 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Directing the research agenda on water and energy technologies with process and economic analysis†
Boreum
Lee
a,
Li
Wang
 a,
Zhangxin
Wang
a,
Zhangxin
Wang
 b,
Nathanial J.
Cooper
a and
Menachem
Elimelech
b,
Nathanial J.
Cooper
a and
Menachem
Elimelech
 *a
*a
aDepartment of Chemical and Environmental Engineering, Yale University, New Haven, Connecticut 06520-8286, USA. E-mail: menachem.elimelech@yale.edu
bSchool of Ecology, Environment and Resources, Guangdong University of Technology, Guangzhou, 510006, China
First published on 1st February 2023
Abstract
Climate change is directly impacting energy consumption, water availability, and agricultural production. Among the global efforts to address the root causes of carbon emissions, numerous emerging technologies have been proposed to accelerate sustainable development for achieving carbon neutrality. While science-based discovery for emerging technologies, such as the development of novel materials, may help enhance sustainable development, analyzing the system design and economic viability is imperative for assessing the feasibility of the technology for upscaling and successful commercialization. Herein, we demonstrate the crucial importance of process modeling and techno-economic analysis by evaluating three emerging technologies at the water-energy nexus: direct seawater electrolysis, salinity gradient energy harvesting, and membrane-based thermal desalination. We show that the synergistic combination of techno-economic analysis and process modeling can effectively assess the potential feasibility of the emerging technologies at the early development stage. We further discuss the challenges of the three emerging technologies in their current states, indicating that they are not economically viable compared to the existing state-of-art technologies. Our study highlights the urgent need for an improved techno-economic approach—coupling process modeling and economic analysis—for the development of emerging technologies at the energy-water nexus.
Broader contextClimate change is one of the most urgent global challenges of our time, affecting the lives of billions of people. The indiscriminate use of fossil fuels is the primary cause of climate change, threatening human and environmental health, and altering ecosystem composition and function. Achieving “carbon neutrality by 2050”, which is a state of net-zero carbon emissions, is therefore a critical global mission. Many emerging technologies are being explored for sustainable development and to phase out fossil fuels, but studies of innovative system design, process evaluation, and economic feasibility are rarely conducted. Techno-economic analysis combined with process modeling can assess the economic viability of emerging technology and provide guidelines for the most impactful directions for research and development to support decarbonization. Through analysis of three well-studied emerging technologies at the water-energy nexus, this Opinion highlights the paramount importance of process modeling and techno-economic analysis for the development of sustainable and economically viable technologies. |
Introduction
Decarbonizing our society will necessitate the development of sustainable, energy-efficient technologies at the energy-food-water nexus. With the increased pressure to reduce greenhouse gas emissions, various emerging technologies have been studied for enhancing energy efficiency, the use of renewable energy and alternative fuels, and more efficient use and recycling of materials. Extensive research has been done at the water-energy nexus on water electrolysis for a green hydrogen economy,1–3 harvesting salinity gradient energy by mixing ocean water with river water for renewable electricity generation,4,5 and novel desalination technologies to reduce energy consumption.6,7 These recent efforts have focused mainly on the development of novel materials for the newly proposed technologies and, to a lesser extent, on innovative system designs to improve process efficiency. However, these studies, while scientifically exciting, have rarely analyzed the economic viability of the proposed technologies and their feasibility for upscaling and successful application.Techno-economic analysis (TEA) combined with process modeling is crucial for evaluating the feasibility of emerging technologies for potential upscaling and commercialization. Specifically, TEA can be a key enabler to providing meaningful directions for research and development as well as identifying important perspectives on the implementation of emerging technologies as part of an innovative business model. For example, the U.S. Department of Energy recently studied the economics of variable-duration energy storage and flexible power generation technologies for future national energy grids with high penetration of renewables.8 They established that the most cost-effective storage choice depends on the length of storage, and that one of the lowest-cost options is a novel application of an existing technology. This type of innovative finding reinforces the necessity of holistic assessments of novel technologies and system designs. However, despite the importance of these analyses, the assessment of the viability of emerging technologies has often been overlooked, even when these tools can provide indispensable insights into the feasibility of the proposed technology.
Herein, we analyze three emerging technologies at the water-energy nexus to exemplify the critical importance of process modeling and techno-economic analysis. The first example is water electrolysis to produce hydrogen using seawater without pretreatment or purification steps, a process referred to as direct seawater electrolysis (DSE).8,9 The second example is the harvesting of energy by the mixing of salty water (primarily seawater) with fresh water (primarily river water), an active area of research commonly referred to as salinity gradient energy (SGE) or blue energy.10–12 The third process analyzed here is membrane distillation (MD), an emerging thermal desalination technology to convert saline waters to fresh water.13,14 Through our analysis, we demonstrate that these technologies are not economically viable compared to existing, conventional technologies—an outcome that may be difficult to change because of the inherent limitations of these technologies. Our results underscore the critical importance of process modeling and techno-economic analysis, which could help to redirect resources for research on more promising technologies.
