Open Access Article
Open Access ArticleFilling the gaps of uranium oxide hydrates with magnesium(II) ions: unique layered structures and the role of additional sodium(I) ions†
Yingjie
Zhang
 *a,
Kimbal T.
Lu
ab,
Tao
Wei
a,
I.
Karatchevtseva
a and
Rongkun
Zheng
*a,
Kimbal T.
Lu
ab,
Tao
Wei
a,
I.
Karatchevtseva
a and
Rongkun
Zheng
 b
b
aAustralian Nuclear Science and Technology Organisation, Locked Bag 2001, Kirrawee DC, NSW 2232, Australia. E-mail: yzx@ansto.gov.au
bSchool of Physics and Advanced Materials, University of Sydney, Ultimo, New South Wales 2007, Australia
First published on 9th November 2023
Abstract
Alkaline earth metal ions play an important role in the formation of secondary uranium minerals due to their abundance in the Earth's crust. Although uranium oxide hydrate (UOH) minerals and synthetic phases with calcium, strontium and barium ions have been investigated, their counterparts with magnesium ions are much less studied. In this work, synthetic UOH materials with magnesium ions have been investigated with three new compounds being synthesised and characterised. Compound Mg2(H3O)2(H2O)6[(UO2)3O4(OH)]2 (U-Mg1 with a U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio of 3
Mg ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) crystallises in the monoclinic P21/c space group having a layered crystal structure, constructed by β-U3O8 layers with 6-fold coordinated Mg2+ ions as interlayer cations. Compound Na2Mg(H2O)4[(UO2)3O3(OH)2]2 (U-Mg2p with U
1) crystallises in the monoclinic P21/c space group having a layered crystal structure, constructed by β-U3O8 layers with 6-fold coordinated Mg2+ ions as interlayer cations. Compound Na2Mg(H2O)4[(UO2)3O3(OH)2]2 (U-Mg2p with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na ratios of 6
Na ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) crystallises in the triclinic P
2) crystallises in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group having a layered structure, constructed by a unique type of uranium oxide hydroxide layer containing both α-U3O8 and β-U3O8 features, with alternating layers of 6-fold coordinated Mg2+ and 6-/8-fold coordinated Na+ interlayer cations. Compound Na2Mg(H2O)4[(UO2)4O3(OH)4]2 (U-Mg2n with U
space group having a layered structure, constructed by a unique type of uranium oxide hydroxide layer containing both α-U3O8 and β-U3O8 features, with alternating layers of 6-fold coordinated Mg2+ and 6-/8-fold coordinated Na+ interlayer cations. Compound Na2Mg(H2O)4[(UO2)4O3(OH)4]2 (U-Mg2n with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na ratios of 8
Na ratios of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) crystallises in the triclinic P
2) crystallises in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group having a corrugated layer structure, constructed by a unique type of uranium oxide hydroxide layer with mixed 6-fold coordinated Mg2+ and 7-fold coordinated Na+ interlayer cations. The structural diversity in the UOH-Mg system was achieved by adjusting the solution pH using NaOH, highlighting the importance of solution pH control and the additional Na+ ions in the formation of UOH phases. The extra structural flexibility offered by the Na+ ions emphasizes the opportunity for synthesising UOHs with dual-cations to further improve our understanding of the alteration products of spent nuclear fuel under geological disposal.
space group having a corrugated layer structure, constructed by a unique type of uranium oxide hydroxide layer with mixed 6-fold coordinated Mg2+ and 7-fold coordinated Na+ interlayer cations. The structural diversity in the UOH-Mg system was achieved by adjusting the solution pH using NaOH, highlighting the importance of solution pH control and the additional Na+ ions in the formation of UOH phases. The extra structural flexibility offered by the Na+ ions emphasizes the opportunity for synthesising UOHs with dual-cations to further improve our understanding of the alteration products of spent nuclear fuel under geological disposal.
1. Introduction
Nuclear energy has resumed a growing momentum worldwide owing to the increasing demand for cleaner energy.1 However, the major technical challenge for the nuclear energy sector is the safe treatment and disposal of spent nuclear fuel (SNF).2,3 The most acceptable approach is the direct geological disposal of the SNF in a stable underground repository.4–6 Although UO2 as the primary component of SNF is stable under the reducing environment in an underground repository, it can undergo severe alterations (oxidation and hydration) if exposed to air.7,8It is well understood that uraninite (UO2+x) as a primary uranium mineral or UO2 as the primary component of SNF will be gradually oxidised from U4+ to U6+ if exposed to oxidative conditions.9–11 As the most stable U6+ form, the uranyl [(UO2)2+] ion with two strongly bonded axial oxygen atoms is ready to coordinate with O/OH ligands in the equatorial positions forming tetragonal, pentagonal and hexagonal bipyramids, which connect each other via both corner- and edge-sharing to form various uranyl-containing compounds, normally in the form of layered structures with various interlayer cations adopted from the surroundings.12–15
Uranium oxide hydrate (UOH) minerals are a group of secondary uranium minerals formed in the early stage of uraninite weathering.3,14–16 They provide a direct natural analogue to the SNF alterations under geological disposal. The recent campaign for a better understanding of UOH materials has led to the discovery of dozens of UOH minerals14–16 and about two dozen synthetic UOH compounds.14–16 Most of these UOH materials have layered structures containing uranium oxide hydroxide layers with interlayer cations. As such, they differ mainly in two aspects: the O/OH ratio in the uranium oxide hydroxide layers and the type of interlayer cation. For UOH minerals, the secondary cations are mainly alkali,17,18 alkaline earth and p-block cations such as Pb due to their natural abundance or are located at the end of the U decay chain.19–22 In addition, the uranyl oxide hydroxide layer topologies for various UOH minerals have been comprehensively reviewed.23 In addition, synthetic UOH materials with a wide range of secondary cations including alkali,24,25 alkaline earth,26–29 lead,30 transition metals31–33 and lanthanide ions34–36 have been reported. Furthermore, synthetic UOH phases with interlayer anions are also possible, although less studied.37
Apart from the dominant layered UOH structures, several types of complicated three-dimensional (3D) structures have also been discovered.30,38,39 Among them is a framework-type structure with uranyl species acting as bridging ligands between the uranium oxide hydroxide layers to form uranium oxide hydrate frameworks (UOHFs).40 The main feature of UOHFs is their structural flexibility as the large framework channels are capable of incorporating a range of secondary cations from 1+ to 4+ including (NH4)+, Pb2+, Sr2+, Y3+, Er3+, Sm3+, Eu3+, Gd3+ and U4+.39–43 The complexity arising from UOHFs highlights the need to study these materials further to better comprehend the uranium hydrolysis chemistry in the presence of various secondary cations.
