Specifications of storage silos or tanks assumed for the small- and large-scale storage systems are included in Table S1 (ESI†). Physical footprint was estimated based on the storage equipment diameter plus spacing of tanks based on NFPA 30 safety codes. This value was divided by the stored CO2 to estimate land footprint intensities in units of m2 per tCO2.Results and discussion
Sorbent carrier potential
Solid porous adsorbents have been extensively studied and developed to separate CO2 from dilute sources where CO2 concentrations are typically below 30% and as low as 0.04% if capturing from ambient air. This requires sorbents to have high CO2 selectivity and uptake capacity as well as fast kinetics at sub-atmospheric partial pressure which typically requires surface functionalization with alkaline molecules such as amines. Implicit in these parameters are other critical parameters such as the enthalpy of adsorption of CO2, which determines both the selectivity and energy required for regeneration. Binding CO2 too strongly may enhance selectivity and kinetics of adsorption but can lead to costly regeneration. While there are many types of porous materials under investigation for CO2 capture, we identified MOF,41 activated carbon,42 and zeolite43,44 adsorbents that have CO2 uptake data at pressures greater than 3 MPa at near room temperature. The properties of the identified adsorbents are shown in Table 1. A number of materials can adsorb CO2 at high temperatures, including CaO and MgO, which are discussed in the following section, hydrotalcites, and lithium-based materials.45 We do not provide further details due to their expected high costs.Zeolites
Zeolites are porous aluminosilicate materials that have relatively low material costs, very high stability and have demonstrated rapid adsorption and low energy penalties in post-combustion applications.27 Zeolite 13x is commercially available and has an excess CO2 uptake, which is defined as the amount of CO2 bonded to the surface and can be thought of as the gravimetric capacity, of 0.16 g g−1 at 4.5 MPa (excluding void spaces). The reference values shown in Table 1 are based on the NIST reference zeolite material, ammonium ZSM-5 zeolite. The measured values are based on a standardized high-pressure CO2 measurement, harmonizing results across measurement techniques and procedures being practiced. Notably, zeolites have been challenged by the presence of water, where CO2 adsorption capacity drops over time as water competes for binding sites. As such, research has focused on how to add highly charged species on the surface to increase affinity for CO2, such as alkali and alkaline earth metal cations.46 Future research for tuning zeolites for storage applications would need to study whether the lack of water may lead to higher cycling capacity and whether this increase in affinity subsequently increases the energy required for regeneration.Activated carbon
Enhanced activated carbons are amorphous porous forms of carbon that have low material costs, very high surface areas, and require relatively low temperatures for regeneration. Overcoming the relatively low capacities of CO2 at low pressure has been a focus for separation applications, however, due to the high surface areas, activated carbons can have very high CO2 capacities at higher pressures that would be used for storage applications.47 That being said, predicting performance at specific conditions from result reported in literature is challenging as reporting methods are not standardized and the correlation between surface chemistry and capacity is still not well understood. Maxsorb, a commercially available high-surface area activated carbon, has the highest surface area and CO2 adsorption at elevated pressure amongst activated carbon as shown in Table 1.Metal–organic frameworks
Metal–organic frameworks consist of metal-based nodes bridged by organic linking groups to form a network. They have ultra-high surface areas compared to the materials just discussed, and have highly tuneable structures and surface chemistries. In particular, tuning structures in the M2(dobdc)(M = Mg, Mn, Fe, Co, Ni, Zn) series has been an area of focus for CO2 capture. The highest excess CO2 uptake has been demonstrated relatively recently in MOF-200 and MOF-210 structures. MOF-210 has an ultrahigh surface area of 6240 m2 g−1 and a crystal pore volume of 3.6 cm3 g−1.41 The volume-specific internal surface area (2060 m2 cm−3) nears for the ultimate adsorption limit for solid materials. Unlike zeolites which have degraded performance in the presence of water, MOFs show increased chemical stability in the presence of steam. Carbon dioxide storage could be an attractive application for MOFs due to their high capacities, however, several challenges must be overcome, including high material costs and potentially weak mechanical strength.Down-selection of sorbents
Future experimental work is needed to characterize material performance as some key challenges, such as amine degradation for MOFs, low uptake at low pressures for activated carbons, or low uptake in the presence of water for zeolites, may not arise in storage applications. Still, sorbents offer better capacities than compressed gas tanks.For the analysis, we selected MOF-210 which uses an octahedral metal-ion cluster (Zn4O(CO2)6) with 4,4′,4′′-[benzene-1,3,5-triyl-tris(ethyne-2,1-diyl)]tribenzoate (BTE) and biphenyl-4,4′-dicarboxylate (BPDC) linkers to serve as a representative material due to the availability of single-component gas adsorption isotherm data for making predictions on CO2 uptake under higher pressures. MOF-200 has also been demonstrated for CO2 separation, but was not considered for transport and storage due to its instability that limits its cyclic use. At 298 K, the density of gaseous CO2 is 1.8–162 kg m−3 between 0.1–5.6 MPa without adsorbents. The total CO2 density inside the volume increases to 6–436 kg m−3 at the same pressure if MOF-210 pellets are used to facilitate CO2 uptake through adsorption. While at this operational range, the volumetric storage capacity of MOF-210 is larger than compressed CO2, it remains smaller than conventional approaches of storing CO2 at liquid or supercritical state. However, storing CO2 at liquid or supercritical state comes with higher energy demand due to heating/cooling as well as compression requirements. Thus, adsorbents may provide a more energy-efficient storage solution if the CO2 capacity can be further improved.
A theoretical upper bound of CO2 uptake of adsorbents can be estimated by assuming the crystal pore space can be saturated with CO2via condensation, so that the density of CO2 in the pore volume reaches the density of liquid CO2. We refer to this hypothetical case as future MOF. The total CO2 uptake using future MOF is estimated by adjusting the CO2 density inside the pore volume of MOF-210 with a multiplier so that the CO2 density inside the pore volume at 5.6 MPa reaches that of liquid CO2 at around 730 kg m−3. This is equivalent to increasing the absolute quantity of adsorbed CO2 on MOF-210 by 69%. While increasing the uptake capacity of adsorbents is not a trivial task, this simplified example illustrates potential benefits of adsorbent-based CO2 storage.