Direct seawater electrolysis for the hydrogen energy economy
Water electrolysis is a process utilizing electricity to produce hydrogen (H2) from water. Most commercially mature electrolysis technologies require the use of highly purified water, typically provided from freshwater sources that are often treated and deionized before use. However, freshwater resources may be limited for the widespread use of electrolysis, as these sources are typically reserved for agricultural and potable use. Considering the vast volume of water in oceans and seawaters, direct seawater electrolysis (DSE) has been proposed as an alternative to meet the increasing global H2 demands.15–17Because of the complex ionic composition of seawater, such as the presence of Cl−, SO42−, Mg2+, and Ca2+, many challenges must be overcome for the development of the technology, some of which are illustrated in Fig. 1.15–17 The oxygen evolution reaction (OER) is a four-electron transfer process with an equilibrium potential of 1.23 V vs. reversible hydrogen electrode (RHE), while the chlorine evolution reaction (CER) requires the transfer of two electrons with an equilibrium potential of 1.36 V vs standard hydrogen electrode (SHE), indicating OER is more thermodynamically favorable than CER.18,19 However, because CER has faster kinetics, it prevails at potentials above 1.36 V. Hence, the OER on the anode suffers from competition from the more kinetically favorable CER. Further, the production of chlorine in CER can corrode the steel of the bipolar plates, resulting in system degradation and significant economic and environmental damage.20 Chloride and other ions in seawater can also poison platinum-based catalysts, or cause catalyst detachment from the electrode.17 The membrane itself is susceptible to degradation from the corrosive nature of seawater, as well, and may further suffer from suspended particles and sparingly soluble salt ions in seawater precipitating or adhering to it.21 In addition, both Cl2 and O2 may cross the membrane to the cathode, where they can compete with the hydrogen evolution reaction, or even react with evolved hydrogen.
 | ||
| Fig. 1 Schematic diagram of H2 production from direct seawater electrolysis (DSE) using an anion exchange membrane and an alkaline electrolyte. A DSE has two main half-reactions: hydrogen evolution reaction (HER) in the cathode and oxygen evolution reaction (OER) in the anode. Reactions 3–11 show the challenges encountered during seawater electrolysis due to the presence of common ions and substances in seawater.15,16,25 | ||
The net impact of the reactions discussed above is a relatively short lifespan of DSE as well as low performance and production rates. Further, DSE would require expensive materials and catalysts to overcome these challenges, which will significantly increase the capital costs. Based on this discussion, it is clear that commercialization of DSE would be significantly hampered by the physically and chemically challenging problems arising from the complexity of seawater components.
Economic motives are a key aspect of commercializing any technology. DSE faces challenges in both operating costs and capital costs. A comparison of the specific energy consumption (SEC) by seawater desalination and conventional polymer electrolyte membrane water electrolysis (PEM WE) is presented in Table 1. The SEC of seawater desalination to provide suitable water accounts for a very small fraction (0.05–0.14%) of the total SEC for H2 production. A similar result can be expected for the relative contribution to the operating costs and the levelized cost of H2 (LCOH), since electricity is the primary contributor to the economic and environmental load of H2 production.22 Further, recent work has reported capital costs for DSE (i.e., over 6000 $ kW−1,21), which are more than double the capital costs for conventional electrolyzers (i.e., 920–1725 USD kW−1 for alkaline water electrolysis, AWE, and 1610–2668 USD kW−1 for PEM WE).23 As a result of the composition of seawater, DSE is also capital intensive due to chlorine crossover and corrosion,24 leading to frequent replacements of membranes, catalysts, and system components.
| Ref. | Water demand (kgH2O kgH2−1) | SEC of water demand via desalinationa (kW h kgH2−1) | SEC for H2 generation (kW h kgH2−1) | Percentage energy for water demand (%) |
|---|---|---|---|---|
| a Calculated based on the energy consumption of 3.5 kW h m−3 for seawater reverse osmosis plants.29 b Average specific energy consumption of PEM water electrolysis30 | ||||
| Beswick et al.26 | 9.0 | 0.03 | 63.97b | 0.05 |
| Lampert et al.27 | 25.7 | 0.09 | 63.97 | 0.14 |
| Hydrogenics28 | 11.126 | 0.04 | 74.54 | 0.05 |
| Siemens28 | 16.689 | 0.06 | 61.86 | 0.09 |
The primary conclusion that can be drawn from these process and economic analyses is that DSE cannot compete with state-of-the-art water purification and electrolysis technologies. It is unclear if all these technical and economic challenges could be overcome, even with significant research efforts and financial investment. Even with major technological improvements, such as pretreatment, to address all the challenges, DSE would save only ∼0.1% of the cost and energy usage over a combination of water purification and conventional electrolyzers. Given the daunting challenges in improving and commercializing DSE, and the extremely limited benefit in using it, we conclude that sustainable H2 production from seawater in the future should be done by coupling an industrially mature desalination technology, such as reverse osmosis, with conventional water electrolysis.13
Salinity gradient energy for green electricity generation