Magnesium (Mg), as the eighth most abundant element in the Earth's crust (∼2%), exists in more than 60 minerals.44 Consequently, it is the third most plentiful element dissolved in seawater.44 Unlike the other larger alkaline earth cations (Ca2+/Sr2+/Ba2+), the relatively smaller ionic radius of Mg2+ makes it behave quite differently. In fact, it adopts 6-fold coordination in an octahedral geometry similar to bivalent transition metal ions.45 Despite the fact that the Mg2+ ion has been found in more than 30 uranyl minerals46 such as silicates, sulphates, phosphates etc., and also in the UOH mineral richetite,47 its exact role in the formation of UOH minerals and synthetic phases has not been well established. Earlier works were focused on the synthesis of UOH-Mg by hydrothermal reactions of schoepite with magnesium nitrate/sulphate, leading to the formation of two types of UOHs with Mg2+ ions, one with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 6
Mg = 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48 and the other with U
48 and the other with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg = 3
Mg = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.49 However, the detailed crystal structures remain unknown. In addition, some UOH minerals have been found to contain M2+ (M = Ca, Sr, Ba, Pb) ions together with Na+ ions,50,51 and the role of the additional Na+ ions in the formation and stabilisation of such UOH structures requires further research.
1.49 However, the detailed crystal structures remain unknown. In addition, some UOH minerals have been found to contain M2+ (M = Ca, Sr, Ba, Pb) ions together with Na+ ions,50,51 and the role of the additional Na+ ions in the formation and stabilisation of such UOH structures requires further research.
In this work, we report the synthesis and characterisation of three novel UOH compounds containing Mg2+ ions with/without Na+ ions. They have three different types of layered structures revealed by synchrotron single crystal X-ray diffraction. The diversity of uranium oxide hydroxide layers has been achieved at nearly neutral solution pH values adjusted with a diluted NaOH solution, highlighting the importance of controlling the solution pH and the additional Na+ ions in the formation and stabilisation of UOH phases with Mg2+ ions. Subsequently, their microstructures and spectroscopic properties have been investigated using scanning and transmission electron microscopy, Raman spectroscopy and diffuse reflectance spectroscopy.
2. Experimental
2.1. Syntheses of materials
Uranyl nitrate hexahydrate with uranium in natural isotopic abundance was used. Materials containing uranium are radioactive and should be handled with care in regulated facilities. Other chemicals of A.R. grade were purchased from Sigma-Aldrich (Merck).2.2. Characterisation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 56 using the Olex2 graphical user interface.57 All but hydrogen atoms were located on the electron density map and refined anisotropically. The one-circle goniometer on the MX2 beamline offered less redundant data for effective absorption corrections. As such, some strong Q-peaks exist around U atoms, which is quite common for uranium oxide materials. The residual peaks could be due to unmodeled disorder or twinning. Such artifacts have consequences including systematic errors in bond distances (which affect BVS analysis) and possible element misidentification in some cases.
56 using the Olex2 graphical user interface.57 All but hydrogen atoms were located on the electron density map and refined anisotropically. The one-circle goniometer on the MX2 beamline offered less redundant data for effective absorption corrections. As such, some strong Q-peaks exist around U atoms, which is quite common for uranium oxide materials. The residual peaks could be due to unmodeled disorder or twinning. Such artifacts have consequences including systematic errors in bond distances (which affect BVS analysis) and possible element misidentification in some cases.
3. Results and discussion
3.1. Material synthesis and characterisation
All compounds were synthesised hydrothermally at 200 °C for 24 h with uranyl and magnesium nitrates, and the solution pH was adjusted to above neutral using a dilute NaOH solution. When the initial solution pH values were below 7.0, only meta-schoepite was identified based on the SEM-EDS analysis. U-Mg1 was formed with the final solution pH of 6.52 while U-Mg2p and U-Mg2n were formed in one pot with the final solution pH of 8.06. Both syntheses were repeated with good reproducibility. The synthesis conditions and final products are summarised in Table 1.| Compound | Precursors | Synthesis conditions | Final product | ||||
|---|---|---|---|---|---|---|---|
U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg |
Initial | Temp. | Formula | pH | U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na Na |
||
| pH | Time | O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) OH OH |
|||||
| U-Mg1 | UO2(NO3)2·6H2O | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
7.50 | 200 °C, 24 h | Mg2(H2O)8[(UO2)3O2(OH)3]2 | 6.52 | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||||
| U-Mg2p | Mg(NO3)2·6H2O | 8.08 | Na2Mg(H2O)4[(UO2)3O3(OH)2]2 | 8.06 | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
|||||||
| U-Mg2n | NaOH | 8.08 | Na2Mg(H2O)4[(UO2)4O3(OH)4]2 | 8.06 | 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
||
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
|||||||
SEM-EDS examination of U-Mg1 confirmed the thin plate crystal morphology (Fig. 1a) and the presence of U, Mg and O, with a U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg atomic ratio of 3
Mg atomic ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S1, ESI†). Similarly, SEM-EDS analysis of U-Mg2p (plate crystals in Fig. 1b) and U-Mg2n (needle crystals in Fig. 1b) confirmed the presence of U, Mg, Na and O with U
1 (Fig. S1, ESI†). Similarly, SEM-EDS analysis of U-Mg2p (plate crystals in Fig. 1b) and U-Mg2n (needle crystals in Fig. 1b) confirmed the presence of U, Mg, Na and O with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na atomic ratios of 6
Na atomic ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for U-Mg2p (Fig. S2, ESI†) and 8
2 for U-Mg2p (Fig. S2, ESI†) and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for U-Mg2n (Fig. S3, ESI†), respectively.
