Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of hydration equilibria on the relaxometric properties of Gd(III) complexes: new insights into old systems†
Alessandro
Nucera
a,
Carlos
Platas-Iglesias
 *b,
Fabio
Carniato
*b,
Fabio
Carniato
 a and
Mauro
Botta
a and
Mauro
Botta
 *a
*a
aDipartimento di Scienze e Innovazione Tecnologica, Università del Piemonte Orientale, Viale T. Michel 11, 15121 Alessandria, Italy. E-mail: mauro.botta@uniupo.it
bUniversidade da Coruña, Centro de Interdisciplinar de Química e Bioloxía (CICA) and Departamento de Química, Facultade de Ciencias, 15071, A Coruña, Galicia, Spain. E-mail: carlos.platas.iglesias@udc.es
First published on 9th November 2023
Abstract
We present a detailed relaxometric and computational investigation of three Gd(III) complexes that exist in solution as an equilibrium of two species with a different number of coordinated water molecules: [Gd(H2O)q]3+ (q = 8, 9), [Gd(EDTA)(H2O)q]− and [Gd(CDTA)(H2O)q]− (q = 2, 3). 1H nuclear magnetic relaxation dispersion (NMRD) data were recorded from aqueous solutions of these complexes using a wide Larmor frequency range (0.01–500 MHz). These data were complemented with 17O transverse relaxation rates and chemical shifts recorded at different temperatures. The simultaneous fit of the NMRD and 17O NMR data was guided by computational studies performed at the DFT and CASSCF/NEVPT2 levels, which provided information on Gd⋯H distances, 17O hyperfine coupling constants and the zero-field splitting (ZFS) energy, which affects electronic relaxation. The hydration equilibrium did not have a very important effect in the fits of the experimental data for [Gd(H2O)q]3+ and [Gd(CDTA)(H2O)q]−, as the hydration equilibrium is largely shifted to the species with the lowest hydration number (q = 8 and 2, respectively). The quality of the analysis improves however considerably for [Gd(EDTA)(H2O)q]− upon considering the effect of the hydration equilibrium. As a result, this study provides for the first time an analysis of the relaxation properties of this important model system, as well as accurate parameters for [Gd(H2O)q]3+ and [Gd(CDTA)(H2O)q]−.
Introduction
Relaxometry plays a key role among the techniques for the characterisation of paramagnetic species in solution.1,2 The technique is based on the measurement of properties of solvent molecules in order to obtain information on the metal complex with which they interact chemically and magnetically. Typically, relaxometric studies involve measuring the 1H longitudinal (T1) and/or transverse relaxation times (T2) of solvent water molecules, which are shortened by the presence of the paramagnetic solute.3,4 Relaxometric data can be acquired at different temperatures to gain information on the dynamics of the system. Furthermore, the fast field cycling relaxometric technique allows recording relaxation data over a rather wide range of magnetic field strengths, typically from 0.01 MHz up to 60 or even 120 MHz.5 The effect of the applied magnetic field is generally analysed using nuclear magnetic relaxation dispersion (NMRD) profiles, which are plots of the relaxation rates (R1,2) or relaxivities (r1,2) versus the proton Larmor frequency. The r1,2 values correspond to the relaxation rate enhancement effect of bulk water protons normalized to a 1 mM concentration of the paramagnetic solute, expressed in mM−1 s−1 units.6Relaxometry has played a critical role in developing and characterizing contrast agents for magnetic resonance imaging (MRI) based on paramagnetic ions such as Gd(III),4,7,8 Fe(III),9–11 V(IV)12,13 or Mn(II).14–16 MRI contrast agents used in clinical practice enhance image contrast by shortening the longitudinal relaxation times of water nuclei present in the tissues where the agent is distributed. The shortening of T1 enhances signal intensity when using short repetition times in the MRI experiment, which facilitates the diagnoses of different pathologies.17 Many fundamental studies were devoted over the last two decades to understand the correlation between the structure of the complex and the dynamic and structural parameters that determine the observed relaxivity.18 As a result, we have now a rather accurate understanding of the relationship between the coordination chemistry of these paramagnetic metal ions and their relaxation properties. Besides the use of relaxometry in the MRI field, this technique is very appropriate to undertake more fundamental studies on paramagnetic species in solution.
The relaxivity of a paramagnetic complex experiences contribution from both inner-sphere (IS) and outer-sphere (OS) mechanisms.19,20 The latter arises from the diffusion of water molecules in the vicinity of the paramagnetic centre,21 and can be barely affected by changing ligand design. The IS mechanism is directly proportional to the number of water molecules present in the inner coordination sphere q, as well as on the mean residence time of water molecules in the first coordination sphere (τM = 1/kex, where kex is the exchange rate), and their relaxation time T1M:
 | (1) |
The presence of a hydration equilibrium is likely to affect significantly relaxometric studies, as q affects both relaxivity and the 17O NMR relaxation and chemical shift data used to evaluate water exchange dynamics. Thus, herein we have undertaken a detailed relaxometric characterization of the [Gd(H2O)q]3+, [Gd(EDTA)(H2O)q]− and [Gd(CDTA)(H2O)q]− complexes to assess the relevance of considering hydration equilibria in the analysis of relaxometric data.
Results and discussion
17O NMR studies
The hydration equilibrium may be expressed as:| [GdL(H2O)N] + H2O ⇄ [GdL(H2O)N+1] | (2) |
 | (3) |
These data show that the hydration number of the aqua-ion is rather insensitive to temperature within the range used for relaxometric studies (ca. 278–353 K), with the population of the nine coordinated species varying in the range 22–33%. This is in line with the general view that the main aqua complex present in solution for Gd(III) is the eight-coordinate form.40,41 On this basis, NMRD and 17O NMR studies considered a value of q = 8 for the analysis of the data,4 though earlier work assumed q = 9,42,43 affording kex values that differed by ∼20%. For the complex with EDTA4− both the populations of q = 2 and q = 3 species are significant over the 278–353 K temperature range, with nearly equal populations of the two species at 278 K. However, the CDTA4− analogue displays q values close to 2, with the tris-hydrated species accounting for 10% or less of the overall population.
With the hydration parameters in hand, we have undertaken a variable temperature 17O NMR study in which transverse relaxation rates and chemical shifts were measured. These data are generally expressed as reduced relaxation rates (1/T2r) and chemical shifts (Δωr) by normalizing the data to the mole fraction of bound water molecules Pm. The latter can be calculated from the values of q at the given temperature as Pm = (cq)/55.55. The values of 1/T2r measured for the three complexes decrease with temperature, a behaviour that is characteristic of the fast exchange regime (Fig. 1).44 Under these conditions, the values of 1/T2r are mainly determined by the 17O relaxation rate (1/T1M) of coordinated water molecules, which is proportional to the square of the 17O hyperfine coupling constant A/ħ and is affected by both water exchange and electron relaxation. One can notice that the 1/T2r values at a given temperature follow the order [Gd(H2O)q]3+ < [Gd(EDTA)(H2O)q]− ∼ [Gd(CDTA)(H2O)q]−, which implies that [Gd(H2O)q]3+ has the fastest water exchange within this series.