Salinity gradient energy (SGE) refers to the generation of electricity from the chemical potential difference between solutions with different salinities.31,32 SGE has been studied extensively in the past decade in terms of material advancement, system innovation, and process mechanisms.4,33,34 The harnessed energy in SGE technologies is a fraction of the Gibbs free energy of mixing that is determined by thermodynamics (Fig. 2A, see ESI,† Note S1, for details on the calculation of extractable energy and Table S1 (ESI†) for summary of extractable energies for each technology).35,36 The three main SGE technologies—pressure-retarded osmosis (PRO), reverse electrodialysis (RED), and nanopore-based or nanofluidic power generation (NPG)—are membrane-based processes. Among the SGE technologies, PRO and RED have reached a significantly high technology readiness level.4,37 More recently, NPG has attracted heightened research attention.10–12 | ||
Fig. 2 Analysis of harvesting salinity gradient energy. (A) Process schematic for harnessing salinity gradient energy by controlling the mixing of two streams with different salinities. Three processes are highlighted in this analysis: pressure-retarded osmosis (PRO), reverse electrodialysis (RED), and nanofluidic/nanopore-based power generation (NPG). (B) Comparison of membrane coupon-scale power density of PRO,43–46 RED,42,43 and NPG,65–71 based on literature reported data. (C) Extractable energy as a function of high salinity solution (brine) concentration. The extractable energy is normalized by the total volume of the high salinity and low salinity solutions. The volume (or flow rate) ratio of the low and high concentration solution streams is assumed to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The ideal extractable energy (blue line) is based on the Gibbs free energy of mixing, while the two realistic scenarios conceptually show the practical extractable energy with an energy efficiency of 50% and 12.5%, respectively. The filled circles represent literature reported extractable energy for PRO (purple), RED (blue), and NPG (orange).35,36,51,62,72 (D) Comparison of the levelized cost of energy (LCOE) of processes for harvesting salinity gradient energy from the mixing of seawater and river water (PRO,52,60 RED,53 and NPG), renewable energy (solar52,54–57 and wind58,59,61), and conventional energy generation from coal and nuclear power plants. 1. The ideal extractable energy (blue line) is based on the Gibbs free energy of mixing, while the two realistic scenarios conceptually show the practical extractable energy with an energy efficiency of 50% and 12.5%, respectively. The filled circles represent literature reported extractable energy for PRO (purple), RED (blue), and NPG (orange).35,36,51,62,72 (D) Comparison of the levelized cost of energy (LCOE) of processes for harvesting salinity gradient energy from the mixing of seawater and river water (PRO,52,60 RED,53 and NPG), renewable energy (solar52,54–57 and wind58,59,61), and conventional energy generation from coal and nuclear power plants. | ||
In PRO, water molecules transport through a semipermeable membrane from the low-salinity side to the pressurized high-salinity side, converting hydraulic pressure to electric power by driving a turbine.38,39 RED relies on the selective transport of counter-ions (i.e., the ions bearing the opposite charge to the membrane) through respective ion exchange membranes (IEMs), resulting in electrical current via redox reactions occurring at the two end electrode plates.35,40 In contrast to RED, NPG utilizes one highly charged and ultrathin nanoporous membrane instead of two IEMs.11,12,41 The mechanisms of the current generation and energy harvesting, however, are similar to RED.
The power density achieved by RED (i.e., 0.7–3.5 W m−2)42,43 is generally smaller than that achieved by PRO (i.e., 2–21 W m−2), based on laboratory-scale experiments with small membrane coupons43–46 (Fig. 2B) – with further details on power density for each technology provided in the ESI† (Table S2). The increased interest in developing NPG for harnessing salinity gradient energy is primarily attributed to the high-power density that NPG achieves. We note that a single nanopore power generator usually reports the power density normalized by the cross-sectional area of the nanopore, resulting in a power density on the order of 106 W m−2.47,48 The power densities of NPG summarized here consider the area as the projected area of the membrane coupon. Process modeling is critically important to identify the limitations of power density that the technology can achieve. For instance, process modeling on the module scale demonstrates that reducing the flow velocity in the channels of the RED module enhances the power density,49 and that internal concentration polarization in PRO is detrimental to achieving high power densities.50 Recently, multiscale process modeling of NPG revealed that the power density at a module-scale is dramatically reduced from the coupon-scale due to concentration polarization and varying salt concentrations along the module, casting doubt about the usefulness of coupon-level power desnities.51
Extractable energy is a critical performance metric that is usually neglected by the research community.43 Evaluating the extractable energy is challenging, as bench-scale research with membrane coupons cannot achieve mixing of the high and low salinity solutions; that is, no mass transfer occurs between the solutions. In this regard, process modeling represents a useful tool to approximate the energy efficiency of the SGE technologies. In Fig. 2C, the Gibbs free energy of mixing represents the ideal case, and the extracted energy is only a fraction of the Gibbs free energy of mixing. Specifically, the energy efficiency (i.e., the ratio of extractable energy to the Gibbs free energy of mixing) is less than unity. Process modeling reveals that the typical energy efficiencies of PRO and RED are ∼60% and ∼35%, respectively.43,52 A recent process modeling study demonstrated that the maximum theoretical energy efficiency for NPG is 50% (assuming monovalent salts) because only cations migrate through the nano-porous membranes,51 rendering the practical energy efficiency much lower than PRO and RED.