2 for U-Mg2n (Fig. S3, ESI†), respectively.
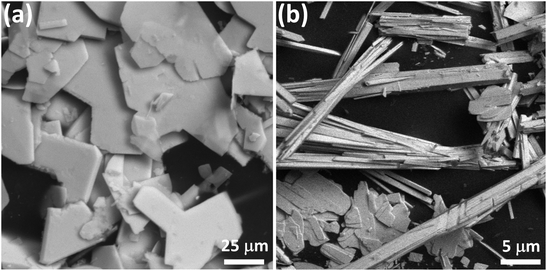 | ||
| Fig. 1 Backscattered SEM images of U-Mg1 (a), U-Mg2p (plate crystals) and U-Mg2n (needle crystals) (b). | ||
3.2. Crystal structures and discussion
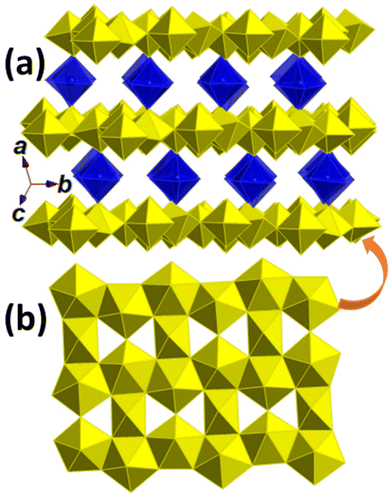
The crystal data and structure refinement details for U-Mg1, U-Mg2p and U-Mg2n are summarised in Table 2, with selected bond lengths (Å) and angles (°) listed in Tables 3–5, respectively. U-Mg1 crystallises in the monoclinic P21/c space group. It has a layered crystal structure (Fig. 2) constructed with β-U3O8 type uranium oxide hydroxide layers (Fig. 2b) and interlayer Mg2+ species (Fig. 2a). There are six distinct U centres, two in 6-fold coordination (U1 and U4) and four in 7-coordination (U2, U3, U5 and U6), and two Mg centres both in 6-fold coordination. Although U1 and U4 are in 6-fold coordination, they differ remarkably. U1 lacks uranyl nature with six U–O bonds ranging from 2.055(18) to 2.136(18) Å while U4 has two shorter U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O distances from 1.887(19) to 1.960(19) Å with an O
O distances from 1.887(19) to 1.960(19) Å with an O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angle of 178.7(7)° and four longer U–O distances in the equatorial plane ranging from 2.154(18) to 2.192(18) Å, suggesting the presence of a uranyl unit with elongated U
O angle of 178.7(7)° and four longer U–O distances in the equatorial plane ranging from 2.154(18) to 2.192(18) Å, suggesting the presence of a uranyl unit with elongated U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds. All U2, U3, U5 and U6 are typical uranyl centres in a pentagonal bipyramidal geometry, with U
O bonds. All U2, U3, U5 and U6 are typical uranyl centres in a pentagonal bipyramidal geometry, with U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths for the uranyl moieties ranging from 1.797(18) to 1.835(18) Å and O
O bond lengths for the uranyl moieties ranging from 1.797(18) to 1.835(18) Å and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angles from 174.9(8)° to 178.3(8)°. The U–O distances in the equatorial planes range from 2.252(18) to 2.383(18) Å. The two Mg centres are separated without any direct interaction, both 6-fold coordinated in an octahedral geometry with Mg–O distances ranging from 2.00(2) to 2.09(2) Å. These U–O and Mg–O bonds are consistent with literature data.45 The distance between layers is ∼4.05 Å measured via the two apical oxygen atoms of the Mg octahedra.
O angles from 174.9(8)° to 178.3(8)°. The U–O distances in the equatorial planes range from 2.252(18) to 2.383(18) Å. The two Mg centres are separated without any direct interaction, both 6-fold coordinated in an octahedral geometry with Mg–O distances ranging from 2.00(2) to 2.09(2) Å. These U–O and Mg–O bonds are consistent with literature data.45 The distance between layers is ∼4.05 Å measured via the two apical oxygen atoms of the Mg octahedra.