Chemical solid carbon dioxide carrier potential
Chemical solid CO2 carriers are discussed in this section and classified as alkali carbonates, alkaline earth metal carbonates, and other non-sorbent chemical solids. Properties of the reviewed materials are provided in Table 2. Some of the most abundant elements found in the earth crust are silicon, aluminium, iron, titanium and manganese which makes them potentially interesting for storing and transporting large quantities of CO2 as carbonates. Also, ammonium is a common compound that forms carbonate and bicarbonate. However, none of these carbonates shows promising characteristics for temporary CO2 storage. A more detailed description of these materials and why they are not suitable for temporary CO2 storage is provided in the ESI.†Alkali carbonates
Lithium carbonate only exists in anhydrous form and has a solid density of 2110 kg m−3, which results in a CO2 mass fraction of 59.6 CO2 wt% and a CO2-density of 1257 kg m−3 (in powder form this number is expected to decrease by roughly 50%). When heated to about 800 °C (melting point 723 °C) Li2CO3 starts to decompose into Li2O; however, much higher temperatures are needed for complete conversion.48 After regeneration, Li2O can be used directly to capture CO2 or converted to LiOH which is used as a solid sorbent in submarines to capture CO2 from breathing air49 or can be dissolved in water for gas scrubbing (similarly to industrial gas scrubbing process using NaOH or KOH). Lithium hydroxide is an order of magnitude more soluble than Li2CO3 (12.9 g L−1) which can be recovered by precipitation or crystallization. This property and the formation of metastable LiHCO3 in aqueous solutions under slightly elevated CO2 partial pressures is industrially used in the Quebec process to obtain technical grade LiCO3;50 however, is considered to be energy intensive.48 Other regeneration processes for the release of CO2 after storage include the use of Ca(OH)2 to produce CaCO3 and LiOH. Due to the hygroscopic nature of dry Li2CO3, it needs to be stored in a cool and dry environment and shelf life is limited to about 1–2 years. The chemistry around LiHCO3 is still not well understood and it is believed that the bicarbonate only exists as metastable ion in aqueous solutions.Sodium carbonate has similar properties as Li2CO3. In its anhydrous form it has a solid density of 2540 kg m−3. However, as sodium atoms are more than 3-times heavier than lithium atoms, this results in a 16% reduction in CO2-density to 1055 kg m−3 (again, in powder form this number is expected to decrease by roughly 50%). The corresponding CO2 mass fraction is 41.5 CO2 wt%. While the production and storage of Na2CO3 is relatively straight forward – it can be obtained from the reaction of NaOH with air as commonly done in DAC systems51 – the regeneration of CO2 from Na2CO3 is challenging. Sodium carbonate does not thermally decompose into CO2 and Na2O even at temperatures as high as 2000 °C (very high reaction enthalpy 319.3 kJ mol−1). Instead Ca(OH)2 can be used to restore the NaOH while producing CaCO3 which can be separated and thermally decomposed into CaO and CO2 (more discussion about this reaction later).51 Depending on the regeneration logistics with Ca(OH)2, the CO2 can be stored as either Na2CO3 or CaCO3. The use of Ca(OH)2 for CO2 recovery from the absorbent has been successfully demonstrated and is currently commercially pursued by companies like Carbon Engineering who adopted this process from the pulp and paper industry (Kraft process).20 If Na2CO3 is produced from an aqueous solution, it is likely that hydrated and bicarbonate species form.52 Sodium carbonate is known to form monohydrate, heptahydrate and decahydrate. The co-crystallization of water substantially reduces CO2 mass fraction and CO2-density, which are 15.4 CO2 wt% and 225 kg m−3 for the decahydrate (other hydrates are listed in Table 2). Due to the higher water content, hydrated forms of Na2CO3 are expected to have longer shelf lives. Sodium bicarbonate is an interesting by-product with a density of 2200 kg m−3 and CO2 mass fraction that is higher than that of Na2CO3 (52.4 CO2 wt% if all CO2 is removed from sodium, e.g. using Ca(OH)2 to form CaCO3 which then can be thermally decomposed to obtain CO2). However, when considering the energetically attractive low temperature thermal decomposition of NaHCO3 to Na2CO3 at 100–200 °C, only 50% of the CO2 is released leading to an effective CO2 mass fraction of 26.2 CO2 wt% and a CO2-density of 576 kg m−3. The preparation of NaHCO3 is relatively simple as it can be precipitated by bubbling CO2 through aqueous NaCO3 brines/solutions. This way of producing NaHCO3 has become a significant industrial process in the US as part of Na2CO3 production from Trona.53
Potassium carbonate is the last alkali metal carbonate we consider, as rubidium and caesium are too costly. Anhydrous K2CO3 has a density of 2430 kg m−3, and due to the heavier metal cation, a relatively low CO2 mass fraction of 31.8 CO2 wt%, and a CO2-density of 774 kg m−3. Similar to Na2CO3, K2CO3 does not decompose to CO2 and K2O upon heating, which is an extremely endothermic reaction with a reaction enthalpy of 393.5 kJ mol−1. Thus, K2CO3 is thermally extremely stable; however, it is very hygroscopic and dissolves 1103 g L−1. This strong hygroscopicity typically limits its shelf life to about 1–5 years depending on the storage conditions. The regeneration of K2CO3 can be accomplished in a similar manner to Na2CO3 by using Ca(OH)2 to form KOH and CaCO3 which is a mildly exothermic process and likely to be exergonic at ambient conditions. When K2CO3 is produced in aqueous solutions or directly from KOH, the formation of sesquihydrate is possible (K2CO3·3/2H2O) which reduces the CO2 mass fraction to 26.6 CO2 wt% and the CO2-density to 645 kg m−3. To obtain the anhydrous form and drive off the water, the sesquihydrate can be dried at 200–350 °C. The reaction of gaseous CO2 with aqueous KOH to obtain pure K2CO3 is a commercial process whereby the K2CO3·3/2H2O is separated via crystallization under vacuum with cooling and subsequently dried to achieve a purity of 98–100%.54 In some industrial K2CO3 production processes, KHCO3 is obtained as a precursor.54 For KHCO3, two CO2 regeneration routes exist. If KHCO3 is converted to K2CO3 at around 100–120 °C, the effective CO2 mass fraction of the CO2 storage medium is 22.0 CO2 wt% and the CO2-density is 477 kg m−3. If complete CO2 recovery is considered, i.e. with Ca(OH)2, the effective CO2 mass fraction of the CO2 storage medium is 44.0 CO2 wt% and the CO2-density is 954 kg m−3.
Alkaline earth metal carbonates
Magnesium carbonate is the lightest alkaline earth metal discussed here and has a density of 2958 kg m−3. With a CO2 mass fraction of 52.2 CO2 wt%, this results in an effective CO2 storage density of 1544 kg m−3. The solubility of MgCO3 is low with only 0.1 g L−1, which is certainly an advantage when producing MgCO3 from aqueous solutions. In aqueous solutions MgCO3 can be produced via the reaction with aqueous NaHCO3; however, if reacted with Na2CO3, basic MgCO3 is formed (a hydrated complex of MgCO3 and Mg(OH)2). In the presence of water, MgCO3 may form dihydrate, trihydrate and pentahydrate crystals which have significantly lower CO2-densities as seen in Table 2. To reduce the water content, drying at temperatures above 157 °C is necessary but drying is not complete until a temperature of 300 °C is reached.55 Alternatively, the anhydrous salt can be produced from a Mg(OH)2 slurry directly if high CO2 partial pressures are available (0.4–0.5 MPa), which is the preferred industrial process for high purity MgCO3 production. This pathway includes the formation of aqueous bicarbonate under moderate temperatures and pressures before being vacuum dried. Magnesium bicarbonate is not a stable side-product, only exists in aqueous solution, and decomposes into CO2 and H2O during drying while forming MgCO3.56 The regeneration of the magnesium carrier and release of CO2 can be achieved by thermal decomposition over a temperature range of 360–800 °C (800 °C are needed for complete conversion).56 In the industrial calcination process for the production of MgO from MgCO3 minerals, temperatures between 700–2000 °C are used. However, lower calcination temperatures of 540–800 °C lead to more reactive MgO which is more suitable for CO2 adsorption.57 The reaction enthalpy with 116.9 kJ mol−1 is relatively low and presents an interesting “low energy” pathway for CO2 storage compared to some of the other carbonate carriers. Other regeneration routes include acid treatment with HCl or H2SO4. A recent review offers insights into several modifications to MgO that can improve its use as an adsorbent for CO2, including the use of promoters, hybrid materials, and amine functionalization; however, benefits such as cyclic stability are more relevant for CO2 capture.58Calcium carbonate has a higher molecular weight than MgCO3. While the density of CaCO3 is similar to MgCO3, the higher mass of calcium leads to a lower CO2 mass fraction and CO2-density with 44.0 CO2 wt% and 1244 kg m−3 respectively. Calcium carbonate is highly insoluble, only 0.013 g dissolve in 1 L of water at 25 °C. Furthermore, CaCO3 does not form hydrates even in aqueous solutions. A monohydrate of CaCO3 exists but does not form under normal circumstances (forms via a Mg-rich amorphous calcium carbonate precursor)59 and the hexahydrate is only stable below 7 °C.60 Calcium carbonate is an intermediate product in DAC systems where aqueous NaOH is used to capture the CO2 followed by the reaction of solid Ca(OH)2 with aqueous Na2CO3 to form solid CaCO3.51 Calcium carbonate is easy to separate from the NaOH solution due to its low solubility and lack of bicarbonate and hydrate side reactions. Thus, when heating CaCO3 all energy goes towards the actual regeneration of CaCO3 and not into reversing side reactions. The regeneration of CaCO3 occurs at temperatures greater than 650 °C (industrial quicklime processes use >900 °C to achieve appreciable reaction rates)61,62 and the reaction enthalpy of the reaction is 178.4 kJ mol−1. This is substantially higher than the reaction enthalpy of MgCO3 and one might wonder why DAC systems use Ca instead of Mg. Besides the higher solubility of MgCO3 and formation of hydrates (drying hydrates would increase energy demand), Mg(OH)2 is weaker than Ca(OH)2 and the reaction of Mg(OH)2 with aqueous Na2CO3 is endergonic (ΔGMg = 34.8 kJ mol−1vs. ΔGCa = −18.4 kJ mol−1) and would not happen spontaneously at ambient conditions.