The ultimate goal of developing SGE technologies is to alleviate the energy crisis and possibly replace conventional energy generation technologies. Since TEA requires a large-scale implementation of the technologies, process modeling can fill the gap between the lab research advancements and the process scale. Specifically, process modeling evaluates the power density and extractable energy under various operating conditions and membrane properties. The output from process modeling is the input to the TEA, enabling the analysis of levelized cost of electricity (LCOE) over a wide range of conditions. The LCOEs of RED and PRO have been evaluated mostly in this fashion, as they are emerging technologies with only a few practical-scale testing.52–61 Both technologies typically show an LCOE > 1 $ kW h−1 (Fig. 2D, see ESI,† Table S3, for summary of LCOE for each technology). On the other hand, the TEA of NPG—a relatively new process—is challenging, as it utilizes an ultra-thin membrane that is not commercially available. Furthermore, the intrinsic low energy efficiency of NPG leads to extremely small (Fig. 2C) or even net negative energy generation due to energy losses from pretreatment and pumping, casting doubt on the viability of NPG.51,62 It is reasonable to assume that the LCOE of NPG is at least double that of RED, as the maximum energy efficiency of NPG is only half of the Gibbs free energy of mixing.51
Solar and wind power plants are two types of representative renewable energy generation. The LCOE of these technologies has dramatically dropped over the past decades, primarily due to cost reduction of the energy generation unit (i.e., capital expenditure). The LCOE of solar and wind are well below 1 $ kW h−1,63,64 rendering them promising alternatives to conventional coal and nuclear power generation. It is critical to note that SGE technologies cannot follow the successful cost reduction pathway of solar and wind power generation, because the viability of harvesting SGE is limited by the energy efficiency—a thermodynamic barrier that cannot be overcome. Specifically, the maximum SGE from the mixing of seawater and river water is the Gibbs free energy of mixing (i.e., 0.28 kW h m−3). The practical extractable energy is only a fraction of Gibbs free energy of mixing (Fig. 2C). To produce a meaningful quantity of energy, large amounts of seawater and river water must be pumped and pretreated, further reducing the net energy generation. More importantly, the development of process optimization and material advancement would not increase the amount of extractable energy. Therefore, harvesting SGE would never be economically feasible.
Desalination for sustainable water production
Process modeling and TEA can also be used to assess the competitiveness of state-of-the-art desalination technologies and emerging desalination technologies for sustainable water production from saline waters. In this study, we compared two conventional technologies, reverse osmosis (RO) and mechanical vapor compression (MVC), with an emerging desalination technology, membrane distillation (MD). The working principles of the desalination technologies are shown in Fig. 3A. In RO, fresh water is produced by forcing the pressurized saline feed to permeate through a semi-permeable RO membrane.72 In MVC, fresh water is produced from saline feed water evaporation, and the required energy is provided by mechanically compressing the generated vapor.73 In MD, a microporous hydrophobic membrane is used to separate a hot saline feed stream and a cold fresh distillate stream, and as the membrane allows the transport of vapor while prohibiting the permeation of salt, fresh water is produced from the temperature-difference-induced feed evaporation.73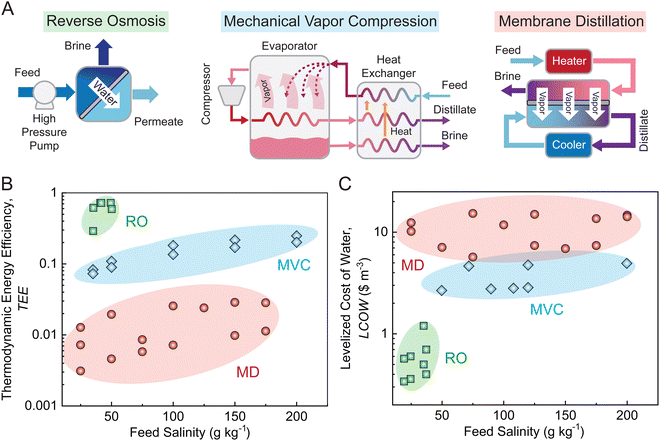 | ||
| Fig. 3 Systematic analysis of desalination processes. (A) Schematic illustrations of conventional and emerging desalination technologies, including reverse osmosis (RO), mechanical vapor compression (MVC), and membrane distillation (MD). (B) Comparison of thermodynamic energy efficiency (TEE) of the desalination technologies as a function of feed water salinity. Here, TEE is the theoretical minimum energy for freshwater production (i.e., Gibbs free energy of separation) normalized by the actual specific energy consumption (SEC) for freshwater production. This metric enables a fair comparison of different desalination technologies with varied feed salinities, water recoveries, and product water qualities. (C) Comparison of the levelized cost of water (LCOW) as a function of feed salinity. Here, LCOW is the average cost to produce a unit volume of fresh water over the lifetime of a desalination facility. This metric can be used as an economic indicator of the viability of a desalination technology because both the capital cost and operation cost are included in the calculation. All data points in panels (B) and (C) were acquired from literature.74–82 | ||
As an emerging desalination technology, MD has attracted heightened attention in the past decades.83–85 Tremendous research efforts have been devoted to the development of novel membrane materials and/or configurations, aiming at facilitating the practical application of MD technology.85,86 For instance, novel MD membranes with ultrahigh vapor permeabilities have been fabricated using covalent organic frameworks or ultrathin nanoporous graphene,87,88 and solar or electricity driven MD systems have been developed to improve the energy efficiency.89,90 Despite these technological innovations, MD is still an emerging technology under lab-scale development.86 Therefore, to assess the future of MD technology, the energy efficiency and economic feasibility of MD need to be compared with those of the state-of-the-art desalination technologies (i.e., RO and MVC) using process modeling and TEA analysis.
Through process modeling, the energy efficiency of the desalination technologies can be compared using thermodynamic energy efficiency (TEE) (Fig. 3B) – with further details on the calculation of the thermodynamic energy efficiency provided in the ESI† (Note S2).81 For seawater desalination, RO has a substantially higher TEE than MD or MVC. As desalination technologies involving phase change of water, MD and MVC have large SEC, thereby leading to low TEE.91 For hypersaline brine desalination, MVC has a larger TEE than MD. The relatively large TEE of MVC can be ascribed to the efficient latent heat recovery which reduces SEC.73 From the TEE comparison, in terms of energy efficiency, the state-of-the-art desalination technologies (i.e., RO and MVC) significantly outperform the emerging MD desalination technology.