| a R 1 = ∑||Fo| − |Fc||/|Fo|. b wR2 = {∑[w(Fo2 − Fc2)2]/∑[w(Fo2)2]}1/2. | |||
|---|---|---|---|
| Compound | U-Mg1 | U-Mg2p | U-Mg2n |
| CCDC | 2289968 | 2289969 | 2289970 |
| Empirical formula | Mg2O30U6 | MgNa2O26U6 | MgNa2O34U8 |
| Formula weight | 1956.80 | 1914.47 | 2518.53 |
| Crystal system | Monoclinic | Triclinic | Triclinic |
| Space group | P21/c |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a (Å) | 8.6310(17) | 7.0150(14) | 8.2140(16) |
| b (Å) | 28.231(6) | 12.067(2) | 8.3980(17) |
| c (Å) | 10.601(2) | 13.411(3) | 10.769(2) |
| α/(°) | 90 | 91.22(3) | 77.62(3) |
| β/(°) | 105.99(3) | 100.49(3) | 89.01(3) |
| γ/(°) | 90 | 90.36(3) | 75.83(3) |
| Volume (Å3) | 2483.1(9) | 1116.0(4) | 703.0(3) |
| Z/μ (mm−1) | 4/39.162 | 2/43.558 | 1/46.071 |
| Min./max. θ [°] | 1.443/24.997 | 1.545/24.995 | 1.935/24.995 |
| d calcd (g cm−3) | 5.234 | 5.697 | 5.949 |
| GOF | 1.125 | 1.076 | 1.019 |
Final R1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a [I > 2σ(I)] a [I > 2σ(I)] |
0.0645 | 0.0401 | 0.0424 |
Final wR2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b [I > 2σ(I)] b [I > 2σ(I)] |
0.1583 | 0.1023 | 0.1058 |
| Bond | Length (Å) | Bond | Length (Å) | Bond | Length (Å) |
|---|---|---|---|---|---|
| a 1/2 + X, 3/2 − Y, −1/2 + Z. b −1/2 + X, 3/2 − Y, −1/2 + Z. c 1 + X, +Y, +Z. d 1 − X, 1 − Y, 1 − Z. e −1 + X, +Y, +Z. f 2 − X, 1 − Y, 1 − Z. g +X, +Y, −1 + Z. h −1/2 + X, 3/2 − Y, 1/2 + Z. | |||||
| U1–O1 | 2.055(18) | U2–O8 | 1.816(18) | U3–O9 | 1.815(18) |
| U1–O4 | 2.057(18) | U2–O7 | 1.835(18) | U3–O10 | 1.841(18) |
| U1–O3 | 2.087(18) | U2–O11 | 2.261(18) | U3–O12d | 2.264(18) |
| U1–O2 | 2.090(18) | U2–O6 | 2.287(18) | U3–O12 | 2.305(18) |
| U1–O5 | 2.105(18) | U2–O20c | 2.330(18) | U3–O11 | 2.317(18) |
| U1–O6 | 2.136(18) | U2–O3b | 2.341(18) | U3–O5b | 2.334(18) |
| U2–O5b | 2.365(18) | U3–O19e | 2.335(18) | ||
O7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O8 O8 |
178.2(8) | O9![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O10 O10 |
177.3(8) | ||
| U4–O14 | 1.887(19) | U5–O17 | 1.797(18) | U6–O22 | 1.805(18) |
| U4–O13 | 1.960(19) | U5–O18 | 1.829(19) | U6–O21 | 1.840(18) |
| U4–O15 | 2.154(18) | U5–O15f | 2.282(18) | U6–O16e | 2.252(18) |
| U4–O11 | 2.165(18) | U5–O19 | 2.328(18) | U6–O20 | 2.311(18) |
| U4–O16 | 2.169(18) | U5–O15g | 2.337(17) | U6–O3e | 2.353(18) |
| U4–O12 | 2.192(18) | U5–O16g | 2.342(18) | U6–O4h | 2.363(18) |
O13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O14 O14 |
178.7(7) | U5–O4a | 2.383(18) | U6–O6h | 2.365(18) |
O17![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O18 O18 |
174.9(8) | O21![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O22 O22 |
178.3(8) | ||
| Mg1–O23 | 2.028(19) | Mg1–O26 | 2.064(19) | Mg2–O28 | 2.036(19) |
| Mg1–O25 | 2.033(19) | Mg1–O24 | 2.075(19) | Mg2–O30 | 2.049(19) |
| Mg1–O9 | 2.05(2) | Mg2–O21 | 2.00(2) | Mg2–O29 | 2.08(2) |
| Mg1–O18 | 2.05(2) | Mg2–O8 | 2.03(2) | Mg2–O27 | 2.09(2) |
| Bond | Length (Å) | Bond | Length (Å) | Bond | Length (Å) |
|---|---|---|---|---|---|
| a 1 + X, +Y, +Z. b 2 − X, 1 − Y, 2 − Z. c 1 − X, 1 − Y, 1 − Z. d 1 − X, 1 − Y, 2 − Z. e −1 + X, +Y, +Z. f 1 − X, −Y, 1 − Z. g −X, −Y, 1 − Z. h 1 − X, −Y, 2 − Z. i −1 + X, −1 + Y, +Z. j 2 − X, 1 − Y, 1 − Z. k +X, +Y, 1 + Z. l 1 + X, +Y, 1 + Z. | |||||
| U1–O1 | 1.840(10) | U2–O7 | 1.819(9) | U3–O19 | 1.808(9) |
| U1–O2 | 1.847(9) | U2–O6 | 1.851(9) | U3–O18 | 1.813(10) |
| U1–O4 | 2.228(9) | U2–O5 | 2.226(8) | U3–O10 | 2.234(9) |
| U1–O3 | 2.235(8) | U2–O10 | 2.226(9) | U3–O5e | 2.238(9) |
| U1–O5 | 2.244(8) | U2–O4e | 2.266(8) | U3–O4e | 2.379(8) |
| U1–O22c | 2.260(9) | U2–O8 | 2.333(9) | U3–O9e | 2.386(9) |
O1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U1 U1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2 O2 |
177.8(4) | U2–O9 | 2.743(11) | U3–O14 | 2.470(9) |
O6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O7 O7 |
178.1(4) | O18![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U3 U3![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O19 O19 |