Other solids
Oxalic acid is a commercially available solid dicarboxylic acid with the formula H2C2O4. Oxalic acid has a density of 1900 kg m−3 and an extremely high CO2 mass fraction of 97.8 CO2 wt% leading to a remarkably high CO2-density of 1857 kg m−3. In aqueous solution H2C2O4 may form a dihydrate crystal which can be reduced to its anhydrous form by drying at modest temperatures of 100 °C.63 These properties make it a very interesting compound for CO2 transport and storage.While there are other carboxylic acids, such as citric acid that are interesting from a CO2 storage perspective, the challenge is to find reaction pathways for the production of these chemicals using CO2. At the same time a viable CO2 regeneration pathway is necessary to recover and reuse the carrier if a non-ubiquitous reactant is used as carrier. Oxalic acid is a compound that possesses both of these characteristics. Traditionally, H2C2O4 is produced from carbohydrates using nitric acid, a process which was discovered in 1776. Nevertheless, commercial processes for a CO2-based synthesis exist via the reverse water gas shift reaction, the dialkyl oxalate process and traditional acidification; however, recently new CO2-based reaction pathways have been discovered. Oxalate can be produced directly from CO2via electrochemical routes using aprotic solvents with faradaic efficiencies up to 80%.64 Alternatively, oxalate can be produced from CO (e.g. from CO2 electrolysis), biomass or the coupling of formate (which can be produced from CO or CO2). The oxalate can then be converted to H2C2O4via traditional acidification or electrodialysis.65 When H2C2O4 is heated to about 125–175 °C it decomposes to CO2 and formic acid (ΔH = 11.4 kJ mol−1).66 Formic acid can be further decomposed to CO2 and H2 at ambient temperatures using catalysts (ΔH = 42.9 kJ mol−1) as described in the following sections.67 Oxalic acid is already a C2 molecule and can become a new base chemical for polymers, enabling numerous new CO2 utilization pathways that offer long term storage.65
Down-selection of solids
Among the various solids evaluated, NaHCO3, MgCO3, CaCO3 and H2C2O4 stand out as potentially attractive CO2 storage media. They are stable at ambient conditions and exhibit some of the highest CO2 mass fractions and CO2-densities. If a concentrated CO2 stream is available NaHCO3 is relatively easy to precipitate in aqueous NaOH solutions without any hydrate by-products. For regeneration, NaHCO3 can be integrated with current DAC systems that rely on a NaOH–Ca(OH)2 cycle. If DAC systems run 100% on renewables like wind and solar, this external source of NaHCO3 can be added to bridge intermittencies and increase the overall capacity factor of the DAC systems while improving the economies of scale. Similarly, CaCO3 can be used in combination with DAC systems. Calcium carbonate has the advantage that it can be used with lower CO2 partial pressures and NaOH. Magnesium carbonate is really attractive due to its low regeneration energy requirement and might be an interesting alternative if high CO2 partial pressures are available. One of the most exciting CO2 storage chemicals is H2C2O4 due to its extremely high CO2 mass fraction, extremely high CO2-density as well as its chemistry. The CO2-density of H2C2O4 exceeds the density of liquid CO2; however, it is important to understand that in powder form this density is likely to decrease by approximately 50%. Nevertheless, even in powder form its density is still very attractive for transportation and storage applications.Liquid organic carbon dioxide carriers
Liquid organic carriers with high carbon contents are discussed in this section and properties are included in Table 2. Urea was reviewed but does not show promise as a candidate. Additional details on urea are provided in the ESI.†Methanol
Methanol stores CO rather than CO2, and would require additional oxygen if it was necessary to produce CO2 upon decommissioning CO2 storage systems. Methanol, which has a H2 content of 12.5 H2 wt% (99 kg m−3), can be stored as a liquid at ambient conditions, and offers a CO content of 87.4 CO2 wt% (which for the sake of comparison can be translated into 137.4 CO2 wt% and 1088 kg m−3). Storing CO2via methanol has been demonstrated commercially via different pathways, such as direct catalytic conversion using H2, conversion via hydrides, and integration of amine-captured CO2 or biomass conversion.68–70 Recently methanol production using CO2 and green H2 has been demonstrated by Carbon Recycling International, making it one of the most mature liquid carriers for both CO2 and H2.71 Recent reviews highlight the ways in which techniques such as photochemical, electrochemical,72 and biological processes can increase the rate, efficiency, and selectivity of conversion.73 For methanol or other alcohols, CO2 can be recovered during the decommissioning of a storage facility, or upon delivery, in a controlled manner.74 For example, steam reforming for dehydrogenation under elevated pressures (2 MPa) and temperatures (250–250 °C) has been widely investigated (eqn (5)).75 As evident in the reaction, the fixation process for methanol forms H2O as a by-product, and its regeneration process requires H2O. Dehydrogenation temperatures vary widely in literature and have been reported as high as 450 °C in commercial systems.76| CH3OH + H2O ⇌ CO2 + 3H2 | (5) |
Formic acid and formate salts
Formic acid and formate salts are stable H2 carriers that can be used to store and transport CO and CO2. Formic acid has a high CO2 capacity (95.6 CO2 wt% and 1152 kg m−3) and low H2 capacity (4.4 H2 wt% and 53 kg m−3). It is already stored and transported in bulk quantities for a number of industrial uses, particularly in Europe, and tends to be transported in a nonpure form to eliminate issues relating to venting and flammability. With proper storage, formic acid can have a shelf life of two years or more.77 The potential safety and stability of formic acid is highlighted by companies such as OCOchem that are developing catalytic approaches for converting CO2 to products under mild conditions.76 Formate salts (HCOOM; M = Li+, Na+, K+, Cs+, NH4+) use a formate bicarbonate cycle for storage.78 Formate salts will have different features depending on the cation, as noted in Table 2. For example, when the cation is sodium, CO2 capacity is 64.7 CO2 wt% with CO2-density of 1229 kg m−3 in comparison to when the cation is potassium and the CO2 capacity is 52.3 CO2 wt% with CO2-density of 994 kg m−3. The direct hydrogenation of CO2 into FA or FS is challenging due to the unfavorable phase change (eqn (6)), but the thermodynamic preference can be altered when the reaction is carried out in aqueous forms, with ΔG of −4 kJ mol−1.79 There are multiple pathways of storing CO or CO2 in the form of FA or FS other than direct hydrogenation (e.g. via methanol), but commercialization requires improvements to selectivity, product separations and stability. As noted in a recent review by Duarah et al., emerging electro-chemical pathways have been developed to improve these processes under ambient conditions, with faradaic efficiencies achieved above 90%.80 One advantage of FA and FS is that they can be dehydrogenated under mild conditions or used directly upon decommissioning of a storage facility to manufacture a wide range of chemicals.81| CO2(g) + H2(g) ⇌ H2CO2(l) ΔG (298 K) = +32.9 kJ mol−1 | (6) |