By performing TEA, the economic feasibility of the desalination technologies can be compared using the levelized cost of water (LCOW) (Fig. 3C).92 In Fig. 3C, the trend of LCOW for the desalination technologies is opposite to that of TEE (Fig. 3B). Specifically, RO has the lowest LCOW, whereas MD has the highest LCOW. The negative correlation between LCOW and TEE can be explained by SEC: a larger TEE indicates a smaller SEC, and thereby corresponds to a lower LCOW. Notably, compared with RO and MVC, although MD does not require high-pressure pumps or high-temperature equipment, its capital cost can still be high due to the large membrane area required for operation and/or the use of heat exchangers.82,93,94 Based on the LCOW comparison, we conclude that MD is not an economically viable desalination technology.
Using process modeling and TEA, we demonstrate that the emerging desalination technology of MD cannot compete with the state-of-the-art desalination technologies (i.e., RO and MVC) on both energy efficiency and economic feasibility. Notably, for off-grid and small-scale desalination purposes, MD could be more attractive than RO and MVC as it might require lower capital costs and be able to use solar energy, but in most scenarios, RO and MVC still dominate the desalination field. Therefore, for sustainable desalination, future research work should focus on improving the performance of state-of-the-art desalination technologies instead of overcoming the potential challenges of emerging desalination technologies, which are inherently limited by thermodynamics. Furthermore, process modeling and TEA can provide important guidance for the improvement of state-of-the-art desalination technologies. For instance, process modeling indicates that one of the keys to improving the performance of RO is to develop membranes with enhanced permselectivity.95 Additionally, TEA suggests that the most effective way to reduce the cost of MVC is to develop inexpensive materials for system construction.96
Perspective and outlook
In this work, we demonstrate the critical importance of process modeling and techno-economic analysis to the development of new technologies for water and energy applications. Significant research and capital investment are required for direct seawater electrolysis to be cost-competitive, but it offers virtually no potential to improve the economic or energetic performance over conventional technologies. Through process modeling, it has been demonstrated that the extractable energy from salinity gradients is too low to be a practically or energetically viable source of energy for the future. Techno-economic analysis and process modeling establish that membrane distillation is outperformed both economically and energetically by existing commercial desalination technologies. These findings illustrate how the use of analysis tools can provide valuable data about the real potential, or lack thereof, of emerging technologies at the water-energy nexus.Our work strongly suggests that the economic, energetic, and environmental viability of new technologies must be considered early in the development cycle, particularly in the context of their suggested application. New technologies need to be evaluated for these viabilities just as early as they are for their thermodynamic efficiency or scientific merit. Each technology will have a slightly different ideal use case, but for technologies where the ideal use is extremely niche, the real value shrinks. For example, direct seawater electrolysis may be useful in situations where space is extremely limited for water purification and seawater is widely available, such as on a ship or submarine, but conventional water purification and electrolysis are still preferable in nearly every other case.
We further suggest that these analyses should be considered for the optimal allocation of research funding for specific research areas. If new technologies require large capital, time, and research investments to offer only marginal improvements over existing technologies, those funds may be better directed to alternative technologies or research areas. Research funds should be targeted to those technologies which can make the greatest impact in the field of interest. Further, those technologies which are not currently economically viable but could be viable with sufficient investment would benefit from government subsidies in the short term to help offset costs. These subsidies are particularly useful for technologies where a major cost barrier is the scale-up of production, or production improvements, such as government subsidies for electric vehicles.
The global challenges of water scarcity, climate change, and energy insecurity must be addressed quickly and efficiently by the research community. Process and economic analyses provide the opportunity to target investments in technologies that may have the most impact in solving these challenges. We suggest these analyses should be used as a fundamental tool for evaluating new technologies to understand the real benefits which they can provide now and projected benefits in the future. Further, such analyses should be revisited regularly to understand how the landscape of technical and economic viability may shift with improvements in technology and changes in cost. The ultimate goal of research on new technologies is to develop cheaper, more efficient, more environmentally friendly, and better technologies. Using these analysis tools would ensure that this goal is achieved effectively.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Science Foundation Nanosystems Engineering Research Center for Nanotechnology-Enabled Water Treatment (EEC-1449500). N. J. C. acknowledges the eFellows Postdoctoral Fellowship from the American Society for Engineering Education (through NSF Grant Number EEC-2127509).References
- W. Tong, M. Förster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5(5), 367–377 CrossRef CAS
.
- M. Ball and M. Wietschel, Int. J. Hydrogen Energy, 2009, 34, 615–627 CrossRef CAS
.
- J. N. Hausmann, R. Schlögl, P. W. Menezes and M. Driess, Energy Environ. Sci., 2021, 14, 3679–3685 RSC
.
- G. Z. Ramon, B. J. Feinberg and E. M. V. Hoek, Energy Environ. Sci., 2011, 4, 4423–4434 RSC
.
- B. E. Logan and M. Elimelech, Nature, 2012, 488(7411), 313–319 CrossRef CAS PubMed
.
- H. Nassrullah, S. F. Anis, R. Hashaikeh and N. Hilal, Desalination, 2020, 491, 114569 CrossRef CAS
.
- S. K. Patel, C. L. Ritt, A. Deshmukh, Z. Wang, M. Qin, R. Epsztein and M. Elimelech, Energy Environ. Sci., 2020, 13, 1694–1710 RSC
.