175.7(4) | ||
| U4–O12 | 1.828(11) | U5–O16 | 1.819(9) | U6–O21 | 1.839(10) |
| U4–O11 | 1.839(11) | U5–O15 | 1.822(10) | U6–O20 | 1.842(9) |
| U4–O13 | 2.192(9) | U5–O13 | 2.203(9) | U6–O13f | 2.231(9) |
| U4–O10 | 2.205(10) | U5–O22g | 2.262(8) | U6–O22 | 2.234(9) |
| U4–O9 | 2.363(9) | U5–O17 | 2.407(9) | U6–O3j | 2.246(8) |
| U4–O17a | 2.380(9) | U5–O3i | 2.436(9) | U6–O8c | 2.337(8) |
| U4–O14 | 2.692(9) | U5–O14 | 2.455(8) | U6–O17g | 2.683(9) |
O11![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U4 U4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O12 O12 |
175.2(5) | O15![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U5 U5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O16 O16 |
176.9(4) | O20![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U6 U6![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O21 O21 |
176.2(4) |
| Mg1–O19 | 2.029(10) | Na1–O18 | 2.345(11) | Na2–O15h | 2.429(10) |
| Mg1–O20 | 2.034(10) | Na1–O6d | 2.384(10) | Na2–O1c | 2.441(11) |
| Mg1–O24 | 2.047(10) | Na1–O1e | 2.414(11) | Na2–O11d | 2.474(10) |
| Mg1–O25 | 2.069(11) | Na1–O21k | 2.452(10) | Na2–O11 | 2.590(11) |
| Mg1–O26 | 2.079(11) | Na1–O1b | 2.600(11) | Na2–O6 | 2.596(10) |
| Mg1–O23 | 2.127(10) | Na1–O6e | 2.672(11) | Na2–O22l | 2.670(12) |
| Na2–O18 | 2.391(11) | Na2–O10 | 2.746(12) | ||
| Bond | Length (Å) | Bond | Length (Å) | Bond | Length (Å) | Bond | Length (Å) |
|---|---|---|---|---|---|---|---|
| a −X, 1 − Y, 2 − Z. b −X, −Y, 2 − Z. c 1 − X, 1 − Y, 2 − Z. d +X, 1 + Y, +Z. e 1 − X, 2 − Y, 1 − Z. f 1 − X, 1 − Y, 1 − Z. g −X, 2 − Y, 1 − Z. h 1 + X, +Y, +Z. | |||||||
| U1–O2 | 1.792(13) | U2–O8 | 1.800(12) | U3–O11 | 1.819(12) | U4–O14 | 1.813(13) |
| U1–O1 | 1.805(12) | U2–O7 | 1.817(11) | U3–O10 | 1.820(13) | U4–O13 | 1.861(13) |
| U1–O5 | 2.168(13) | U2–O5a | 2.243(13) | U3–O12 | 2.247(12) | U4–O15 | 2.207(13) |
| U1–O4 | 2.317(13) | U2–O5 | 2.263(12) | U3–O12e | 2.281(12) | U4–O12 | 2.169(11) |
| U1–O3 | 2.381(12) | U2–O9 | 2.314(13) | U3–O15f | 2.295(12) | U4–O15f | 2.299(12) |
| U1–O6 | 2.566(12) | U2–O3d | 2.483(12) | U3–O9 | 2.336(14) | U4–O6f | 2.477(13) |
| U1–O3b | 2.607(12) | U2–O6 | 2.509(13) | U3–O4d | 2.431(12) | O13![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U5 U5![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O14 O14 |
178.4(5) |
O1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U1 U1![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O2 O2 |
178.5(5) | O7![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U2 U2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O8 O8 |
177.0(5) | O10![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U4 U4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O11 O11 |
175.3(5) | ||
| Mg1–O16g | 2.051(11) | Mg1–O11g | 2.061(12) | Na1–O2h | 2.412(15) | Na1–O7c | 2.501(13) |
| Mg1–O16 | 2.051(11) | Mg1–O17 | 2.120(12) | Na1–O10 | 2.439(15) | Na1–O8h | 2.634(14) |
| Mg1–O11 | 2.061(12) | Mg1–O17g | 2.120(12) | Na1–O14f | 2.450(15) | Na1–O13 | 2.931(16) |
While the bending of the uranyl unit for U5 [174.9(8)°] is obvious, the phenomenon is often observed in uranyl-containing compounds.15 The coordination environment for U1 is unusual in that it does not involve a uranyl species. In fact, it has a tetraoxido core coordination environment and can be either U(V) or U(VI) depending on the six U–O bond lengths.58,59 However, similar U centres have been found in other synthetic UOH systems especially with the presence of β-U3O8 type layers.16
The bond valence sum (BVS) calculations (Table S1, ESI†) with the parameters from the literature60,61 confirmed that all six U centres are present as U6+ [U1 (5.59), U2 (6.09), U3 (5.84), U4 (5.75), U5 (6.07) and U6 (6.05)] and two Mg centres as Mg2+ [Mg1 (2.29) and Mg2 (2.31)]. The asymmetric unit contains 2Mg, 6U and 30O (Table S1, ESI†), with the majority being O, two OH (O19 and O20) and eight H2O (O23–O30). As such, the formula for U-Mg1 was determined to be Mg2(H3O)2(H2O)6[(UO2)3O4(OH)]2.