Down-selection of LOHC
Among the liquid carriers that can simultaneously store CO2 and H2, methanol and FA are the most promising as they have relatively high technology maturities and favourable gravimetric and volumetric CO2 densities. Synthesis of FS has largely been demonstrated for CO and not CO2, and justifies us not evaluating it further in the following analysis. The shelf life of these liquid carriers may be a few years even if stored properly, which is short compared with most of the solid carriers listed in Table 2 and may make them more viable for transportation applications. Additionally, unlike sorbents and solid carriers, LOHC require a deformation of CO2, which requires substantial amounts of energy. We decided to include these materials in our analysis as there have been major advancements in reactor design as well as direct electroreduction of CO2 focused on lowering the cost of CO2 utilization,82,83 which will also lower the cost of LOHC for CO2 storage. For example, Sun et al. review the promise of tuning two-dimensional nanosheets to avoiding the use of catalysts based on costly noble metals.84 Moving towards an electrochemical approach may improve overall synergies with DAC facilities with dedicated renewables or established grid connections.Storage analysis
The importance of gravimetric capacity becomes apparent when estimating the quantity of material required to store the same amount of CO2 (Fig. 4). Oxalic acid and FA require very little material per unit of CO2 stored, while NaHCO3, when removing 0.5 CO2 per Na, requires substantial amounts of material. Methanol technically only stores CO, so an additional amount of oxygen is required to provide CO2. Volumetric capacity and the phase of the material and flammability of material are key variables influencing the physical footprint of the system. At the pilot scale, FA and methanol have the largest footprint per unit of CO2 stored due to the space required for safety, despite their good gravimetric capacities. NFPA 30 requires spatial separation of large-scale storage of Class 1 flammable liquids of at least 15 meters, and we conservatively use this value to estimate safety spacing for methanol and formic acid. Liquid CO2 has the smallest footprint due to its high density and relatively small assumed safety spacing requirement. It offers high densities, but requires thermal insulation and liquefaction has a high energy consumption. Liquified CO2 is energetically costly and subject to boil-off issues, making it unsuitable for long-term CO2 storage.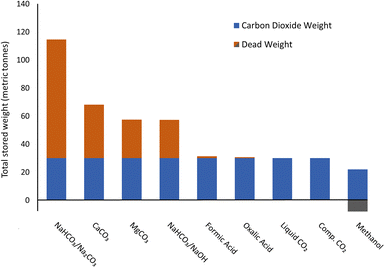 | ||
| Fig. 4 Quantity of carrier weight required to store 30 tCO2. Carbon dioxide weight is excluded for methanol as it stores CO rather than CO2. | ||
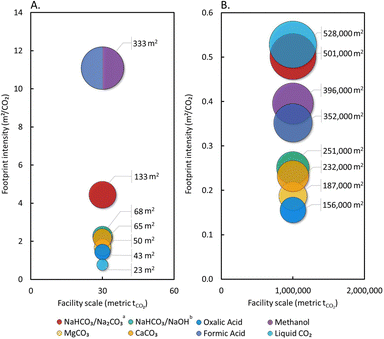
Results are substantially different at the regional hub scale (Fig. 5), as liquid CO2 tanks do not achieve great economies of scale (110 m3 compared to 28 m3 capacity for the pilot facility) relative to other materials. The poor gravimetric capacity of NaHCO3 does not outweigh the great economies of scale achieved with bulk powder silos, and it has a similar footprint intensity as liquid CO2. Flammable liquid storage also scales well with capacity, and methanol and FA require less than 60 storage vessels, compared with the bulk powder materials that require between 100–400, and liquid CO2 which requires nearly 8260 tanks. Oxalic acid, which offers the smallest land footprint for a regional hub, would still require a space the size of approximately 22 soccer fields (7140 m2) or 6.5% of an average landfill in the United States. Carbonate salts require slightly more land area at the regional hub scale than oxalic acid (20–61% more), but still perform far better than methanol and FA at all scales studied.
Transportation analysis
Considering B-train trucks, we find that the quantity of CO2 that can be transported per vehicle is limited by weight, except for sorbents where the volume of the tanks limited CO2 delivery per truck (Table S2, ESI†). Methanol, formic acid, and oxalic acid can deliver more CO2 per truck than liquified CO2 trucks, and all return empty except for oxalic acid which has very little dead weight. Liquid chemical carriers tend to outperform solid carriers due to their high carbon contents and good bulk densities. It is notable that for methanol, the mass of CO2 transported per tonne of methanol is 1.38 tonne, which is higher than the mass of the methanol required. This is due to the fact that additional oxygen (when reforming in the form of steam) is required when releasing CO2 from methanol. Oxalic acid has an extremely high gravimetric capacity of 97.8 CO2 wt%, which leads to similar transport characteristics as liquefied CO2. In comparison, the weight of the returning trucks for other carbonate salts, can be almost as heavy as a full truck which increases energy requirement of CO2 transport. MOFs do not have as significant a deadweight, but are limited in volume as it relies on compressing gaseous CO2.Moving 1 million tonnes of CO2 from a hypothetical plant annually through methanol, formic acid, oxalic acid, and liquid CO2 would require operating 39–56 B-trains each day. Moving the same amount of CO2via MOF or future MOF would need 114 or 71 B-trains daily, respectively. Moving CO2 with other solids can require up to 207 B-trains daily due to limited capacity. Just comparing across a single round-trip, methanol can deliver the most CO2, with only 39 trucks required to move daily CO2 production in the regional hub scenario. No truck transportation technology can match the low transportation energy of supercritical pipelines.