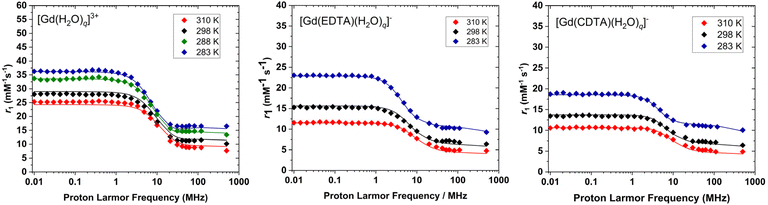
1H NMRD profiles
The relaxivities of the three Gd(III) complexes were measured over a wide range of proton Larmor frequencies using fast field cycling relaxometry (ca. 0.01–120 MHz), with additional points recorded using a high resolution instrument operating at 500 MHz (Fig. 2). The NMRD profiles have the typical shape observed for solutions of small Gd(III) complexes, with a plateau at low field (<1 MHz), a dispersion at intermediate fields (ca. 1–10 MHz) and a second region of fairly constant relaxivity at high field (>20 MHz).1 The NMRD profiles recorded for [Gd(H2O)q]3+ and [Gd(EDTA)(H2O)q]− agree with those measured earlier up to 60 MHz.4,45 The higher relaxivities observed for [Gd(H2O)q]3+ are simply a consequence of the high hydration number. The NMRD profiles of [Gd(EDTA)(H2O)q]− display higher relaxivities at low field compared with [Gd(CDTA)(H2O)q]−, which is likely reflecting some differences in electron spin relaxation. The relaxivities of all three complexes increase when the temperature is lowered. This is a consequence of an increased outer-sphere contribution due to a lower diffusion coefficient at lower temperatures. Furthermore, the IS contribution increases as well at high fields (>2 MHz) due to the longer rotational correlation times (τR) observed at low temperatures.Theoretical calculations
A rather large number of parameters affects the 1H NMRD profiles and 17O NMR data, some of which are common to both sets of data.46 The presence of two complex species in solution with different hydration numbers complicates further the analysis, as it requires incorporating additional parameters. For instance, the two species at equilibrium may have significantly different values of the hyperfine coupling constant A/ħ, or the electronic relaxation times, as a result of differences in zero field splitting (ZFS) energies. Incorporating additional parameters into the model is likely to improve the quality of the fits, but not necessarily be more meaningful. We therefore performed a computational study to guide the strategy to fit the experimental data.We started our computational study by modelling the [Gd(H2O)8]3+ and [Gd(H2O)9]3+ systems. Previous studies on these small complexes were conducted using polarizable continuum models (PCM) to account for solvent effects.40 However, geometry optimizations using PCM suffer from convergence issues, and furthermore they cannot be used to study water exchange reactions. Thus, we started our calculations using a cluster/continuum approach,47,48 in which an explicit second sphere solvation shell is added to the model, while the effects of bulk water are considered with the PCM model.49 The starting geometry had nine coordinated water molecules and 18 second-sphere water molecules (Fig. 3). The hydrogen bond pattern in this cluster is such that the coordinated water molecules are connected by hydrogen bonds through two second sphere water molecules, providing a complete second-sphere shell. This hydrogen bond structure was used previously to study octahedral aqua-complexes.50–52 Our calculations provide a tricapped trigonal prismatic coordination polyhedron (TTP), as observed by X-ray diffraction studies. A potential energy surface scan was subsequently performed by elongating one of the Gd–Ow bonds, leading to a second energy minimum that corresponds to the eight-coordinate complex. The latter displays a square antiprismatic (SAP) coordination environment, as would be expected. The relative free energy of the [Gd(H2O)9]3+·18H2O and [Gd(H2O)8]3+·19H2O systems (at 298.15 K) favour the latter by 4.37 kJ mol−1, in qualitative agreement with the experimental data.
The TTP coordination polyhedron in [Gd(H2O)9]3+·18H2O is characterized by Gd–O bonds involving capping water molecules (Gd–Oc, average at 2.509 Å) being slightly longer than those at the vertexes of the prism (Gd–Op at 2.469 Å). This is in good agreement with the available crystallographic data.53 The average Gd–O distance decreases slightly upon lowering the coordination number from nine to eight, as would be expected (Table 2). This has a slight impact on the Gd⋯H distances involving coordinated water molecules (rGdH), which nevertheless are very close to the value of 3.1 Å obtained using ENDOR measurements.54 The values of the isotropic hyperfine coupling constants A/ħ are also slightly affected by the coordination number, with the shorter Gd–O bonds in the eight-coordinate form resulting in somewhat higher values of A/ħ compared with the nine-coordinate form.55 Nevertheless, the average values of A/ħ calculated for the two eight- and nine-coordinate forms differ by <10% (Table 2).
| r GdO /Å | r GdH /Å | 17O A/ħa/106 rad s−1 | D/cm−1 | E/D | Δ S/cm−1 | Δ S 2/1019 s−2 | |
|---|---|---|---|---|---|---|---|
| a Average values within parenthesis. | |||||||
| [Gd(H2O)8]3+·19H2O | 2.407–2.533 (2.460) | 2.974–3.158 (3.085) | 3.41–6.11 (4.80) | 0.0659 | 0.042 | 0.0539 | 10.3 |
| [Gd(H2O)9]3+·18H2O | 2.449–2.543 (2.482) | 3.054–3.214 (3.119) | 3.73–5.18 (4.40) | −0.0098 | 0.064 | 0.0081 | 0.232 |
| [Gd(EDTA)(H2O)2]−·5H2O | 2.529; 2.540 (2.535) | 3.011–3.111 (3.062) | 3.15; 3.46 (3.31) | −0.0322 | 0.223 | 0.0282 | 2.82 |
| [Gd(EDTA)(H2O)3]−·4H2O | 2.561–2.671 (2.598) | 3.025–3.163 (3.091) | 2.57–2.92 (2.75) | −0.0335 | 0.281 | 0.0304 | 3.29 |
| [Gd(CDTA)(H2O)2]−·5H2O | 2.513; 2.552 (2.533) | 3.024–3.176 (3.095) | 2.94; 2.85 (2.90) | −0.0380 | 0.327 | 0.0357 | 4.52 |
| [Gd(CDTA)(H2O)3]−·4H2O | 2.561–2.570 (2.562) | 3.025–3.177 (3.100) | 2.59–2.94 (2.79) | 0.0377 | 0.294 | 0.0345 | 4.24 |
Cluster-continuum calculations were also performed on the [Gd(EDTA)(H2O)3]−·5H2O and [Gd(CDTA)(H2O)3]−·5H2O systems, with subsequent relaxed potential energy surface scans leading to the corresponding eight-coordinate species (see ESI†). In the case of CDTA, the bis-hydrated form is more stable than the tris-hydrated species, with the relative free energy of the latter being +7.81 kJ mol−1. However, the q = 2 and q = 3 forms possess very similar free energy values (ΔG° ∼ 0.11 kJ mol−1) in the case of EDTA. This is in good qualitative agreement with the experimental data shown in Table 1, which evidence that the tris-hydrated form is more stable for EDTA than CDTA complexes. The average A/ħ vales calculated for EDTA and CDTA complexes are somewhat lower than those obtained for the [Gd(H2O)9]3+·18H2O and [Gd(H2O)8]3+·19H2O systems, which is expected considering the shorter Gd–O bond distances observed for the latter. Of note, the calculated values of A/ħ = Aiso × 2π are all positive, though values obtained from 17O NMR studies are often incorrectly reported as negative.56 The calculated rGdH values are again very close to the of 3.1 Å, which confirms that this parameter is rather insensitive to the nature of the co-ligands present in the Gd(III) coordination environment.
Electron relaxation in Gd(III) complexes is generally attributed to fluctuations of the zero field splitting (ZFS) energy due to transient distortions of the coordination environment, caused by vibrations and collisions with solvent molecules.57,58 Electron relaxation is believed to receive contributions from both static and transient ZFS effects.59,60 The ZFS energy was calculated using the complete active space self-consistent field (CASSCF) approach, with electron correlation incorporated using N-electron valence state perturbation theory (NEVPT2). This multiconfigurational method provides ZFS energies of Gd(III) complexes of the correct order of magnitude, while the results obtained with DFT are heavily dependent on the functional used.58,61 The ZFS can be defined using the common axial (D) and rhombic (E/D) parameters,62 which are listed in Table 2.
The values of D and E/D obtained with NEVPT2 calculations define highly rhombic ZFS tensors, with E/D being close to its maximum value of 1/3. It has been shown that for E/D values >0.2 the prediction of the sign of D becomes problematic for Mn(II) complexes.62 While this may be the case also for the Gd(III) complexes investigated here, we note that electron relaxation is related to fluctuations in the value of Δ2, and thus only the absolute value of Δ is relevant.
The values of |D| obtained for the complexes of EDTA4− and CDTA4− are relatively similar, being close to those obtained for other Gd(III) complexes using CASSCF-based approaches.58,61,63 We note that the pairs of species involved in hydration equilibrium, for the complexes of EDTA4− and CDTA4−, display very similar values of |D| and E/D, and thus very likely similar electronic relaxation times. The aqua complexes [Gd(H2O)9]3+ and [Gd(H2O)8]3+ are characterized by lower E/D values than the EDTA4− and CDTA4− complexes, which can be ascribed to the more symmetrical coordination environments in the former.64 The value of |D| calculated for the eight-coordinate species is one order of magnitude higher than that of the nine-coordinated species. Conversely, the eight-coordinate form [Gd(H2O)8]3+ displays a |D| value that is comparable to those of the CDTA4− and EDTA4− complexes. This suggests that the TTP coordination environment results in particularly small ZFS energies. This is in line with previous studies, which showed that lanthanide complexes with this coordination environment display particularly sharp EPR lines, which is associated to slow electron spin relaxation due to a nearly zero static ZFS.64,65
The static ZFS energy can be approximated using the following expression:20,60
 | (4) |
The trend observed for the values of |D| calculated for this series of complexes is associated with the splitting of the Kramer's doublets arising from the 8S configuration of Gd(III) (Fig. 4). For axially symmetric systems, the Kramer doublets are separated by energies equal to 2D, 4D and 6D with respect to the lowest energy level if D > 0, while the situation is reversed if D < 0.61 For the rhombic systems investigated here, a D > 0 value is obtained when the separation of the Kramer doublets increases on increasing energy (i.e. [Gd(H2O)8]3+), while a negative D value is observed in the opposite case.