U-Mg2p crystallises in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The layered crystal structure (Fig. 3a) is constructed by a unique type of uranium oxide hydroxide layer containing both α-U3O8 and β-U3O8 features (Fig. 3b) with alternating layers of 6-fold coordinated Mg2+ ions and both 6- and 8-fold coordinated Na+ ions (Fig. 3a and c). The structure contains six unique U centres, U1 in 6-fold coordination with a tetragonal bipyramid and U2–U6 in 7-fold coordination with pentagonal bipyramids. All six uranyl moieties exhibit the normal uranyl form with near-linear U
space group. The layered crystal structure (Fig. 3a) is constructed by a unique type of uranium oxide hydroxide layer containing both α-U3O8 and β-U3O8 features (Fig. 3b) with alternating layers of 6-fold coordinated Mg2+ ions and both 6- and 8-fold coordinated Na+ ions (Fig. 3a and c). The structure contains six unique U centres, U1 in 6-fold coordination with a tetragonal bipyramid and U2–U6 in 7-fold coordination with pentagonal bipyramids. All six uranyl moieties exhibit the normal uranyl form with near-linear U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds ranging from 1.808(9) to 1.851(9) Å and O
O bonds ranging from 1.808(9) to 1.851(9) Å and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angles from 175.2(5)° to 1778.1(4)°. The U–O bonds in the equatorial planes range from 2.192(9) to 2.743(11) Å. The longer than normal U–O bonds of 2.743(11) Å for U2–O9 and 2.692(9) Å for U4–O14 are likely due to the deviations of O9 and O14 from the UOH layer. The Mg2+ ion is 6-fold coordinated in an octahedral environment with Mg–O bonds ranging from 2.029(10) to 2.127(10) Å. While Na1 is 6-fold coordinated in an octahedral geometry with Na–O bonds ranging from 2.235(11) to 2.672(11) Å, Na2 is 8-fold coordinated in a distorted cubic geometry with Na–O bonds ranging from 2.391(11) to 2.746(12) Å. All Mg–O and Na–O bond lengths are normal.
O angles from 175.2(5)° to 1778.1(4)°. The U–O bonds in the equatorial planes range from 2.192(9) to 2.743(11) Å. The longer than normal U–O bonds of 2.743(11) Å for U2–O9 and 2.692(9) Å for U4–O14 are likely due to the deviations of O9 and O14 from the UOH layer. The Mg2+ ion is 6-fold coordinated in an octahedral environment with Mg–O bonds ranging from 2.029(10) to 2.127(10) Å. While Na1 is 6-fold coordinated in an octahedral geometry with Na–O bonds ranging from 2.235(11) to 2.672(11) Å, Na2 is 8-fold coordinated in a distorted cubic geometry with Na–O bonds ranging from 2.391(11) to 2.746(12) Å. All Mg–O and Na–O bond lengths are normal.
The distances between uranium oxide hydroxide layers are ∼4.02 Å with interlayer Mg2+ cations and ∼2.65 Å with interlayer Na+ cations. The longer interlayer distance between the layers which sandwich Mg2+ cations is due to the corner-connections to the two most separated apices of the Mg octahedra. The BVS values (Table S2, ESI†) confirmed that all six U centres are present as U6+ [U1 (5.75), U2 (5.97), U3 (5.85), U4 (5.92), U5 and U6 (5.97)], the Mg centre as Mg2+ (2.21) and two Na centres as Na+ [Na1 (1.02) and Na2 (1.14)], with the majority being O, four OH (O8, O9, O14 and O17) and four H2O (O23–O26). The formula for U-Mg2p was then determined to be Na2Mg(H2O)4[(UO2)3O3(OH)2]2.
U-Mg2n crystallises in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The layered structure (Fig. 4a) is constructed by a unique uranium oxide hydroxide layer (Fig. 4b) that is composed of two types of chains containing double U1 and U2, and double U3 and U4 (Fig. 3c) with mixed 6-fold coordinated Mg2+ and 7-fold coordinated Na+ interlayer cations (Fig. 4d). There are four unique U centres, U1–U3 in pentagonal bipyramids and U4 in a tetragonal bipyramid, one 6-fold coordinated Mg and one 7-fold coordinated Na centre. All four uranyl centres are normal with U
space group. The layered structure (Fig. 4a) is constructed by a unique uranium oxide hydroxide layer (Fig. 4b) that is composed of two types of chains containing double U1 and U2, and double U3 and U4 (Fig. 3c) with mixed 6-fold coordinated Mg2+ and 7-fold coordinated Na+ interlayer cations (Fig. 4d). There are four unique U centres, U1–U3 in pentagonal bipyramids and U4 in a tetragonal bipyramid, one 6-fold coordinated Mg and one 7-fold coordinated Na centre. All four uranyl centres are normal with U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths from 1.792(13) to 1.861(13) Å and O
O bond lengths from 1.792(13) to 1.861(13) Å and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) U
U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O angles from 175.3(5)° to 178.5(5)°. The equatorial U–O bond lengths range from 2.168(13) to 2.566(12) Å, which are typical U–O distances as previously reported.16 While all six Mg–O bond lengths are in the normal range of 2.051(11) Å to 2.120(12) Å, the Na–O bond lengths are normal ranging from 2.412(15) Å to 2.931(16) Å.
O angles from 175.3(5)° to 178.5(5)°. The equatorial U–O bond lengths range from 2.168(13) to 2.566(12) Å, which are typical U–O distances as previously reported.16 While all six Mg–O bond lengths are in the normal range of 2.051(11) Å to 2.120(12) Å, the Na–O bond lengths are normal ranging from 2.412(15) Å to 2.931(16) Å.