Energy consumption per truck is a function of weight and increases linearly with round-trip distance (31–40 MJ per km-truck). The total transport energy for trucking shown in Fig. 6 accounts for diesel fuel consumed by a truck fleet moving 1 million tonnes of CO2. Methanol, formic acid, and oxalic acid can deliver CO2 with diesel consumption close to or less than trucking liquified CO2. Emissions associated with diesel consumption derived using our approach were between 45 and 57 grams of CO2 equivalents per tonne-km, which aligns well with emission factors reported for long-haul trucks (57 gCO2 per t-km).85 Emissions associated with diesel consumption and with recompression for pipelines will have a negligible impact, especially over expected transportation distances (<500 km). The emissions breakeven distance shows that pipeline is the most efficient mode of CO2 transport, followed by methanol, formic acid, oxalic acid, liquid CO2 and future MOF trucking. Lowering the speed of trucks from 100 to 80 kg h−1 reduces the transport energy intensity by 15–20% while increasing the breakeven distance by 19–23% in all carriers considered. Smaller changes were observed for solid carriers due to the heavy weight of the returning trucks. However, the relative orders amongst carriers in terms of energy intensity and breakeven distances are unaffected. The breakeven distance of methanol, the best-performing carrier, is 37.8 km when operating trucks at 80 km h−1, which is still considerably shorter than 63.4 km of CO2 pipeline. Trucking CO2 with MOF will create more emissions during delivery compared to conventional means even when its uptake capacity can be increased in the future. This is due to the dead weight and volume occupied by MOF pellets limiting the quantity of the transported CO2. The quantity of CO2 transported using a truck filled with MOFs can be further increased by lowering temperature or increasing pressure during transport, but these strategies were not considered in this analysis due to the lack of data.
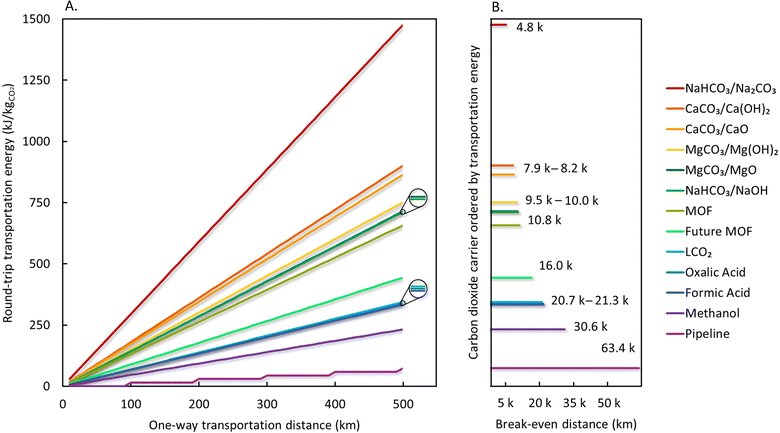 | ||
| Fig. 6 Truck driving distances and (A) estimated energy consumed for transport of CO2 by way of gas, liquid, and solid carriers, and (B) break-even distance before more CO2 is emitted during transportation than is stored. Values are presented in Table S2 (ESI†). | ||
Pathways for capturing CO2, converting it for storage, loading and unloading a vehicle, and recovering the CO2 have very different processes involved, and estimating energy consumption is outside the scope of this analysis. For example, the relatively low diesel consumption required for methanol and FA transport is likely to be overshadowed by the energy intensive processes required for hydrogenation and dehydrogenation, unless these processes can rely on low-carbon electricity. There are concepts to take advantage of high decarbonation temperatures in thermal energy storage, e.g. in thermal solar plants,86 which might present an interesting integration of systems that improves life-cycle performance. Upfront compression requirements for sorbent transportation technologies at the scale of a refuelling station must be compared with relevant scale systems for liquified CO2 or supercritical pipeline CO2, as the behaviour of CO2 varies substantially from ideal in these ranges.35,87,88 Whether liquid carriers outcompete sorbents or solid carriers on a carbon emission or energy intensity basis will need to be assessed through life-cycle assessment that determines impacts from CO2 transportation and from H2 transportation.
Concluding remarks
In this analysis, we reviewed several classes of materials for their viability as storage materials for CO2. Material classes were identified based on carbonate chemistry in nature and industry, consideration in CO2 capture systems, and consideration for H2 storage. Materials were screened based on their ability to offer stable but reversible storage over relevant time scales, their theoretical gravimetric and volumetric storage capacities, compatibility with existing solid, liquid, and gaseous infrastructure currently used in industry, and availability of experimental data describing performance. The quantity of material and subsequent number of trucks and storage units were estimated for storage capacities of 30 tCO2 and 1 MtCO2 and capture rates of 10 tpy and 1 Mtpy.While many materials derived from CO2 have been ruled out as options for permanent sequestration of CO2 due to limited compound stability under ambient storage conditions, the concept of temporary storage in the form of an environmentally friendly cheap compound to bridge temporal, spatial and economic scale gaps may not be that absurd. This is especially true considering the dramatically poor scaling of liquid CO2 stationary storage compared with solid chemical carriers (Fig. 5). Our first-order evaluation of materials for CO2 storage and transportation suggests that the land footprint and number of trucks required to manage CO2 in the absence of geologic storage and pipelines is large, but improved over gaseous and liquified CO2 storage. We also find that the greenhouse gas emissions associated with transporting CO2 by diesel truck is negligible but still greater than emissions associated with recompression in pipelines. Both CO2 pipelines and liquefaction facilities are capital-intensive infrastructure to build, especially at small annual capacities, and would require CO2 sources and sinks to remain relatively fixed. Other storage and transport options – such as chemicals and sorbents – may offer flexibility to emerging distributed and highly mobile CO2 capture technologies that are positioned to tackle our climate crisis.
Carbonates are possible candidates for stationary storage but it is unclear whether the long shelf life of the material will offset challenges associated with acquiring the large initial quantity of carrier material required (Fig. 4). Formic acid, oxalic acid, and methanol provide higher densities than cryogenic liquid CO2, with less starting material required than carbonates. Tradeoffs between material requirements (Fig. 4), land requirements (Fig. 5), storage duration (Table 2) and energy consumption (Fig. 6) will all influence cost. While it is not within the scope of this study to estimate which method may be most economic for long duration CO2 storage, we offer the following thoughts on cost. First, the maturity, long storage duration, and high density of carbonates could lead to low costs. Second, the ultra-high storage capacity of oxalic acid, along with the potential to be readily produced from CO2 and water under mild conditions could also lead to low costs. The same is true for formic acid, and both acids have gained increased attention as valuable products of electroreduction of CO2 at ∼400 $ per t market value.89 Third, the cost of LOHC that require hydrogen will be highly dependent on the price of H2. Fourth, for some of our top candidates, we wish to highlight an exciting benefit, which would be the direct capture of CO2 into liquid or solid materials for storage without the need for CO2 compression, examples of which are noted by Gutierrez-Sanchez et al. for formic acid and methanol.90 Future experimental and engineering design work are needed to advance the integration of storage materials into CO2-capture concepts, and we hope this study will draw attention to this research gap.
Finally, we identify multiple pathways for low-energy recovery of CO2 from storage materials. This is important for recycling solid and liquid carrier materials for reuse or sale. In a circular economy there will be many different CO2-sources and technologies utilized to control CO2 emissions. Thus, it is likely that various CO2 storage media will find use in a circular economy upon decommissioning the CO2 storage system.91 At the same time, given the scale of storage required, it is essential to find alternatives to materials with high supply risk and cost. The issue of material criticality for clean energy systems has led to national investments in domestic production of materials such as lithium. It is notable that our top candidates for storage do not rely on critical materials, however, we do note that sorbents may require cobalt, nickel, or manganese, and that some catalysts for LOHC use critical materials such as iridium. Considering the potential market growth of liquid H2 carriers92,93 with careful planning and road mapping, integrating H2 and CO2 transportation may likely have economic and environmental benefits.10 We envision scenarios where large potential off-takers of CO2, such as the cement industry, use H2 to provide clean heat and electricity.