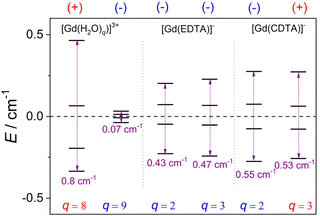 | ||
| Fig. 4 Splitting of the Kramers doublets obtained with CASSCF/NEVPT2 calculations and the signs of D. | ||
Simultaneous fit of the 1H NMRD and 17O NMR data
The simultaneous fit of the experimental relaxation and chemical shift data is the most convenient approach to estimate the parameters that determine the relaxivity of a given complex.46 The 1H relaxivity data were fit to the standard Solomon–Bloembergen–Morgan theory of paramagnetic relaxation,66–68 while the Swift–Connick69 equations were used for 17O NMR. The two sets of data are affected by several common parameters: the exchange rate of the coordinated water molecules expressed by k298ex and its activation enthalpy ΔH‡, and the parameters describing electron relaxation, namely the correlation time for the fluctuation of the ZFS interaction, τV, and the mean-square zero field splitting energy, Δ2. Separate fits of the data generally improve the agreement between experimental and calculated data, but do not necessarily yield more accurate parameters.The equilibrium expressed by eqn (2) implies that the rate constants for the forward (k+) and reverse (k−) processes are related by the equilibrium constant, so that Keq = k+/k−. Thus, we used a single set of k298ex and ΔH‡ parameters for the fits, corresponding to the process in which a water molecule enters the coordination sphere. The number of coordinated water molecules q was expressed by eqn (3), using the thermodynamic data shown in Table 1. The values of 1/T2r and Δωr were also calculated at each temperature from the molar fraction of coordinated water molecules obtained as Pm = (cq)/55.55.
Some parameters had to be fixed to reasonable values for a reliable analysis. The distance between proton nuclei of coordinated water molecules and the metal ion was fixed to 3.0 Å, a value that is close to those obtained by the DFT calculations described above and within the range afforded by ENDOR studies (3.1 ± 0.1 Å).54,70 The distance of closest approach of an outer-sphere water molecule was fixed to 4.0 Å on the grounds of previous studies.71 The parameters related to diffusion were also fixed to reasonable values: the diffusion coefficient was set to D298GdH = 2.3 × 10−9 m2 s−1 and its activation energy was constrained to ED = 20 kJ mol−1.72 Finally, we had to fix the value of the activation energy for the modulation of the zero field splitting (Ev = 1 kJ mol−1), as otherwise unreasonable values were obtained. We fitted a single value for A/ħ, τV and Δ2 to avoid over-parametrization of the model. This approach is supported by our theoretical calculations, which indicate that these parameters differ only slightly for the eight- and nine-coordinate species in the case of EDTA4− and CDTA4− complexes (Table 2). For the aqua complex, the nine-coordinate species accounts for ∼20–35% of the overall population in the temperature range investigated (Table 1). Thus, we performed the fits of the data assuming different values of Δ2 for the eight- and nine-coordinate species.
The agreement between the experimental and calculated 1/T2r and Δωr data is excellent for the three complexes investigated here, as shown in Fig. 1. The calculated 1H NMRD data are also in good agreement with the experiment, considering the large number of parameters fitted and the assumptions made to perform the analysis (Fig. 2). For [Gd(H2O)q]3+ and [Gd(CDTA)(H2O)q]− the eight-coordinate species is either largely dominant ([Gd(CDTA)(H2O)q]−), or its population is barely affected by temperature ([Gd(H2O)q]3+). As a result, the simultaneous fits performed including the hydration equilibrium, or assuming coordination number 8, provide very similar fitted parameters and R2 values (Table 3). The most important difference is the k298ex of [Gd(H2O)q]3+, which decreases by ∼22% upon considering the hydration equilibrium.
| [Gd(H2O)q]3+ | [Gd(EDTA)(H2O)q]− | [Gd(CDTA)(H2O)q]− | ||||
|---|---|---|---|---|---|---|
| q | 8 ⇄ 9 | 8 | 2 ⇄ 3 | 2 | 2 ⇄ 3 | 2 |
| a Parameters fixed during the fitting procedure. b This value corresponds to [Gd(H2O)8]3+. For the minor [Gd(H2O)9]3+ species the fit affords Δ2 = 0.6 × 1019 s−2 with a large statistical error (see text). | ||||||
| 298 Δ 2/1019 s−2 | 19.2 ± 3.2b | 11.3 ± 2.0 | 1.61 ± 0.16 | 0.79 ± 0.07 | 2.49 ± 0.13 | 2.23 ± 0.12 |
| 298 τ V/ps | 6.7 ± 0.9 | 7.1 ± 1.0 | 30.7 ± 2.7 | 54.3 ± 4.7 | 32.4 ± 1.6 | 34.9 ± 1.8 |
| E V/kJ mol−1 | 1.0a | 1.0a | 1.0a | 1.0a | 1.0a | 1.0a |
| A O/ħ/106 rad s−1 | 4.5 ± 0.6 | 4.6 ± 0.6 | 4.0 ± 0.3 | 4.0 ± 0.3 | 4.0 ± 0.2 | 4.0 ± 0.2 |
| C os | 0.1a | 0.1a | 0.1a | 0.1a | 0.1a | 0.1a |
| τ 298M/ns | 2.0 ± 0.7 | 1.6 ± 0.5 | 7.5 ± 1.2 | 5.9 ± 1.0 | 5.8 ± 0.8 | 5.4 ± 0.8 |
| k 298ex/106 s−1 | 490 ± 166 | 632 ± 212 | 134 ± 21 | 169 ± 30 | 173 ± 23 | 184 ± 26 |
| ΔH‡/kJ mol−1 | 16.5 ± 6.1 | 16.1 ± 6.1 | 24.4 ± 2.6 | 23.5 ± 3.0 | 21.9 ± 2.3 | 21.0 ± 2.4 |
| ΔS‡/J mol−1 K−1 | −23.0 ± 5.0 | −22.0 ± 5.0 | −8.0 ± 2.0 | −9.0 ± 3.0 | −14.0 ± 2.0 | −16.0 ± 2.0 |
| 298 τ R/ps | 32.8 ± 0.6 | 33.4 ± 0.6 | 50.8 ± 0.8 | 59.4 ± 0.6 | 64.0 ± 0.6 | 64.7 ± 0.6 |
| E R/kJ mol−1 | 14.5 ± 0.4 | 14.0 ± 0.6 | 24.3 ± 0.3 | 26.9 ± 0.4 | 25.7 ± 0.4 | 26.5 ± 0.4 |
| r GdH/Å | 3.0a | 3.0a | 3.0a | 3.0a | 3.0a | 3.0a |
| a GdH/Å | 4.0a | 4.0a | 4.0a | 4.0a | 4.0a | 4.0a |
| 298 D/10−9 m2 s−1 | 2.3a | 2.3a | 2.3a | 2.3a | 2.3a | 2.3a |
| E D/kJ mol−1 | 20.0a | 20.0a | 20.0a | 20.0a | 20.0a | 20.0a |
| R 2 | 0.99657 | 0.99648 | 0.99808 | 0.99748 | 0.99787 | 0.99761 |
The quality of the fit for [Gd(EDTA)(H2O)q]− deteriorates significantly if the hydration equilibrium is neglected. Furthermore, the values of τV and Δ2 experience a strong impact if neglecting the hydration equilibrium. In particular, τV is an effective correlation time that should be intermediate between that expected for the transient ZFS (<1 ps) and that of the static ZFS, which is modulated by τR.60 The value of τV obtained assuming q = 2 (54.3 ps, Table 3) is virtually identical to that of τ298R (59.4 ps). This would imply that transient ZFS does not contribute to the relaxation of the electron spin, a situation that is extremely unlikely. When considering the hydration equilibrium, τV assumes a more reasonable value of 31 ps, which is close to that obtained for [Gd(CDTA)(H2O)q]−. The values of τ298R and its activation energy ER also vary significantly depending on whether the hydration equilibrium is taken into account or not. In particular, the value of ER for small Gd(III) complexes should be close to 20 kJ mol−1.4,73 Taking this value as a reference, it is clear that the fit of the data neglecting the hydration equilibrium worsens the quality of the value obtained for ER. Thus, we conclude that considering the hydration equilibrium provides more reliable fitted parameters. This appears to be particularly important in cases where the two species at equilibrium present similar populations in solution. Of note, the value of k298ex increases by ∼26% when neglecting the hydration equilibrium.
Rotational dynamics
The 298τR values obtained from the fits of the data and the corresponding activation energies ER are very similar to those determined for small Gd(III) complexes.4,73 For [Gd(H2O)q]3+, different EPR studies afforded 298τR values of 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 74 and 23 ps,75 in good agreement with the present study. Previous NMRD studies gave a somewhat longer 298τR value of 41 ps.4 However, the latter study involved simultaneous fit of NMRD and T117O relaxation data. It was later found that the correlation time for the Gd–Owater vector is sensibly longer than that of the Gd–Hwater vector (τRH/τRO = 0.65),76 explaining the longer τR. The 298τR values obtained for the EDTA4− and CDTA4− complexes are longer than for the aqua ion, as their molecular masses are greater.