The BVS values (Table S3, ESI†) confirmed that all four U centres are present as U6+ [U1 (5.90), U2 (6.00), U3 (5.86) and U4 (5.62)], Mg as Mg2+ (2.13) and Na as Na+ (0.85), with three O (O5, O12 and O15) and four OH (O3, O4, O6 and O9) in the asymmetric unit. Consequently, the formula for U-Mg2n was determined to be Na2Mg(H2O)4[(UO2)4O3(OH)4]2.
| Mineral | Chemical formula | Space group | Cell parameters | Ref. |
|---|---|---|---|---|
| Agrinierite | K2(Ca0.65Sr0.35)[(UO2)3O3(OH)2]2(H2O)5 | Orthorhombic, F2mm | a = 14.094(2), b = 14.127(2), c = 24.106(4) Å (Z = 16), V = 4799.6(1) Å3 | 19 |
| Calciouranoite | (Ca, Ba, Pb, K2, Na2)[(UO2)(O,OH)](H2O)5 | — | — | 50 |
| Metacalciouranoite | (Ca, Ba, Pb, K2, Na2)[(UO2)(O,OH)](H2O)2 | — | — | 50 |
| Clarkeite | (Na, Ca)[(UO2)(O,OH)](H2O)0–1 | Hexagonal, R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) w w |
a = 3.954(4), c = 17.73(1) Å (Z = 3) | 51 |
| Rameauite | K2Ca[(UO2)6O6(OH)4](H2O)6 | Monoclinic, C2/c | a = 13.97, b = 14.26, c = 14.22 Å, β = 121° | 20 |
| Richetite | (Fe,Mg)xPb8.57[(UO2)18O18(OH)12]2(H2O)41 | Triclinic, P1 | a = 20.9391(3), b = 12.1000(2), c = 16.3450(3) Å, α = 103.87(1), β = 115.37(1), γ = 90.27(1)°, V = 3605.2 Å3 | 47 |
Unlike the Mg2+ ion which has a preferred octahedral coordination environment with the typical average Mg–O bond length of around 2.03 Å, the Na+ ion is rather flexible adopting 6-/8-fold coordination geometries with longer Na–O bond lengths ranging from 2.38 to 2.93 Å. In addition, the single charge of the Na+ ion makes it ready to be incorporated in various UOH structures for variable charge compensations.
3.3. TEM characterisation
In general, UOH materials are not stable in the ultra-high vacuum of the TEM and beam damage is likely to occur, making the TEM analysis rather difficult. However, the strategy of minimising working time and working on multiple grains seems to be a good practice often leading to success. Compounds U-Mg1 and U-Mg2n were further examined by TEM. For U-Mg1, a TEM bright field image showed the U-Mg1 grains (Fig. S4a, ESI†). The selected area electron diffraction (SAED) pattern in a single zone axis was not obtained even using the smallest selected aperture; instead, diffraction rings were obtained (Fig. S4b, ESI†). Additionally, several bright spots on the rings indicated that the grain size of U-Mg1 is very fine as the smallest selected aperture contains several grains for diffraction. However, the d-spacing distances measured from the TEM diffraction rings agree with the SC-XRD result for the U-Mg1 crystal structure in the P21/c space group. A high-resolution transmission electron microscopy (HRTEM) image showed lattice fringes in nano-domains (Fig. S4c, ESI†).For U-Mg2n, a TEM bright field image (Fig. 6a) showed the crushed grains. TEM-EDS analysis confirmed the presence of U, Mg, Na and O. The SAED pattern from a grain in the [3 1 1] zone axis was indexed to the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. 6b), in agreement with the SC-XRD pattern. A HRTEM image in the [−1 1 1] zone axis showed lattice fringes with a fast Fourier transform (FFT) image in the inset (Fig. 6c). The d(2 2 0) and d(2 0 2) spacing values of 0.324 nm and 0.312 nm measured from the image (Fig. 6c) are consistent with the crystal data from SC-XRD.
space group (Fig. 6b), in agreement with the SC-XRD pattern. A HRTEM image in the [−1 1 1] zone axis showed lattice fringes with a fast Fourier transform (FFT) image in the inset (Fig. 6c). The d(2 2 0) and d(2 0 2) spacing values of 0.324 nm and 0.312 nm measured from the image (Fig. 6c) are consistent with the crystal data from SC-XRD.
3.4. Electronic structures
Based on BVS calculations, all three compounds are U6+ dominant and the characteristic feature in the DRS spectra (Fig. 7) is the broad and unresolved absorptions in the UV region (300 nm–500 nm) with two broad maxima at ∼350 nm and ∼450 nm, corresponding to the typical charge transfer bands for the U6+ containing materials.64–693.5. Vibrational modes
Micro-Raman spectroscopy has been used to study the vibrational modes of compounds U-Mg1, U-Mg2p and U-Mg2n. Raman spectra for U-Mg1, U-Mg2p and U-Mg2n (Fig. 8) have revealed multiple bands at 850–700 cm−1,70–72 corresponding to a variety of calculated U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths for the uranyl centres between 1.762 Å and 1.854 Å,73 broadly consistent with the U
O bond lengths for the uranyl centres between 1.762 Å and 1.854 Å,73 broadly consistent with the U![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond lengths (1.792 Å–1.861 Å) determined from the SC-XRD analysis. The bands at 520–310 cm−1 have been assigned predominantly to ν(U3O) bridge elongations and γ[U3(OH)3] bending vibrations,70–72 and possibly ν(U–Oligand) vibrations. Multiple weak bands at 295–200 cm−1 are due to ν2(UO2)2+ bending vibrations while lattice vibrations can be seen below 150 cm−1.70–72
O bond lengths (1.792 Å–1.861 Å) determined from the SC-XRD analysis. The bands at 520–310 cm−1 have been assigned predominantly to ν(U3O) bridge elongations and γ[U3(OH)3] bending vibrations,70–72 and possibly ν(U–Oligand) vibrations. Multiple weak bands at 295–200 cm−1 are due to ν2(UO2)2+ bending vibrations while lattice vibrations can be seen below 150 cm−1.70–72
3.6. Implications and perspectives
It is essential to understand the alteration chemistry of uranium oxides in the presence of transition metal ions given their abundance in the environment. With only a few UOH materials containing transition metal ions being reported,31–34 there is an obvious need for further work. Many bivalent 3d/4d-transition metal ions (M2+ = Mn, Co, Ni, Cu, Zn, Cd) have ionic radii ranging from 0.69 to 0.95 Å with the corresponding r(M2+)/r(O2−) ratios in the range 0.73–0.41, characteristic of the octahedral coordination geometry for M2+ ions.49 The Mg2+ ion is of particular interest as its ionic radius also meets the conditions for octahedral coordination [r(Mg2+) = 0.72 Å, r(Mg2+)/r(O2−) = 0.53]. As such, all synthetic UOH-Mg phases will be very useful in predicting the potential formation of UOH phases with bivalent transition metal ions.Many UOH minerals containing dual or multiple cations have been well documented due to their relative abundance in the geological environment. Despite this, only a few synthetic UOH materials with dual cations have been reported, clearly highlighting the need to further explore UOHs with dual cations. The importance of exploring dual-cation systems has been demonstrated in this work by maintaining the same Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio (2
Mg ratio (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) within the two different structures in a one pot synthesis. As such, these dual-cation systems deserve further study.