The ability to recycle solid sorbent materials such as MOFs is unknown, and their use may be limited to CO2 capture and transportation applications where they will undergo frequent cycling. While the projected future cost of MOFs for H2 and natural gas storage ranges between $10–71 per kg-sorbent,94 some of the best performing MOFs for CO2 are unlikely to achieve such low costs. In comparison, activated carbon sells on markets such as Alibaba at as low as $0.45–6 per kg-sorbent. As such, the use of MOFs for small stationary storage, or short duration storage with high cycling, such as for transportation, is more feasible than large-scale bulk storage. It is important to note that our sorbent-based transportation analysis was limited by the lack of high-pressure CO2 adsorption data points; further measurement of MOFs, activated carbons, and zeolites at high-pressure environment relevant to CO2 storage would reduce the technical uncertainty of sorbent-based storage and transportation.
In conclusion, material emplacement of CO2, or material-based storage at large scales, should be taken seriously as a CO2 storage solution as it can be: (1) deployed today; (2) geographically agnostic; (3) used as a form of bulk CO2 transportation, avoiding the construction of pipelines; and (4) decommissioned and monitored. Carbon dioxide storage and transportation are essential components of CO2 removal, and the discovery and development of advanced storage technologies could play a critical role in enabling the deployment of capture technologies in the next decade. As summarized in this study, the benefits and challenges of each material reviewed are highly specific, and will require further analysis to verify storage durability and to identify the most cost-effective solutions. We encourage the community of scientists advancing the use of materials for CO2 capture and for utilization to apply their knowledge to this pressing application.
Author contributions
H. B. conceptualized and conceived the analysis. H. B., T. L., and F. R. developed the methodology. All authors conducted the investigation, curated the data, wrote the original draft, and reviewed and edited the paper. H. B., F. R., and T. L. visualized the results. H. B. supervised and obtained funding and resources for the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge support from the Laboratory-Directed Research and Development program at Lawrence Berkeley National Laboratory (LBNL), and funding from the U.S. Department of Energy Office of Basic Energy Sciences, under Contract Number DE-AC02-05CH11231 with LBNL. The views and opinions of the authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof. Neither the United States Government nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would not infringe privately owned rights. We gratefully thank the two reviewers, Hiroyasu Furukawa for his insights on metal organic framework performance, and Damien Jullié for his constructive review that led to the development of Fig. 1.References
- E. Rubin and H. De Coninck, IPCC special report on carbon dioxide capture and storage, TNO Cost Curves CO2 Storage, Part 2, UK Cambridge Univ. Press, 2005 Search PubMed.
- S. D. Supekar, T. H. Lim and S. J. Skerlos, Costs to achieve target net emissions reductions in the US electric sector using direct air capture, Environ. Res. Lett., 2019, 14, 084013 CrossRef CAS.
- O. Edenhofer, Climate Change. Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Technical Report, 2014.
- GCCSI. Global status report of CCS: 2022. https://status22.globalccsinstitute.com/(2022).
- P. S. Ringrose and T. A. Meckel, Maturing global CO2 storage resources on offshore continental margins to achieve 2DS emissions reductions, Sci. Rep., 2019, 1–10 CAS.
- E. Abramson, D. McFarlane and J. Brown, Transport Infrastructure for Carbon Capture and Storage, Whitepaper on Regional Infrastructure for Midcentury Decarbonization, 2020, https://www.betterenergy.org/wp-content/uploads/2020/06/GPI_RegionalCO2Whitepaper.pdf.
- IEA, CCUS around the world, https://www.iea.org/reports/ccus-around-the-world (2021).
- S. E. Baker, et al., Getting to Neutral: Options for Negative Carbon Emissions in California, http://www.osti.gov/servlets/purl/1597217/ (2019) DOI:10.2172/1597217.
- M. Aresta, Carbon dioxide recovery and utilization, Springer Science & Business Media, 2013 Search PubMed.
- S. M. Jarvis and S. Samsatli, Technologies and infrastructures underpinning future CO2 value chains: A comprehensive review and comparative analysis, Renewable Sustainable Energy Rev., 2018, 85, 46–68 CrossRef CAS.
- T. Lim, B. R. Ellis and S. J. Skerlos, Mitigating CO2 emissions of concrete manufacturing through CO2-enabled binder reduction, Environ. Res. Lett., 2019, 14, 114014 CrossRef CAS.
- Board, Ocean Studies, and National Academies of Sciences, Engineering, and Medicine, Negative emissions technologies and reliable sequestration: A research agenda, 2019 DOI:10.17226/25259.
- L. A. Bullock, A. Yang and R. C. Darton, Kinetics-informed global assessment of mine tailings for CO2 removal, Sci. Total Environ., 2022, 808, 152111 CrossRef CAS PubMed.
- B. C. O’Neill, et al., A new scenario framework for climate change research: The concept of shared socioeconomic pathways, Clim. Change, 2014, 122, 387–400 CrossRef.
- J. Rogelj, et al., Scenarios towards limiting global mean temperature increase below 1.5 °C, Nat. Clim. Change, 2018, 84(8), 325–332 CrossRef.
- IEA. CO2 Capture and Utilisation, https://www.iea.org/reports/co2-capture-and-utilisation (2022).
- M. Ozaki and T. Ohsumi, CCS from multiple sources to offshore storage site complex via ship transport, Energy Procedia, 2011, 4, 2992–2999 CrossRef.
- B. Y. Yoo, S. G. Lee, K. P. Rhee, H. S. Na and J. M. Park, New CCS system integration with CO2 carrier and liquefaction process, Energy Procedia, 2011, 4, 2308–2314 CrossRef.
- T. Barrett, DNV awards KNCC AiP for high pressure LCO2 transport concept, (2022).
- D. W. Keith, G. Holmes, D. St. Angelo and K. Heidel, A Process for Capturing CO2 from the Atmosphere, Joule, 2018, 2, 1573–1594 CrossRef CAS.
- S. Fuss, et al., Negative emissions—Part 2: Costs, potentials and side effects, Environ. Res. Lett., 2018, 13, 063002 CrossRef.
- S. Jackson and E. Brodal, A comparison of the energy consumption for CO2 compression process alternatives, IOP Conf. Ser.: Earth Environ. Sci., 2018, 167, 012031 CrossRef.
- E. De Lena, et al., Techno-economic analysis of calcium looping processes for low CO2 emission cement plants, Int. J. Greenhouse Gas Control, 2019, 82, 244–260 CrossRef CAS.
- M. P. S. Santos, V. Manovic and D. P. Hanak, Unlocking the potential of pulp and paper industry to achieve carbon-negative emissions via calcium looping retrofit, J. Cleaner Prod., 2021, 280, 124431 CrossRef CAS.
- Y. Sun, et al., Decarbonising the iron and steel sector for a 2 °C target using inherent waste streams, Nat. Commun., 2022, 131(13), 1–8 Search PubMed.
- N. McQueen, P. Kelemen, G. Dipple, P. Renforth and J. Wilcox, Ambient weathering of magnesium oxide for CO2 removal from air, Nat. Commun., 2020, 111(11), 1–10 Search PubMed.
- K. Sumida, et al., Carbon dioxide capture in metal–organic frameworks, Chem. Rev., 2011, 112, 724–781 CrossRef PubMed.
- R. Veneman, W. Zhao, Z. Li, N. Cai and D. W. F. Brilman, Adsorption of CO2 and H2O on supported amine sorbents, Energy Procedia, 2014, 63, 2336–2345 CrossRef CAS.