74 and 23 ps,75 in good agreement with the present study. Previous NMRD studies gave a somewhat longer 298τR value of 41 ps.4 However, the latter study involved simultaneous fit of NMRD and T117O relaxation data. It was later found that the correlation time for the Gd–Owater vector is sensibly longer than that of the Gd–Hwater vector (τRH/τRO = 0.65),76 explaining the longer τR. The 298τR values obtained for the EDTA4− and CDTA4− complexes are longer than for the aqua ion, as their molecular masses are greater.
Hyperfine coupling constants
The value of AO/ħ determined for the aqua ion (4.5 × 106 rad s−1) is somewhat lower than those obtained in previous 17O NMR studies (5.3 × 106 rad s−1).4,77 Our value is in turn in excellent agreement with different computational studies,55,78 including the average values obtained with DFT in this work (Table 2). The values of AO/ħ are somewhat lower for the complexes of EDTA4− and CDTA4− (4.0 × 106 rad s−1). This value of AO/ħ is very similar to those obtained for Gd(III) complexes other than the aqua-ion: (3.9 ± 0.3) × 106 rad s−1.55 Our DFT calculations (Table 2) provide AO/ħ values that are somewhat lower than the experimental ones. We attribute this effect to the functional used for geometry optimization (wB97XD), which was chosen for this work because it provides good estimates for activation energies of water exchange reactions.79 In comparison with other functionals like TPPSh, wB97XD affords slightly longer Gd–Owater distances,80 resulting in lower AO/ħ values.Electron relaxation
The fits reported in Table 3 and performed considering hydration equilibria, provide very reasonable values of τV and Δ2. The correlation time τV takes values intermediate between τR and the correlation time expected for the transient ZFS (<1 ps),58,60 suggesting that both the transient and static ZFS have a significant contribution to electron relaxation. Interestingly, the values of Δ2 estimated using theoretical calculations (Table 2) follow very well the trend of the experimental values. In the case of [Gd(H2O)q]3+ the eight-coordinate species is the most abundant one in solution. The fits and NEVPT2 calculations give Δ2 values for [Gd(H2O)8]3+ in good mutual agreement (19.2 × 1019 and 10.3 × 1019 s−2), considering the difficulties associated with the calculation of ZFS parameters.62 Our fits give a Δ2 value for [Gd(H2O)9]3+ that is one order of magnitude lower (Δ2 = 0.6 ± 1.4 × 1019 s−2), though the low abundance of this species resulted in a large statistical error. Nevertheless, these results support the fact that two aqua complexes at equilibrium have very different ZFS energies, in agreement with the theoretical study. The value of Δ2 obtained from the fits for [Gd(CDTA)(H2O)q]− is slightly higher than that of [Gd(EDTA)(H2O)q]− by a factor of ∼1.5, a trend that is also reproduced by the theoretical data.Water exchange rates
The best estimate of [Gd(H2O)q]3+k298ex (804 × 106 s−1) reported to date was obtained from a combined NMRD, 17O NMR and EPR study, assuming that q = 8.4 The analysis of our data incorporating the hydration equilibrium indicates that this value is somewhat lower (k298ex = 490 × 106 s−1), but remains one of the fastest water exchange rates reported to date for a Gd(III) complex.81 Water exchange is somewhat lower for [Gd(EDTA)(H2O)q]− and [Gd(CDTA)(H2O)q]−, following the general trend that coordination of polyaminocarboxylate ligands slows down water exchange with respect to the aqua ion. Water exchange is slightly faster for [Gd(CDTA)(H2O)q]− than for [Gd(EDTA)(H2O)q]−. This is in contrast with previous studies on q = 1 Gd(III) complexes, which showed that rigidifying the ligand structure by replacing an ethyl group by a cyclohexyl moiety slowed water exchange considerably.35,82The values of the entropy of activation (ΔS‡) for water exchange are negative, suggesting associatively activated water exchange mechanisms for the eight-coordinate complexes.83 In the case of [Gd(H2O)q]3+ this was confirmed by variable pressure 17O NMR measurements, which afforded a negative activation volume of ΔV‡ = −3.3 cm3 mol−1.4 The same study reported a ΔS‡ value identical to that obtained in this work. Given the negative ΔS‡ value obtained for [Gd(H2O)q]3+, it may be somewhat surprising the positive reaction entropy reported for the hydration equilibrium expressed as in eqn (2). Most likely this positive ΔS is related to more ordered second-sphere hydration shell in the eight-coordinate [Gd(H2O)8]3+ species.
The water exchange mechanism for [Gd(H2O)8]3+ and [Gd(EDTA)(H2O)2]− were further investigated by computing the transition states responsible for the interconversion between eight- and nine-coordinate species. For the [Gd(H2O)8]3+·19H2O system, one of the second sphere water molecules with a Gd⋯Owater distance of 4.21 Å approaches the Gd(III) ion to 3.43 Å to reach the transition state, while the coordinated water molecules experience very minor changes in Gd–Owater distances. The values of ΔH‡ and ΔS‡ obtained by DFT are 8.4 kJ mol−1 and −14.8 J mol−1 K−1, respectively. For [Gd(EDTA)(H2O)2]−·7H2O DFT affords ΔH‡ = 8.7 kJ mol−1 and ΔS‡ = −30.2 J mol−1 K−1, with a Gd⋯OH2 distance in the transition state involving the entering water molecule of 3.30 Å. Thus, DFT affords activation parameters in qualitative good agreement with the experimental data, and confirms that water exchange in this family of eight-coordinated species is associatively activated.
Conclusions
Over the last twenty years, the increasing availability of commercial instruments for the accurate measurement of the frequency dependence of nuclear magnetic relaxation times of solvent protons in solutions of paramagnetic metal complexes has made the fast field-cycling relaxometry technique a relevant tool for coordination chemists. The most striking example concerns the remarkable knowledge acquired of the properties of metal complexes of Gd(III), Mn(II) and Fe(III) in aqueous solution, which are investigated as MRI probes. The measurement and analysis of NMRD profiles allows obtaining reliable information on the hydration state, the metal–water protons distance, the rotational dynamics, the exchange rate of metal–bound water molecules and the electronic relaxation times of the paramagnetic ion.By simultaneously analysing 1H NMRD profiles with R2 and shift 17O NMR data, measured at high field on a high-resolution spectrometer, more accurate and meaningful results are obtained. However, in the not an uncommon case in which the complexes exist in solution as a mixture of species differing in the hydration state (fractional effective q value), the application of this procedure only provides values of the molecular parameters that are the weighted average of the two forms with different q. The knowledge of the thermodynamic parameters of the exchange process between the two species, obtained via UV-Vis spectroscopy, and the results of computational techniques allow the relaxometric analysis to evaluate with high accuracy the separate contributions of the two species, if their relative populations do not differ excessively. We have used this approach for the first time, applying it to the case of the Gd(III) aqua ion and to complexes with EDTA4− and CDTA4−. The results obtained are very satisfactory both for the better quality of the best-fitting parameters of the relaxometric data and for the new insights into the property–structure relationship, with particular regard to the exchange process of inner-sphere water molecules and electronic relaxation. We think that this approach can provide considerable help to better understand the structure and dynamics in aqueous solution of the numerous paramagnetic Gd(III) complexes characterized by fractional q.