- B. E. Logan, L. Shi and R. Rossi, Joule, 2021, 5, 760–762 CrossRef
.
- S. S. Veroneau and D. G. Nocera, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2024855118 CrossRef CAS PubMed
.
- X. Tong, S. Liu, J. Crittenden and Y. Chen, ACS Nano, 2021, 15, 5838–5860 CrossRef CAS PubMed
.
- M. Macha, S. Marion, V. V. R. Nandigana and A. Radenovic, Nat. Rev. Mater., 2019, 4(9), 588–605 CrossRef CAS
.
- A. Siria, M. L. Bocquet and L. Bocquet, Nat. Rev. Chem., 2017, 1(11), 1–10 Search PubMed
.
- A. Deshmukh, C. Boo, V. Karanikola, S. Lin, A. P. Straub, T. Tong, D. M. Warsinger and M. Elimelech, Energy Environ. Sci., 2018, 11, 1177–1196 RSC
.
- A. M. Alklaibi and N. Lior, Desalination, 2005, 171, 111–131 CrossRef CAS
.
- M. A. Khan, T. Al-Attas, S. Roy, M. M. Rahman, N. Ghaffour, V. Thangadurai, S. Larter, J. Hu, P. M. Ajayan and M. G. Kibria, Energy Environ. Sci., 2021, 14, 4831–4839 RSC
.
- S. Dresp, F. Dionigi, M. Klingenhof and P. Strasser, ACS Energy Lett., 2019, 4, 933–942 CrossRef CAS
.
- W. Zheng, L. Y. S. Lee and K. Y. Wong, Nanoscale, 2021, 13, 15177–15187 RSC
.
- K. S. Exner, ChemElectroChem, 2020, 7, 1448–1455 CrossRef CAS
.
- J. G. Vos, Z. Liu, F. D. Speck, N. Perini, W. Fu, S. Cherevko and M. T. M. Koper, ACS Catal., 2019, 9, 8561–8574 CrossRef CAS
.
- M. A. J. Mazumder, Global J. Eng. Sci., 2020, 4, 1–5 Search PubMed
.
- F. Y. Gao, P. C. Yu and M. R. Gao, Curr. Opin. Chem. Eng., 2022, 36, 100827 CrossRef
.
- B. Lee, D. Lim, H. Lee and H. Lim, Renewable Sustainable Energy Rev., 2021, 143, 110963 CrossRef CAS
.
- B. Lee, L. R. Winter, H. Lee, D. Lim, H. Lim and M. Elimelech, ACS Energy Lett., 2022, 3032–3038 CrossRef CAS
.
- F. Sun, J. Qin, Z. Wang, M. Yu, X. Wu, X. Sun and J. Qiu, Nat. Commun., 2021, 12(1), 1–11 CrossRef PubMed
.
- W. Zheng, L. Y. S. Lee and K. Y. Wong, Nanoscale, 2021, 13, 15177–15187 RSC
.
- R. R. Beswick, A. M. Oliveira and Y. Yan, ACS Energy Lett., 2021, 6, 3167–3169 CrossRef CAS
.
- D. J. Lampert, H. Cai and A. Elgowainy, Energy Environ. Sci., 2016, 9, 787 RSC
.
- A. Mayyas, M. Ruth, B. Pivovar, G. Bender and K. Wipke, Manufacturing Cost Analysis for Proton Exchange Membrane Water Electrolyzers, 2019.
- M. Elimelech and W. A. Phillip, Science, 1979, 2011(333), 712–717 Search PubMed
.
- A. Buttler and H. Spliethoff, Renewable Sustainable Energy Rev., 2018, 82, 2440–2454 CrossRef CAS
.
-
E. Brauns, Current Trends and Future Developments on (Bio-) Membranes: Membrane Desalination Systems: The Next Generation, 2019, pp. 327–348 Search PubMed
.
- K. M. Creer, R. Thompson and L. Molyneux, Science, 1979, 1974(186), 350–352 Search PubMed
.
- J. W. Post, J. Veerman, H. V. M. Hamelers, G. J. W. Euverink, S. J. Metz, K. Nymeijer and C. J. N. Buisman, J. Memb. Sci., 2007, 288, 218–230 CrossRef CAS
.
- J. Gao, W. Guo, D. Feng, H. Wang, D. Zhao and L. Jiang, J. Am. Chem. Soc., 2014, 136, 12265–12272 CrossRef CAS PubMed
.
- N. Y. Yip, D. A. Vermaas, K. Nijmeijer and M. Elimelech, Environ. Sci. Technol., 2014, 48, 4925–4936 CrossRef CAS PubMed
.
- N. Y. Yip and M. Elimelech, Environ. Sci. Technol., 2012, 46, 5230–5239 CrossRef CAS PubMed
.
- K. Nijmeijer and S. Metz, Sustainability Sci. Eng., 2010, 2, 95–139 Search PubMed
.
- F. Helfer, C. Lemckert and Y. G. Anissimov, J. Memb. Sci., 2014, 453, 337–358 CrossRef CAS
.
- S. Sarp, Z. Li and J. Saththasivam, Desalination, 2016, 389, 2–14 CrossRef CAS
.
- J. W. Post, H. V. M. Hamelers and C. J. N. Buisman, Environ. Sci. Technol., 2008, 42, 5785–5790 CrossRef CAS PubMed
.