- E. Southall and L. Lukashuk, Potential Deployment and Integration of Liquid Organic Hydrogen Carrier Technology within Different Industries: Liquid organic hydrogen carrier technology to support on demand hydrogen supply and energy storage, Johnson Matthey Technol. Rev., 2022, 66, 259–270 CrossRef CAS.
- A. Anastasopoulou, Technoeconomic Analysis of Metal–Organic Frameworks for Bulk Hydrogen Transportation, Energy Environ. Sci., 2021, 14, 1083–1094 RSC.
- J. Galos, M. Sutcliffe, D. Cebon, M. Piecyk and P. Greening, Reducing the energy consumption of heavy goods vehicles through the application of lightweight trailers: Fleet case studies, Transp. Res. Part D Transp. Environ., 2015, 41, 40–49 CrossRef.
- E. W. Lemmon, I. H. Bell, M. L. Huber and M. O. McLinden, Thermophysical Properties of Fluid Systems. in Hemistry WebBook, NIST Standard Reference Database Number 69, ed. P. J. Linstrom and W. G. Mallard, 2022 Search PubMed.
- CO2 pipeline infrastructure, http://www.globalccsinstitute.com/archive/hub/publications/120301/CO2-pipeline-infrastructure.pdf (2013).
- S. P. Peletiri, N. Rahmanian and I. M. Mujtaba, CO2 Pipeline Design: A Review, Energies, 2018, 11, 2184 CrossRef.
- V. E. Onyebuchi, A. Kolios, D. P. Hanak, C. Biliyok and V. Manovic, A systematic review of key challenges of CO2 transport via pipelines, Renewable Sustainable Energy Rev., 2018, 81, 2563–2583 CrossRef CAS.
- G. Heddle, H. Herzog and M. Klett, The economics of CO2 storage, 2003 Search PubMed.
- S. Deutz and A. Bardow, Life-cycle assessment of an industrial direct air capture process based on temperature–vacuum swing adsorption, Nat. Energy, 2021, 62(6), 203–213 CrossRef.
- B. Omell and D. Bhattacharyya, Carbon Capture Simulation Initiative (CCSI) Compressor User Manual, 2018.
- Inventory of U.S. Greenhouse Gas Emissions and Sinks: Tables A-32, A-38, and A-232, http://www.eia.gov/environment/emissions/CO2_vol_mass.php.
- EIA-923 emissions survey data, http://www.eia.gov/tools/faqs/faq.php?id=74&t=11 (2021).
- H. Furukawa, et al., Ultrahigh porosity in metal-organic frameworks, Science, 2010, 329, 424–428 CrossRef CAS PubMed.
- S. Himeno, T. Komatsu and S. Fujita, High-Pressure Adsorption Equilibria of Methane and Carbon Dioxide on Several Activated Carbons, J. Chem. Eng. Data, 2005, 50, 369–376 CrossRef CAS.
- H. G. T. Nguyen, et al., A reference high-pressure CO2 adsorption isotherm for ammonium ZSM-5 zeolite: results of an interlaboratory study, Adsorption, 2018, 24, 531–539 CrossRef CAS PubMed.
- Y. Wang and M. D. LeVan, Adsorption Equilibrium of Carbon Dioxide and Water Vapor on Zeolites 5A and 13X and Silica Gel: Pure Components, J. Chem. Eng. Data, 2009, 54, 2839–2844 CrossRef CAS.
- S. M. Amorim, M. D. Domenico, T. L. P. Dantas, H. J. José and R. F. P. M. Moreira, Lithium orthosilicate for CO2 capture with high regeneration capacity: Kinetic study and modeling of carbonation and decarbonation reactions, Chem. Eng. J., 2016, 283, 388–396 CrossRef CAS.
- J. Zhang, R. Singh and P. A. Webley, Alkali and alkaline-earth cation exchanged chabazite zeolites for adsorption based CO2 capture, Microporous Mesoporous Mater., 2008, 111, 478–487 CrossRef CAS.
- C. F. Martín, et al., On the limits of CO2 capture capacity of carbons, Sep. Purif. Technol., 2010, 74, 225–229 CrossRef.
- C. W. Kamienski, D. P. McDonald, M. W. Stark and J. R. Papcun, Lithium and lithium compounds, Kirk- Othmer Encyclopedia of Chemical Technology, 2000.
- H. M. Baek, Possibility of military service-regeneration of LiOH for submarines and improvement in CO2 scrubbing performance of LiOH canisters, J. Adv. Mar. Eng. Technol., 2022, 46, 115–121 CrossRef.
- F. Meng, J. McNeice, S. S. Zadeh and A. Ghahreman, Review of Lithium Production and Recovery from Minerals, Brines, and Lithium-Ion Batteries, Miner. Process. Extr. Metall. Rev., 2021, 42, 123–141 CrossRef CAS.
- A. Gambhir and M. Tavoni, Direct Air Carbon Capture and Sequestration: How It Works and How It Could Contribute to Climate-Change Mitigation, One Earth, 2019, 1, 405–409 CrossRef.
- M. Mahmoudkhani and D. W. Keith, Low-energy sodium hydroxide recovery for CO2 capture from atmospheric air-Thermodynamic analysis, Int. J. Greenhouse Gas Control, 2009, 3, 376–384 CrossRef CAS.
- C. Thieme, Sodium Carbonates, Ullmann's Encyclopedia of Industrial Chemistry, 2000, DOI:10.1002/14356007.A24_299.
- H. Schultz, G. Bauer, E. Schachl, F. Hagedorn and P. Schmittinger, Potassium Compounds, Ullmann's Encyclopedia of Industrial Chemistry, 2000 DOI:10.1002/14356007.A22_039.
- V. Vágvölgyi, et al., Conventional and controlled rate thermal analysis of nesquehonite Mg(HCO3)(OH)·2(H2O), J. Therm. Anal. Calorim., 2008, 942(94), 523–528 CrossRef.
- R. C. Ropp, Group 14 (C, Si, Ge, Sn, and Pb) Alkaline Earth Compounds, Encycl. Alkaline Earth Compd., 2013, 351–480, DOI:10.1016/B978-0-444-59550-8.00005-3.
- R. C. Ropp, Group 16 (O, S, Se, Te) Alkaline Earth Compounds, Encycl. Alkaline Earth Compd., 2013, 105–197, DOI:10.1016/B978-0-444-59550-8.00003-X.
- A. H. Ruhaimi, M. A. A. Aziz and A. A. Jalil, Magnesium oxide-based adsorbents for carbon dioxide capture: Current progress and future opportunities, J. CO2 Util., 2021, 43, 101357, DOI:10.1016/j.jcou.2020.101357.
- J. D. Rodriguez-Blanco, S. Shaw, P. Bots, T. Roncal-Herrero and L. G. Benning, The role of Mg in the crystallization of monohydrocalcite, Geochim. Cosmochim. Acta, 2014, 127, 204–220 CrossRef CAS.
- J. M. Huggett, B. P. Schultz, D. J. Shearman and A. J. Smith, The petrology of ikaite pseudomorphs and their diagenesis, Proc. Geol. Assoc., 2005, 116, 207–220 CrossRef.
- G. S. Kumar, A. Ramakrishnan and Y.-T. Hung, Lime Calcination, Adv. Physicochem. Treat. Technol., 2007, 611–633, DOI:10.1007/978-1-59745-173-4_14.