Experimental and computational section
General considerations
All reagents used were purchased from commercial sources and were of reagent grade quality. The [Gd(H2O)q]3+ sample was prepared by dissolving GdCl3·6H2O in water at pH = 4. The [GdL(H2O)q]− (L = EDTA, CDTA) complexes were prepared by adding 1.1 equiv. of the Gd(III) salt to an aqueous solution of the L ligand at pH = 6. After the addition, the pH was adjusted to 6.0 with a diluted NaOH solution and the solution was stirred at room temperature for 5 h. Then, the pH was increased to 10 with 0.1 M NaOH, and the solution was stirred for 3 h, to promote the precipitation of the free Gd(III) as hydroxides. The solution was finally filtered through 0.2 μm filters and neutralized with 0.1 M HCl.1H NMRD and 17O NMR measurements
1/T11H Nuclear Magnetic Relaxation Dispersion (NMRD) profiles were collected with a Fast-Field Cycling (FFC) Stelar SmarTracer Relaxometer (Stelar s.r.l., Mede, PV, Italy) over a continuum of proton Larmor frequencies from 9.97 × 10−3 to 10 MHz. Additional data in the range 20–120 MHz proton Larmor frequency were measured with a High Field Relaxometer (Stelar) equipped with the HTS-110 3T Metrology Cryogen-free Superconducting Magnet. The analyses were carried out by using the standard inversion recovery sequence (20 experiments, 2 scans) with a typical 90° pulse width of 3.5 μs and the reproducibility of the data was within ±0.5%. The temperature was controlled with a Stelar VTC-91 heater airflow equipped with a copper–constantan thermocouple (uncertainty of ±0.1 K). R1 values at 500 MHz were measured with Bruker Avance III spectrometer (11.7 T) equipped with a 5 mm probe.17O NMR measurements were recorded on a Bruker Avance III spectrometer (11.7 T) equipped with a 5 mm probe and standard temperature control unit. Aqueous solutions of the complexes were enriched to reach 2.0% of the 17O isotope (Cambridge Isotope). The transverse relaxation rates were calculated at different temperatures (278–353 K) from the signal width at a half-height. The concentration of the metal complexes was assessed by 1H-NMR measurements (Bruker Avance III Spectrometer equipped with a wide bore 11.7 Tesla magnet), by using the well-established bulk magnetic susceptibility method.84
Computational details
The geometries of the Gd(III) complexes were optimized with the Gaussian 16 program package (revision C.01)85 using the wB97XD functional, which is a long-range corrected hybrid density functional incorporating atom–atom dispersion corrections.86 In these calculations we employed a large-core effective core potential for the lanthanide (LCECP) including 53 electrons in the core for Gd(III)87 and the Def2-TZVPP basis set for all ligand atoms.88 The integration grid was set with the integral = superfinegrid command. Bulk water solvent effects were considered using a polarizable continuum model49 using scrf = (pcm, solvent = water) and the default options implemented in Gaussian, except the radii of O and H, which were taken as 1.925 and 1.5873 Å, respectively.52 Frequency calculations were used to confirm that the optimized structures correspond to stationary points on the potential energy surface. Transition states were located using the Transit-Guided Quasi-Newton (STQN3) method,89 and characterized by a single negative frequency with atom displacements that signal the approach of the entering water molecule to the eight-coordinate complex.The ORCA program package (version 5.0.3)90,91 was used to calculate isotropic 17O hyperfine coupling constants and the ZFS tensor. Relativistic effects were incorporated using the Douglas–Kroll–Hess (DKH2) approximation.92,93 The SARC2-DKH-QZVP94 and DKH-def2-TZVPP95 basis sets were used for Gd and ligand atoms, respectively. The resolution of identity and chain of spheres (RIJCOSX) method96–98 was used throughout with the SARC2-DKH-QZVP/JK94 auxiliary basis set for Gd and auxiliary basis sets generated with the Autoaux99 method for ligand atoms. Hyperfine coupling constants were obtained using DFT with the TPSSh functional,100 which was found to perform well for this specific problem.55,101,102 The quasi-restricted orbitals103 generated from these calculations were used as starting orbitals for complete active space self-consistent field (CASSCF) calculations,104,105 in which dynamic correlation was incorporated using the quasi-degenerate106 strongly contracted variant of N-electron valence state perturbation theory (SC-NEVPT2).106,107 Spin–orbit coupling effects were introduced using quasi-degenerate perturbation theory (QDPT).108,109 The active space of the state-averaged CASSCF calculations consisted of seven electrons distributed over the seven Gd-based 7f orbitals CAS(7,7), including one octet and 48 sextet roots. Bulk water solvent effects in all ORCA calculations were included with the SMD solvation model.110
Author contributions
A. N.: synthesis, purification, and characterization of the complexes; NMRD profiles. F. C.: 17O NMR data, relaxivity measurements, data analysis. C. P.-I.: DFT calculations, NMR data analysis, resources, conceptualization and manuscript preparation. M. B.: project supervision, conceptualization, data analysis resources and manuscript preparation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. N. acknowledges Università del Piemonte Orientale for the PhD grant. C. P.-I. thanks Ministerio de Ciencia e Innovación (grants PID2019-104626GB-I00 and PID2022-138335NB-I00) and Xunta de Galicia (grant ED431C 2023/33) for generous financial support. C. P.-I. also thanks Centro de Supercomputación de Galicia (CESGA) for providing the computer facilities.References
- S. Aime, M. Botta, D. Esteban-Gómez and C. Platas-Iglesias, Characterisation of magnetic resonance imaging (MRI) contrast agents using NMR relaxometry, Mol. Phys., 2019, 117, 898–909 CrossRef CAS.
- G. Parigi, E. Ravera, M. Fragai and C. Luchinat, Unveiling protein dynamics in solution with field-cycling NMR relaxometry, Prog. Nucl. Magn. Reson. Spectrosc., 2021, 124–125, 85–98 CrossRef CAS PubMed.
- S. H. Koenig, C. M. Baglin and R. D. Brown, Magnetic field dependence of solvent proton relaxation in aqueous solutions of Fe3+ complexes, Magn. Reson. Med., 1985, 2, 283–288 CrossRef CAS PubMed.
- D. H. Powell, O. M. N. Dhubhghaill, D. Pubanz, L. Helm, Y. S. Lebedev, W. Schlaepfer and A. E. Merbach, Structural and Dynamic Parameters Obtained from 17O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd3+ Complexes of Interest in Magnetic Resonance Imaging: An Integrated and Theoretically Self-Consistent Approach, J. Am. Chem. Soc., 1996, 118, 9333–9346 CrossRef CAS.
- Y. Gossuin, Z. Serhan, L. Sandiford, D. Henrard, T. Marquardsen, R. T. M. De Rosales, D. Sakellariou and F. Ferrage, Sample Shuttling Relaxometry of Contrast Agents: NMRD Profiles above 1 T with a Single Device, Appl. Magn. Reson., 2016, 47, 237–246 CrossRef CAS PubMed.
- S. Dumas, V. Jacques, W.-C. Sun, J. S. Troughton, J. T. Welch, J. M. Chasse, H. Schmitt-Willich and P. Caravan, High Relaxivity Magnetic Resonance Imaging Contrast Agents Part 1 Impact of Single Donor Atom Substitution on Relaxivity of Serum Albumin-Bound Gadolinium Complexes, Invest. Radiol., 2010, 45, 13 CrossRef PubMed.
- S. Aime, A. Barge, J. I. Bruce, M. Botta, J. A. K. Howard, J. M. Moloney, D. Parker, A. S. de Sousa and M. Woods, NMR, Relaxometric, and Structural Studies of the Hydration and Exchange Dynamics of Cationic Lanthanide Complexes of Macrocyclic Tetraamide Ligands, J. Am. Chem. Soc., 1999, 121, 5762–5771 CrossRef CAS.
- S. Aime, M. Botta, G. Ermondi, F. Fedeli and F. Uggeri, Synthesis and NMRD studies of gadolinium(3+) complexes of macrocyclic polyamino polycarboxylic ligands bearing .beta.-benzyloxy-alpha-propionic residues, Inorg. Chem., 1992, 31, 1100–1103 CrossRef CAS.
- I. Bertini, F. Capozzi, C. Luchinat and Z. Xia, Nuclear and electron relaxation of hexaaquairon(3+), J. Phys. Chem., 1993, 97, 1134–1137 CrossRef CAS.
- Z. Baranyai, F. Carniato, A. Nucera, D. Horváth, L. Tei, C. Platas-Iglesias and M. Botta, Defining the conditions for the development of the emerging class of FeIII-based MRI contrast agents, Chem. Sci., 2021, 12, 11138–11145 RSC.
- R. Uzal-Varela, F. Lucio-Martínez, A. Nucera, M. Botta, D. Esteban-Gómez, L. Valencia, A. Rodríguez-Rodríguez and C. Platas-Iglesias, A systematic investigation of the NMR relaxation properties of Fe(III)-EDTA derivatives and their potential as MRI contrast agents, Inorg. Chem. Front., 2023, 10, 1633–1649 RSC.
- I. Bertini, Z. Xia and C. Luchinat, Solvent water 1H NMRD study of oxovanadium(IV) aquo ion, J. Magn. Reson. (1969–1992), 1992, 99, 235–246 CrossRef CAS.
- V. Lagostina, F. Carniato, D. Esteban-Gómez, C. Platas-Iglesias, M. Chiesa and M. Botta, Magnetic and relaxation properties of vanadium(IV) complexes: an integrated 1H relaxometric, EPR and computational study, Inorg. Chem. Front., 2023, 10, 1999–2013 RSC.
- E. Balogh, Z. He, W. Hsieh, S. Liu and É. Tóth, Dinuclear Complexes Formed with the Triazacyclononane Derivative ENOTA4−: High-Pressure 17O NMR Evidence of an Associative Water Exchange on [MnII2(ENOTA)(H2O)2], Inorg. Chem., 2007, 46, 238–250 CrossRef CAS PubMed.
- I. Bertini, F. Briganti, Z. Xia and C. Luchinat, Nuclear Magnetic Relaxation Dispersion Studies of Hexaaquo Mn(II) ions in Water-Glycerol Mixtures, J. Magn. Reson., Ser. A, 1993, 101, 198–201 CrossRef CAS.