- L. Cao, Q. Wen, Y. Feng, D. Ji, H. Li, N. Li, L. Jiang, W. Guo, L. Cao, H. Li, N. Li, Q. Wen, Y. Feng, D. Ji, L. Jiang and W. Guo, Adv. Funct. Mater., 2018, 28, 1804189 CrossRef
.
- D. A. Vermaas, E. Guler, M. Saakes and K. Nijmeijer, Energy Procedia, 2012, 20, 170–184 CrossRef CAS
.
- N. Y. Yip and M. Elimelech, Environ. Sci. Technol., 2014, 48, 11002–11012 CrossRef CAS PubMed
.
- X. Song, Z. Liu and D. D. Sun, Energy Environ. Sci., 2013, 6, 1199–1210 RSC
.
- S. E. Skilhagen, J. E. Dugstad and R. J. Aaberg, Desalination, 2008, 220, 476–482 CrossRef CAS
.
- A. Tiraferri, N. Y. Yip, W. A. Phillip, J. D. Schiffman and M. Elimelech, J. Memb. Sci., 2011, 367, 340–352 CrossRef CAS
.
- J. Feng, M. Graf, K. Liu, D. Ovchinnikov, D. Dumcenco, M. Heiranian, V. Nandigana, N. R. Aluru, A. Kis and A. Radenovic, Nature, 2016, 536(7615), 197–200 CrossRef CAS PubMed
.
- A. Siria, P. Poncharal, A. L. Biance, R. Fulcrand, X. Blase, S. T. Purcell and L. Bocquet, Nature, 2013, 494(7438), 455–458 CrossRef CAS PubMed
.
- R. Long, B. Li, Z. Liu and W. Liu, Energy, 2018, 151, 1–10 CrossRef
.
- A. P. Straub, S. Lin and M. Elimelech, Environ. Sci. Technol., 2014, 48, 12435–12444 CrossRef CAS PubMed
.
- L. Wang, Z. Wang, S. K. Patel, S. Lin and M. Elimelech, ACS Nano, 2021, 15, 4093–4107 CrossRef CAS PubMed
.
- N. Y. Yip, D. Brogioli, H. V. M. Hamelers and K. Nijmeijer, Environ. Sci. Technol., 2016, 50, 12072–12094 CrossRef CAS PubMed
.
- F. Giacalone, M. Papapetrou, G. Kosmadakis, A. Tamburini, G. Micale and A. Cipollina, Energy, 2019, 181, 532–547 CrossRef CAS
.
- R. Musi, B. Grange, S. Sgouridis, R. Guedez, P. Armstrong, A. Slocum and N. Calvet, AIP Conf. Proc., 2017, 1850, 160018 CrossRef
.
- N. E. Benti, Y. S. Mekonnen, A. A. Asfaw, M. D. Lerra, T. A. Woldegiyorgis, C. A. Gaffe and A. B. Aneseyee, Cogent Eng., 2022, 9, 1–21 Search PubMed
.
- Y. Kalinci, A. Hepbasli and I. Dincer, Int. J. Hydrogen Energy, 2015, 40, 7652–7664 CrossRef CAS
.
- S. Ud-Din Khan, I. Wazeer, Z. Almutairi and M. Alanazi, Alexandria Eng. J., 2022, 61, 6739–6753 CrossRef
.
- C. Dao, B. Kazemtabrizi and C. Crabtree, Wind Energy, 2019, 22, 1848–1871 CrossRef
.
- T. Ashuri, M. B. Zaaijer, J. R. R. A. Martins, G. J. W. van Bussel and G. A. M. van Kuik, Renew Energy, 2014, 68, 893–905 CrossRef
.
- A. N. Newby, T. v Bartholomew and M. S. Mauter, ACS ES&T Engg, 2021, 1, 1113–1121 Search PubMed
.
- Lazard.com | Levelized Cost Of Energy, Levelized Cost Of Storage, and Levelized Cost Of Hydrogen, https://www.lazard.com/perspective/levelized-cost-of-energy-levelized-cost-of-storage-and-levelized-cost-of-hydrogen/, (accessed 14 August 2022).
- Z. Wang, L. Wang and M. Elimelech, Engineering, 2022, 9, 51–60 CrossRef CAS
.
- Renewable Power Generation Costs in 2021, International Renewable Energy Agency, 2022, ISBN: 978-92-9260-452-3, https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2022/Jul/IRENA_Power_Generation_Costs_2021.pdf?rev=34c22a4b244d434da0accde7de7c73d8.
- Lazard.com | Levelized Cost Of Energy, Levelized Cost Of Storage, and Levelized Cost Of Hydrogen, https://www.lazard.com/perspective/levelized-cost-of-energy-levelized-cost-of-storage-and-levelized-cost-of-hydrogen/, (accessed 1 October 2022).
- S. Hong, F. Ming, Y. Shi, R. Li, I. S. Kim, C. Y. Tang, H. N. Alshareef and P. Wang, ACS Nano, 2019, 13, 8917–8925 CrossRef CAS PubMed
.
- J. Chen, W. Xin, X. Y. Kong, Y. Qian, X. Zhao, W. Chen, Y. Sun, Y. Wu, L. Jiang and L. Wen, ACS
Energy Lett., 2020, 5, 742–748 CrossRef CAS
.
- Z. Zhang, L. He, C. Zhu, Y. Qian, L. Wen and L. Jiang, Nat. Commun., 2020, 11(1), 1–8 CrossRef PubMed
.
- J. Hwang, S. Kataoka, A. Endo and H. Daiguji, Lab Chip, 2016, 16, 3824–3832 RSC
.