- M. Bilton, A. P. Brown and S. J. Milne, Investigating the optimum conditions for the formation of calcium oxide, used for CO2 sequestration, by thermal decomposition of calcium acetate, J. Phys. Conf. Ser., 2012, 371, 012075 CrossRef.
- ICSC 0707 - Oxalic Acid Dihydrate.
- M. König, S. H. Lin, J. Vaes, D. Pant and E. Klemm, Integration of aprotic CO2 reduction to oxalate at a Pb catalyst into a GDE flow cell configuration, Faraday Discuss., 2021, 230, 360–374 RSC.
- E. Schuler, M. Demetriou, N. R. Shiju and G. J. M. Gruter, Towards Sustainable Oxalic Acid from CO2 and Biomass, ChemSusChem, 2021, 14, 3636–3664 CrossRef CAS PubMed.
- G. Lapidus, D. Barton and P. E. Yankwich, Kinetics and stoichiometry of the gas-phase decomposition of oxalic acid, J. Phys. Chem., 1964, 68, 1863–1865 CrossRef CAS.
- K. Tedsree, et al., Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst, Nat. Nanotechnol., 2011, 6, 302–307 CrossRef CAS PubMed.
- S. Kar, A. Goeppert and G. K. S. Prakash, Combined CO2 Capture and Hydrogenation to Methanol: Amine Immobilization Enables Easy Recycling of Active Elements, ChemSusChem, 2019, 12, 3172–3177 CrossRef CAS PubMed.
- E. Alberico and M. Nielsen, Towards a methanol economy based on homogeneous catalysis: methanol to H2 and CO2 to methanol, Chem. Commun., 2015, 51, 6714–6725 RSC.
- R. Andika, et al., Co-electrolysis for power-to-methanol applications, Renewable Sustainable Energy Rev., 2018, 95, 227–241 CrossRef CAS.
- Carbon dioxide-to-methanol catalyst ignites ‘fuel from air’ debate | Research | Chemistry World, https://www.chemistryworld.com/news/carbon-dioxide-to-methanol-catalyst-ignites-fuel-from-air-debate/9339.article.
- S. Zhang, X. Jing, Y. Wang and F. Li, Towards Carbon-Neutral Methanol Production from Carbon Dioxide Electroreduction, ChemNanoMat, 2021, 7, 728–736 CrossRef CAS.
- S. Bhardwaj, A. Biswas, M. Das and R. S. Dey, Nanostructured Cu foam and its derivatives: emerging materials for the heterogeneous conversion of CO2 to fuels, Sustainable Energy Fuels, 2021, 5, 2393–2414 RSC.
- G. Garcia, E. Arriola, W. H. Chen and M. D. De Luna, A comprehensive review of hydrogen production from methanol thermochemical conversion for sustainability, Energy, 2021, 217, 119384 CrossRef CAS.
- A. M. Ranjekar and G. D. Yadav, Steam Reforming of Methanol for Hydrogen Production: A Critical Analysis of Catalysis, Processes, and Scope, Ind. Eng. Chem. Res., 2021, 60, 89–113 CrossRef CAS.
- B. S. Crandall, T. Brix, R. S. Weber and F. Jiao, Techno-Economic Assessment of Green H2Carrier Supply Chains, Energy Fuels, 2022, 37, 1441–1450, DOI:10.1021/acs.energyfuels.2c03616.
- Y. K. A. Migdadi, A. A. Khalifa, A. Al-Swidi, A. I. Amhamed and M. H. El-Naas, A Conceptual Framework of Customer Value Proposition of CCU-Formic Acid Product, Sustainability, 2022, 14, 16351, DOI:10.3390/su142416351.
- B. Zaidman, H. Wiener and Y. Sasson, Formate salts as chemical carriers in hydrogen storage and transportation, Int. J. Hydrogen Energy, 1986, 11, 341–347 CrossRef CAS.
- S. Chatterjee, I. Dutta, Y. Lum, Z. Lai and K. W. Huang, Enabling storage and utilization of low-carbon electricity: power to formic acid, Energy Environ. Sci., 2021, 14, 1194–1246 RSC.
- P. Duarah, D. Haldar, V. S. K. Yadav and M. K. Purkait, Progress in the electrochemical reduction of CO2 to formic acid: A review on current trends and future prospects, J. Environ. Chem. Eng., 2021, 9, 106394 CrossRef CAS.
- D. Wei, R. Sang, P. Sponholz, H. Junge and M. Beller, Reversible hydrogenation of carbon dioxide to formic acid using a Mn-pincer complex in the presence of lysine, Nat. Energy, 2022, 75(7), 438–447 CrossRef.
- W. B. Li and C. Yu, et al., Recent advances in the electroreduction of carbon dioxide to formic acid over carbon-based materials, New Carbon Mater., 2022, 37, 277–289 CrossRef.
- L. Fan, C. Xia, P. Zhu, Y. Lu and H. Wang, Electrochemical CO2 reduction to high-concentration pure formic acid solutions in an all-solid-state reactor, Nat. Commun., 2020, 111(11), 1–9 Search PubMed.
- Z. Sun, T. Ma, H. Tao, Q. Fan and B. Han, Fundamentals and Challenges of Electrochemical CO2 Reduction Using Two-Dimensional Materials, Chem, 2017, 3, 560–587 CAS.
- P.-L. Ragon and F. Rodríguez, CO2 emissions from trucks in the EU: An analysis of the heavy-duty CO2 standards baseline data, https://trid.trb.org/view/1893383 (2021).
- N. Amghar, et al., The SrCO3/SrO system for thermochemical energy storage at ultra-high temperature, Sol. Energy Mater. Sol. Cells, 2022, 238, 111632 CrossRef CAS.
- S. T. McCoy and E. S. Rubin, An engineering-economic model of pipeline transport of CO2 with application to carbon capture and storage, Int. J. Greenhouse Gas Control, 2008, 2, 219–229 CrossRef CAS.
- F. Chen and T. Morosuk, Exergetic and Economic Evaluation of CO2 Liquefaction Processes, Energies, 2021, 14, 7174, DOI:10.3390/en14217174.
- N. Dawass, et al., Solubilities and Transport Properties of CO2, Oxalic Acid, and Formic Acid in Mixed Solvents Composed of Deep Eutectic Solvents, Methanol, and Propylene Carbonate, J. Phys. Chem. B, 2022, 3572–3584 CrossRef CAS PubMed.
- O. Gutiérrez-Sánchez, et al., Electrochemical Conversion of CO2 from Direct Air Capture Solutions, Energy Fuels, 2022, 21, 13115–13123 CrossRef.
- M. Aresta, A. Dibenedetto and A. Angelini, Catalysis for the valorization of exhaust carbon: from CO2 to chemicals, materials, and fuels. Technological use of CO2, Chem. Rev., 2014, 114, 1709–1742 CrossRef CAS PubMed.
- R. Singh, M. Singh and S. Gautam, Hydrogen economy, energy, and liquid organic carriers for its mobility, Mater. Today Proc., 2021, 46, 5420–5427 CrossRef CAS.
- Q. Song, et al., A comparative study on energy efficiency of the maritime supply chains for liquefied hydrogen, ammonia, methanol and natural gas, Carbon Capture Sci. Technol., 2022, 4, 100056 CrossRef CAS.
- D. DeSantis, et al., Techno-economic analysis of metal–organic frameworks for hydrogen and natural gas storage, Energy Fuels, 2017, 31, 2024–2032 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ee03623a |
| This journal is © The Royal Society of Chemistry 2023 |