- D. Esteban-Gómez, C. Cassino, M. Botta and C. Platas-Iglesias, 17O and 1H relaxometric and DFT study of hyperfine coupling constants in [Mn(H2O)6]2+, RSC Adv., 2014, 4, 7094 RSC.
- B.-T. Doan, S. Meme and J.-C. Beloeil, in The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, ed. A. Merbach, L. Helm and É. Tóth, John Wiley & Sons, Ltd, Chichester, UK, 2013, pp. 1–23 Search PubMed.
- J. Wahsner, E. M. Gale, A. Rodríguez-Rodríguez and P. Caravan, Chemistry of MRI Contrast Agents: Current Challenges and New Frontiers, Chem. Rev., 2019, 119, 957–1057 CrossRef CAS PubMed.
- P. Caravan, J. J. Ellison, T. J. McMurry and R. B. Lauffer, Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications, Chem. Rev., 1999, 99, 2293–2352 CrossRef CAS PubMed.
- L. Helm, Relaxivity in paramagnetic systems: Theory and mechanisms, Prog. Nucl. Magn. Reson. Spectrosc., 2006, 49, 45–64 CrossRef CAS.
- J. H. Freed, Dynamic effects of pair correlation functions on spin relaxation by translational diffusion in liquids. II. Finite jumps and independent T1 processes, J. Chem. Phys., 1978, 68, 4034–4037 CrossRef CAS.
- A. Vágner, E. Gianolio, S. Aime, A. Maiocchi, I. Tóth, Z. Baranyai and L. Tei, High kinetic inertness of a bis-hydrated Gd-complex with a constrained AAZTA-like ligand, Chem. Commun., 2016, 52, 11235–11238 RSC.
- S. Aime, L. Calabi, C. Cavallotti, E. Gianolio, G. B. Giovenzana, P. Losi, A. Maiocchi, G. Palmisano and M. Sisti, [Gd-AAZTA]−: A New Structural Entry for an Improved Generation of MRI Contrast Agents, Inorg. Chem., 2004, 43, 7588–7590 CrossRef CAS PubMed.
- C. Ferroud, H. Borderies, E. Lasri, A. Guy and M. Port, Synthesis of a novel amphiphilic GdPCTA-[12] derivative as a potential micellar MRI contrast agent, Tetrahedron Lett., 2008, 49, 5972–5975 CrossRef CAS.
- C. S. Bonnet, S. Laine, F. Buron, G. Tircsó, A. Pallier, L. Helm, F. Suzenet and É. Tóth, A Pyridine-Based Ligand with Two Hydrazine Functions for Lanthanide Chelation: Remarkable Kinetic Inertness for a Linear, Bishydrated Complex, Inorg. Chem., 2015, 54, 5991–6003 CrossRef CAS PubMed.
- J. Costa, É. Tóth, L. Helm and A. E. Merbach, Dinuclear, Bishydrated GdIII Polyaminocarboxylates with a Rigid Xylene Core Display Remarkable Proton Relaxivities, Inorg. Chem., 2005, 44, 4747–4755 CrossRef CAS PubMed.
- Z. Baranyai, M. Botta, M. Fekete, G. B. Giovenzana, R. Negri, L. Tei and C. Platas-Iglesias, Lower Ligand Denticity Leading to Improved Thermodynamic and Kinetic Stability of the Gd3+ Complex: The Strange Case of OBETA, Chem. – Eur. J., 2012, 18, 7680–7685 CrossRef CAS PubMed.
- J. Hao, P. Bourrinet and P. Desché, Assessment of Pharmacokinetic, Pharmacodynamic Profile, and Tolerance of Gadopiclenol, A New High Relaxivity GBCA, in Healthy Subjects and Patients With Brain Lesions (Phase I/IIa Study), Invest. Radiol., 2019, 54, 396–402 CrossRef CAS PubMed.
- C. Robic, M. Port, O. Rousseaux, S. Louguet, N. Fretellier, S. Catoen, C. Factor, S. Le Greneur, C. Medina, P. Bourrinet, I. Raynal, J.-M. Idée and C. Corot, Physicochemical and Pharmacokinetic Profiles of Gadopiclenol: A New Macrocyclic Gadolinium Chelate With High T1 Relaxivity, Invest. Radiol., 2019, 54, 475–484 CrossRef CAS PubMed.
- A. Beeby, I. M. Clarkson, R. S. Dickins, S. Faulkner, D. Parker, L. Royle, A. S. de Sousa, J. A. G. Williams and M. Woods, Non-radiative deactivation of the excited states of europium, terbium and ytterbium complexes by proximate energy-matched OH, NH and CH oscillators: an improved luminescence method for establishing solution hydration states, J. Chem. Soc., Perkin Trans. 2, 1999, 493–504 RSC.
- R. M. Supkowski and W. D. Horrocks, On the determination of the number of water molecules, q, coordinated to europium(III) ions in solution from luminescence decay lifetimes, Inorg. Chim. Acta, 2002, 340, 44–48 CrossRef CAS.
- K. Djanashvili and J. A. Peters, How to determine the number of inner-sphere water molecules in lanthanide(III) complexes by 17O NMR spectroscopy. A technical note, Contrast Media Mol. Imaging, 2007, 2, 67–71 CrossRef CAS PubMed.
- N. Graeppi, D. Hugh Powell, G. Laurenczy, L. Zékány and A. E. Merbach, Coordination equilibria and water exchange kinetics of lanthanide(III) propylenediaminetetraacetates and other magnetic resonance imaging related complexes, Inorg. Chim. Acta, 1995, 235, 311–326 CrossRef CAS.
- É. Tóth, O. M. N. Dhubhghaill, G. Besson, L. Helm and A. E. Merbach, Coordination equilibrium—a clue for fast water exchange on potential magnetic resonance imaging contrast agents?, Magn. Reson. Chem., 1999, 701–708 CrossRef.
- E. Balogh, M. Mato-Iglesias, C. Platas-Iglesias, É. Tóth, K. Djanashvili, J. A. Peters, A. de Blas and T. Rodríguez-Blas, Pyridine- and Phosphonate-Containing Ligands for Stable Ln Complexation. Extremely Fast Water Exchange on the GdIII Chelates, Inorg. Chem., 2006, 45, 8719–8728 CrossRef CAS PubMed.
- M. Mato-Iglesias, A. Roca-Sabio, Z. Pálinkás, D. Esteban-Gómez, C. Platas-Iglesias, É. Tóth, A. de Blas and T. Rodríguez-Blas, Lanthanide Complexes Based on a 1,7-Diaza-12-crown-4 Platform Containing Picolinate Pendants: A New Structural Entry for the Design of Magnetic Resonance Imaging Contrast Agents, Inorg. Chem., 2008, 47, 7840–7851 CrossRef CAS PubMed.
- C. Platas-Iglesias, D. M. Corsi, L. V. Elst, R. N. Muller, D. Imbert, J.-C. G. Bünzli, É. Tóth, T. Maschmeyer and J. A. Peters, Stability, structure and dynamics of cationic lanthanide(III) complexes of N,N′-bis(propylamide)ethylenediamine-N,N′-diacetic acid, Dalton Trans., 2003, 727–737 RSC.
- F. Yerly, F. A. Dunand, É. Tóth, A. Figueirinha, Z. Kovács, A. D. Sherry, C. F. G. C. Geraldes and A. E. Merbach, Spectroscopic Study of the Hydration Equilibria and Water Exchange Dynamics of Lanthanide(III) Complexes of 1,7-Bis(carboxymethyl)-1,4,7,10-tetraazacyclododecane (DO2A), Eur. J. Inorg. Chem., 2000, 1001–1006 CrossRef CAS.
- R. Janicki and A. Mondry, Structural and thermodynamic aspects of hydration of Gd(III) systems, Dalton Trans., 2019, 48, 3380–3391 RSC.
- K. Djanashvili, C. Platas-Iglesias and J. A. Peters, The structure of the lanthanide aquo ions in solution as studied by 17O NMR spectroscopy and DFT calculations, Dalton Trans., 2008, 602–607 RSC.
- C. Cossy, L. Helm, D. H. Powell and A. E. Merbach, A change in coordination number from nine to eight along the lanthanide(III) aqua ion series in solution: a neutron diffraction study, New J. Chem., 1995, 19, 27–35 CAS.
- C. Cossy, L. Helm and A. E. Merbach, Oxygen-17 nuclear magnetic resonance kinetic study of water exchange on the lanthanide(III) aqua ions, Inorg. Chem., 1988, 27, 1973–1979 CrossRef CAS.
- R. V. Southwood-Jones, W. L. Earl, K. E. Newman and A. E. Merbach, Oxygen-17 NMR and EPR studies of water exchange from the first coordination sphere of gadolinium(III) aquoion and gadolinium(III) propylenediaminetetra-acetate, J. Chem. Phys., 1980, 73, 5909–5918 CrossRef CAS.