- D. K. Kim, C. Duan, Y. F. Chen and A. Majumdar, Microfluid. Nanofluid., 2010, 9, 1215–1224 CrossRef CAS
.
- C. Li, L. Wen, X. Sui, Y. Cheng, L. Gao and L. Jiang, Sci. Adv., 2021, 7, 1–7 Search PubMed
.
- X. Liu, M. He, D. Calvani, H. Qi, K. B. S. S. Gupta, H. J. M. de Groot, G. J. A. Sevink, F. Buda, U. Kaiser and G. F. Schneider, Nat. Nanotechnol., 2020, 15(4), 307–312 CrossRef CAS PubMed
.
- M. Qin, A. Deshmukh, R. Epsztein, S. K. Patel, O. M. Owoseni, W. S. Walker and M. Elimelech, Desalination, 2019, 455, 100–114 CrossRef CAS
.
- E. Shaulsky, Z. Wang, A. Deshmukh, V. Karanikola and M. Elimelech, Desalination, 2020, 496, 114694 CrossRef CAS
.
- G. P. Thiel, E. W. Tow, L. D. Banchik, H. W. Chung and J. H. Lienhard, Desalination, 2015, 366, 94–112 CrossRef CAS
.
- S. R. Osipi, A. R. Secchi and C. P. Borges, Desalination, 2018, 430, 107–119 CrossRef CAS
.
- A. v Dudchenko, T. v Bartholomew and M. S. Mauter, J. Memb. Sci., 2021, 627, 119228 CrossRef CAS
.
- M. A. Jamil and S. M. Zubair, Desalination, 2017, 420, 292–307 CrossRef CAS
.
- R. Ullah, M. Khraisheh, R. J. Esteves, J. T. McLeskey, M. AlGhouti, M. Gad-el-Hak and H. Vahedi Tafreshi, Desalination, 2018, 433, 56–67 CrossRef CAS
.
- T. Tong and M. Elimelech, Environ. Sci. Technol., 2016, 50, 6846–6855 CrossRef CAS PubMed
.
- D. Akgul, M. Çakmakci, N. Kayaalp and I. Koyuncu, Desalination, 2008, 220, 123–131 CrossRef CAS
.
- S. Lin, Environ. Sci. Technol., 2020, 54, 76–84 CrossRef CAS PubMed
.
- T. v Bartholomew, A. v Dudchenko, N. S. Siefert and M. S. Mauter, J. Memb. Sci., 2020, 611, 118370 CrossRef CAS
.
- K. W. Lawson and D. R. Lloyd, J. Memb. Sci., 1997, 124, 1–25 CrossRef CAS
.
- A. Alkhudhiri, N. Darwish and N. Hilal, Desalination, 2012, 287, 2–18 CrossRef CAS
.
- P. Wang and T. S. Chung, J. Memb. Sci., 2015, 474, 39–56 CrossRef CAS
.
- T. Horseman, Y. Yin, K. S. Christie, Z. Wang, T. Tong and S. Lin, ACS ES&T Engg, 2020, 1, 117–140 Search PubMed
.
- S. Zhao, C. Jiang, J. Fan, S. Hong, P. Mei, R. Yao, Y. Liu, S. Zhang, H. Li, H. Zhang, C. Sun, Z. Guo, P. Shao, Y. Zhu, J. Zhang, L. Guo, Y. Ma, J. Zhang, X. Feng, F. Wang, H. Wu and B. Wang, Nat. Mater., 2021, 20(11), 1551–1558 CrossRef CAS PubMed
.
- D. Lu, Z. Zhou, Z. Wang, D. T. Ho, G. Sheng, L. Chen, Y. Zhao, X. Li, L. Cao, U. Schwingenschlögl, J. Ma and Z. Lai, Adv. Mater., 2022, 34, 2109718 CrossRef CAS PubMed
.
- P. D. Dongare, A. Alabastri, S. Pedersen, K. R. Zodrow, N. J. Hogan, O. Neumann, J. Wud, T. Wang, A. Deshmukh, M. Elimelech, Q. Li, P. Nordlander and N. J. Halas, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6936–6941 CrossRef CAS PubMed
.
- K. Zuo, W. Wang, A. Deshmukh, S. Jia, H. Guo, R. Xin, M. Elimelech, P. M. Ajayan, J. Lou and Q. Li, Nat. Nanotechnol., 2020, 15(12), 1025–1032 CrossRef CAS PubMed
.
- Z. Wang, T. Horseman, A. P. Straub, N. Y. Yip, D. Li, M. Elimelech and S. Lin, Sci. Adv., 2019, 5, eaax0763 CrossRef CAS PubMed
.
- X. Chen and N. Y. Yip, Environ. Sci. Technol., 2018, 52, 2242–2250 CrossRef CAS PubMed
.
- V. Karanikola, S. E. Moore, A. Deshmukh, R. G. Arnold, M. Elimelech and A. E. Sáez, Desalination, 2019, 472, 114164 CrossRef CAS
.
- Y. Lu and J. Chen, Ind. Eng. Chem. Res., 2012, 51, 6798–6810 CrossRef CAS
.
- J. R. Werber, A. Deshmukh and M. Elimelech, Environ. Sci. Technol. Lett., 2016, 3, 112–120 CrossRef CAS
.
- A. Panagopoulos, Chem. Eng. Process., 2020, 152, 107934 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d2ee03271f |
| This journal is © The Royal Society of Chemistry 2023 |