- J. Maigut, R. Meier, A. Zahl and R. van Eldik, Triggering Water Exchange Mechanisms via Chelate Architecture. Shielding of Transition Metal Centers by Aminopolycarboxylate Spectator Ligands, J. Am. Chem. Soc., 2008, 130, 14556–14569 CrossRef CAS PubMed.
- S. H. Koenig, C. Baglin, R. D. Brown and C. Fred Brewer, Magnetic field dependence of solvent proton relaxation induced by Gd3+ and Mn2+ complexes, Magn. Reson. Med., 1984, 1, 496–501 CrossRef CAS PubMed.
- J. A. Peters, The reliability of parameters obtained by fitting of 1H NMRD profiles and 17O NMR data of potential Gd3+-based MRI contrast agents: Fitting of 1H NMRD profiles and 17O NMR data, Contrast Media Mol. Imaging, 2016, 11, 160–168 CrossRef CAS PubMed.
- D. Riccardi, H.-B. Guo, J. M. Parks, B. Gu, L. Liang and J. C. Smith, Cluster-Continuum Calculations of Hydration Free Energies of Anions and Group 12 Divalent Cations, J. Chem. Theory Comput., 2013, 9, 555–569 CrossRef CAS PubMed.
- K. E. Gutowski and D. A. Dixon, Predicting the Energy of the Water Exchange Reaction and Free Energy of Solvation for the Uranyl Ion in Aqueous Solution, J. Phys. Chem. A, 2006, 110, 8840–8856 CrossRef CAS PubMed.
- J. Tomasi, B. Mennucci and R. Cammi, Quantum Mechanical Continuum Solvation Models, Chem. Rev., 2005, 105, 2999–3094 CrossRef CAS PubMed.
- C. W. Bock, G. D. Markham, A. K. Katz and J. P. Glusker, The Arrangement of First- and Second-shell Water Molecules Around Metal Ions: Effects of Charge and Size, Theor. Chem. Acc., 2006, 115, 100–112 Search PubMed.
- G. D. Markham, J. P. Glusker and C. W. Bock, The Arrangement of First- and Second-Sphere Water Molecules in Divalent Magnesium Complexes: Results from Molecular Orbital and Density Functional Theory and from Structural Crystallography, J. Phys. Chem. B, 2002, 106, 5118–5134 CrossRef CAS.
- M. Regueiro-Figueroa, D. Esteban-Gómez, R. Pujales-Paradela, L. Caneda-Martínez, A. De Blas and C. Platas-Iglesias, Water exchange rates and mechanisms in tetrahedral [Be(H2O)4]2+ and [Li(H2O)4]+ complexes using DFT methods and cluster-continuum models, Int. J. Quantum Chem., 2016, 116, 1388–1396 CrossRef CAS.
- I. Persson, P. D'Angelo, S. De Panfilis, M. Sandström and L. Eriksson, Hydration of Lanthanoid(III) Ions in Aqueous Solution and Crystalline Hydrates Studied by EXAFS Spectroscopy and Crystallography: The Myth of the “Gadolinium Break”, Chem. – Eur. J., 2008, 14, 3056–3066 CrossRef CAS PubMed.
- A. V. Astashkin, A. M. Raitsimring and P. Caravan, Pulsed ENDOR Study of Water Coordination to Gd3+ Complexes in Orientationally Disordered Systems, J. Phys. Chem. A, 2004, 108, 1990–2001 CrossRef CAS.
- D. Esteban-Gómez, A. de Blas, T. Rodríguez-Blas, L. Helm and C. Platas-Iglesias, Hyperfine Coupling Constants on Inner-Sphere Water Molecules of GdIII-Based MRI Contrast Agents, ChemPhysChem, 2012, 13, 3640–3650 CrossRef PubMed.
- O. V. Yazyev, L. Helm, V. G. Malkin and O. L. Malkina, Quantum Chemical Investigation of Hyperfine Coupling Constants on First Coordination Sphere Water Molecule of Gadolinium(III) Aqua Complexes, J. Phys. Chem. A, 2005, 109, 10997–11005 CrossRef CAS PubMed.
- P. H. Fries, Computing Electronic Spin Relaxation for Gd3+-Based Contrast Agents – Practical Implementation, Eur. J. Inorg. Chem., 2012, 2156–2166 CrossRef CAS.
- S. Khan, R. Pollet, R. Vuilleumier, J. Kowalewski and M. Odelius, An ab initio CASSCF study of zero field splitting fluctuations in the octet ground state of aqueous [Gd(III)(HPDO3A)(H2O)], J. Chem. Phys., 2017, 147, 244306 CrossRef PubMed.
- E. Belorizky and P. H. Fries, Simple analytical approximation of the longitudinal electronic relaxation rate of Gd(III) complexes in solutions, Phys. Chem. Chem. Phys., 2004, 6, 2341 RSC.
- P. H. Fries and E. Belorizky, Determination of the Static Zero-Field Splitting of Gd3+ Complexes in Solution from the Shifts of the Central Magnetic Fields of Their EPR Spectra, ChemPhysChem, 2012, 13, 2074–2081 CrossRef CAS PubMed.
- S. Khan, A. Kubica-Misztal, D. Kruk, J. Kowalewski and M. Odelius, Systematic theoretical investigation of the zero-field splitting in Gd(III) complexes: Wave function and density functional approaches, J. Chem. Phys., 2015, 142, 034304 CrossRef PubMed.
- S. Zein and F. Neese, Ab Initio and Coupled-Perturbed Density Functional Theory Estimation of Zero-Field Splittings in MnII Transition Metal Complexes, J. Phys. Chem. A, 2008, 112, 7976–7983 CrossRef CAS PubMed.
- C. Charpentier, J. Salaam, A. Nonat, F. Carniato, O. Jeannin, I. Brandariz, D. Esteban-Gómez, C. Platas-Iglesias, L. J. Charbonnière and M. Botta, pH-Dependent Hydration Change in a Gd-Based MRI Contrast Agent with a Phosphonated Ligand, Chem. – Eur. J., 2020, 26, 5407–5418 CrossRef CAS PubMed.
- A. Borel, H. Kang, C. Gateau, M. Mazzanti, R. B. Clarkson and R. L. Belford, Variable Temperature and EPR Frequency Study of Two Aqueous Gd(III) Complexes with Unprecedented Sharp Lines, J. Phys. Chem. A, 2006, 110, 12434–12438 CrossRef CAS PubMed.
- E. A. Suturina, K. Mason, M. Botta, F. Carniato, I. Kuprov, N. F. Chilton, E. J. L. McInnes, M. Vonci and D. Parker, Periodic trends and hidden dynamics of magnetic properties in three series of triazacyclononane lanthanide complexes, Dalton Trans., 2019, 48, 8400–8409 RSC.
- I. Solomon, Relaxation Processes in a System of Two Spins, Phys. Rev., 1955, 99, 559–565 CrossRef CAS.
- N. Bloembergen, Proton Relaxation Times in Paramagnetic Solutions, J. Chem. Phys., 1957, 27, 572–573 CrossRef CAS.
- N. Bloembergen and L. O. Morgan, Proton Relaxation Times in Paramagnetic Solutions. Effects of Electron Spin Relaxation, J. Chem. Phys., 1961, 34, 842–850 CrossRef CAS.
- T. J. Swift and R. E. Connick, NMR-Relaxation Mechanisms of O17 in Aqueous Solutions of Paramagnetic Cations and the Lifetime of Water Molecules in the First Coordination Sphere, J. Chem. Phys., 1962, 37, 307–320 CrossRef CAS.
- S. G. Zech, W.-C. Sun, V. Jacques, P. Caravan, A. V. Astashkin and A. M. Raitsimring, Probing the Water Coordination of Protein-Targeted MRI Contrast Agents by Pulsed ENDOR Spectroscopy, ChemPhysChem, 2005, 6, 2570–2577 CrossRef CAS PubMed.
- L. Leone, G. Ferrauto, M. Cossi, M. Botta and L. Tei, Optimizing the Relaxivity of MRI Probes at High Magnetic Field Strengths With Binuclear GdIII Complexes, Front. Chem., 2018, 6, 158 CrossRef PubMed.
- L. Vander Elst, A. Sessoye, S. Laurent and R. N. Muller, Can the Theoretical Fitting of the Proton-Nuclear-Magnetic-Relaxation-Dispersion (Proton NMRD) Curves of Paramagnetic Complexes Be Improved by Independent Measurement of Their Self-Diffusion Coefficients?, Helv. Chim. Acta, 2005, 88, 574–587 CrossRef CAS.
- H. Lammers, F. Maton, D. Pubanz, M. W. van Laren, H. van Bekkum, A. E. Merbach, R. N. Muller and J. A. Peters, Structures and Dynamics of Lanthanide(III) Complexes of Sugar-Based DTPA-bis(amides) in Aqueous Solution: A Multinuclear NMR Study, Inorg. Chem., 1997, 36, 2527–2538 CrossRef CAS.
- S. Rast, P. H. Fries and E. Belorizky, Static zero field splitting effects on the electronic relaxation of paramagnetic metal ion complexes in solution, J. Chem. Phys., 2000, 113, 8724–8735 CrossRef CAS.
- S. Rast, A. Borel, L. Helm, E. Belorizky, P. H. Fries and A. E. Merbach, EPR Spectroscopy of MRI-Related Gd(III) Complexes: Simultaneous Analysis of Multiple Frequency and Temperature Spectra, Including Static and Transient Crystal Field Effects, J. Am. Chem. Soc., 2001, 123, 2637–2644 CrossRef CAS PubMed.
- F. A. Dunand, A. Borel and A. E. Merbach, How Does Internal Motion Influence the Relaxation of the Water Protons in LnIII DOTA-like Complexes?, J. Am. Chem. Soc., 2002, 124, 710–716 CrossRef CAS PubMed.
- K. Micskei, D. H. Powell, L. Helm, E. Brücher and A. E. Merbach, Water exchange on [Gd(H2O)8]3+ and [Gd(PDTA)(H2O)2]− in aqueous solution: A variable-pressure, -temperature and -magnetic field 17O NMR study, Magn. Reson. Chem., 1993, 31, 1011–1020 CrossRef CAS.
- O. V. Yazyev and L. Helm, Gadolinium(III) ion in liquid water: Structure, dynamics, and magnetic interactions from first principles, J. Chem. Phys., 2007, 127, 084506 CrossRef PubMed.
- L. Leone, S. Camorali, A. Freire-García, C. Platas-Iglesias, D. Esteban Gomez and L. Tei, Scrutinising the role of intramolecular hydrogen bonding in water exchange dynamics of Gd(III) complexes, Dalton Trans., 2021, 50, 5506–5518 RSC.
- A. Roca-Sabio, M. Regueiro-Figueroa, D. Esteban-Gómez, A. de Blas, T. Rodríguez-Blas and C. Platas-Iglesias, Density functional dependence of molecular geometries in lanthanide(III) complexes relevant to bioanalytical and biomedical applications, Comput. Theor. Chem., 2012, 999, 93–104 CrossRef CAS.
- P. Caravan, D. Esteban-Gómez, A. Rodríguez-Rodríguez and C. Platas-Iglesias, Water exchange in lanthanide complexes for MRI applications. Lessons learned over the last 25 years, Dalton Trans., 2019, 48, 11161–11180 RSC.
- F. Lucio-Martínez, Z. Garda, B. Váradi, F. K. Kálmán, D. Esteban-Gómez, É. Tóth, G. Tircsó and C. Platas-Iglesias, Rigidified Derivative of the Non-macrocyclic Ligand H4OCTAPA for Stable Lanthanide(III) Complexation, Inorg. Chem., 2022, 61, 5157–5171 CrossRef PubMed.
- L. Helm and A. E. Merbach, Inorganic and Bioinorganic Solvent Exchange Mechanisms, Chem. Rev., 2005, 105, 1923–1960 CrossRef CAS PubMed.
- D. M. Corsi, C. Platas-Iglesias, H. van Bekkum and J. A. Peters, Determination of paramagnetic lanthanide(III) concentrations from bulk magnetic susceptibility shifts in NMR spectra, Magn. Reson. Chem., 2001, 39, 723–726 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 (revision C.01), Gaussian, Inc., 2016 Search PubMed.
- J.-D. Chai and M. Head-Gordon, Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- M. Dolg, H. Stoll, A. Savin and H. Preuss, Energy-adjusted pseudopotentials for the rare earth elements, Theor. Chim. Acta, 1989, 75, 173–194 CrossRef CAS.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- C. Peng and H. B. Schlegel, Combining Synchronous Transit and Quasi-Newton Methods to Find Transition States, Isr. J. Chem., 1993, 33, 449–454 CrossRef CAS.
- F. Neese, The ORCA program system, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2012, 2, 73–78 CAS.
- F. Neese, Software update: the ORCA program system, version 4.0, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2018, 8, e1327 Search PubMed.
- M. Reiher, Douglas–Kroll–Hess Theory: a relativistic electrons-only theory for chemistry, Theor. Chem. Acc., 2006, 116, 241–252 Search PubMed.
- M. Barysz and A. J. Sadlej, Two-component methods of relativistic quantum chemistry: from the Douglas–Kroll approximation to the exact two-component formalism, J. Mol. Struct.: THEOCHEM, 2001, 573, 181–200 CrossRef CAS.
- D. Aravena, F. Neese and D. A. Pantazis, Improved Segmented All-Electron Relativistically Contracted Basis Sets for the Lanthanides, J. Chem. Theory Comput., 2016, 12, 1148–1156 CrossRef CAS PubMed.
- D. A. Pantazis, X.-Y. Chen, C. R. Landis and F. Neese, All-Electron Scalar Relativistic Basis Sets for Third-Row Transition Metal Atoms, J. Chem. Theory Comput., 2008, 4, 908–919 CrossRef CAS PubMed.
- F. Neese, An improvement of the resolution of the identity approximation for the formation of the Coulomb matrix, J. Comput. Chem., 2003, 24, 1740–1747 CrossRef CAS PubMed.
- S. Kossmann and F. Neese, Comparison of two efficient approximate Hartee–Fock approaches, Chem. Phys. Lett., 2009, 481, 240–243 CrossRef CAS.
- R. Izsák and F. Neese, An overlap fitted chain of spheres exchange method, J. Chem. Phys., 2011, 135, 144105 CrossRef PubMed.
- G. L. Stoychev, A. A. Auer and F. Neese, Automatic Generation of Auxiliary Basis Sets, J. Chem. Theory Comput., 2017, 13, 554–562 CrossRef CAS PubMed.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Climbing the Density Functional Ladder: Nonempirical Meta-Generalized Gradient Approximation Designed for Molecules and Solids, Phys. Rev. Lett., 2003, 91, 146401 CrossRef PubMed.
- S. Karimi and L. Helm, Water Exchange on [Ln(DO3A)(H2O)2] and [Ln(DTTA–Me)(H2O)2]− Studied by Variable Temperature, Pressure, and Magnetic Field NMR, Inorg. Chem., 2016, 55, 4555–4563 CrossRef CAS PubMed.
- M. Regueiro-Figueroa and C. Platas-Iglesias, Toward the Prediction of Water Exchange Rates in Magnetic Resonance Imaging Contrast Agents: A Density Functional Theory Study, J. Phys. Chem. A, 2015, 119, 6436–6445 CrossRef CAS PubMed.
- F. Neese, Importance of Direct Spin–Spin Coupling and Spin-Flip Excitations for the Zero-Field Splittings of Transition Metal Complexes: A Case Study, J. Am. Chem. Soc., 2006, 128, 10213–10222 CrossRef CAS PubMed.
- P.-Å. Malmqvist and B. O. Roos, The CASSCF state interaction method, Chem. Phys. Lett., 1989, 155, 189–194 CrossRef CAS.
- C. Kollmar, K. Sivalingam, B. Helmich-Paris, C. Angeli and F. Neese, A perturbation-based super-CI approach for the orbital optimization of a CASSCF wave function, J. Comput. Chem., 2019, 40, 1463–1470 CrossRef CAS PubMed.
- C. Angeli, S. Borini, M. Cestari and R. Cimiraglia, A quasidegenerate formulation of the second order n-electron valence state perturbation theory approach, J. Chem. Phys., 2004, 121, 4043–4049 CrossRef CAS PubMed.
- C. Angeli, R. Cimiraglia, S. Evangelisti, T. Leininger and J.-P. Malrieu, Introduction of n-electron valence states for multireference perturbation theory, J. Chem. Phys., 2001, 114, 10252–10264 CrossRef CAS.
- D. Maganas, S. Sottini, P. Kyritsis, E. J. J. Groenen and F. Neese, Theoretical Analysis of the Spin Hamiltonian Parameters in Co(II)S4 Complexes, Using Density Functional Theory and Correlated ab initio Methods, Inorg. Chem., 2011, 50, 8741–8754 CrossRef CAS PubMed.
- M. Atanasov, D. Aravena, E. Suturina, E. Bill, D. Maganas and F. Neese, First principles approach to the electronic structure, magnetic anisotropy and spin relaxation in mononuclear 3d-transition metal single molecule magnets, Coord. Chem. Rev., 2015, 289–290, 177–214 CrossRef CAS.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Additional relaxometric data and optimized geometries obtained with DFT. See DOI: https://doi.org/10.1039/d3dt03413e |
| This journal is © The Royal Society of Chemistry 2023 |