1) within the two different structures in a one pot synthesis. As such, these dual-cation systems deserve further study.
Uranyl hydrolysis is heavily pH dependent. Hydrolysed uranyl species such as [UO2(OH)]+, [(UO2)(OH)2], [(UO2)2(OH)2]2+ and [(UO2)3(OH)5]+ increase when the solution pH is above 5.33 Most of the UOH phases were synthesised hydrothermally with solution pH values from 3 to 6. However, UOH phase formation in slightly alkaline solutions has been less investigated. This was briefly addressed in this work by furthering uranyl hydrolysis at solution pH values from 6.5 to 8.0. The success in synthesising the three novel UOHs with Mg2+ ions highlights the delicate balance of reaction conditions which leads to the formation of preferred structure types. Future synthesis work targeting higher solution pH values (from 8 to 10) close to the pH range for underground water is essential towards elucidating the conditions that drive the selective formation of new UOH phases.
4. Conclusions
Three novel UOH materials with Mg2+ or Mg2+ and Na+ ions have been successfully synthesised hydrothermally, with the solution pH adjusted by adding a dilute NaOH solution. Although they are all layered structures with interlayer secondary cations, they differ substantially in the uranium oxide hydroxide layers and the arrangements of interlayer cations. While U-Mg1 crystallises in the P21/c space group with a U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg ratio of 3
Mg ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, both U-Mg2p and U-Mg2n crystallise in the P
1, both U-Mg2p and U-Mg2n crystallise in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group with U
space group with U![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mg
Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na ratios of 6
Na ratios of 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 8
2 and 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, respectively. The structures differ in that U-Mg1 has β-U3O8-type uranyl oxide hydroxide layers with Mg2+ interlayer cations while U-Mg2p has unique layers containing both α-U3O8 and β-U3O8 features, and U-Mg2n has corrugated layers but with different ways of incorporating mixed Mg2+ and Na+ interlayer cations.
2, respectively. The structures differ in that U-Mg1 has β-U3O8-type uranyl oxide hydroxide layers with Mg2+ interlayer cations while U-Mg2p has unique layers containing both α-U3O8 and β-U3O8 features, and U-Mg2n has corrugated layers but with different ways of incorporating mixed Mg2+ and Na+ interlayer cations.
The structure diversity in the U–Mg system has been achieved in a narrow solution pH range from 6.5 to 8.0, highlighting the complex uranium chemistry which drives the formation and stabilisation of UOH phases at near neutral solution pH values via the subtle evolution of uranium oxide hydroxide layers and the incorporation of single-/dual-secondary cations. While the Mg2+ ion adopts 6-fold coordination in an octahedral geometry similar to some 3d transition metal ions (such as Co2+ and Ni2+), the Na+ ion adopts more flexible coordination environments with coordination numbers ranging from 6 to 8. Therefore, this work not only fills the knowledge gaps in synthetic UOH phases with Mg2+ ions, but also sheds light on the possible UOH structures with some 3d transition metal ions (a similar coordination environment to the Mg2+ ion). Although the structural flexibility induced by the addition of Na+ ions has been briefly discussed, further work on dual-cation systems is still necessary to better rationalise the complex structural nature of these UOH phases. In addition, further experiments such as X-ray absorption could resolve ambiguities in these complex structures, such as whether they have variable compositions.
Author contributions
Y. Zhang: conceptualization, data curation, formal analysis, project administration, resources, supervision, writing – original draft, and writing – review & editing; K. T. Lu: data curation, formal analysis, and writing – review & editing; T. Wei: data curation, formal analysis, and writing – review & editing; I. Karatchevtseva: data curation, formal analysis, and writing – review & editing; R. Zheng: supervision and writing – review & editing.Conflicts of interest
The authors are not aware of any conflict of interest.Acknowledgements
The synthesis and characterization of materials were carried out in the facilities under Nuclear Science and Technology (NST) at ANSTO. The crystallographic data for compounds U-Mg1, U-Mg2p and U-Mg2n were collected on the MX2 beamline at the Australian Synchrotron, a part of ANSTO, and the Australian Cancer Research Foundation (ACRF) detector was used.References
- V. Popa and O. Cocoş, Cent. Eur. J. Geogr. Sustainable Dev., 2021, 3, 17–25 Search PubMed.
- Y. Zhang, L. Kong, M. Ionescu and D. J. Gregg, J. Eur. Ceram. Soc., 2022, 42(5), 1852–1876 CrossRef CAS.
- R. J. Baker, Coord. Chem. Rev., 2014, 266, 123–136 CrossRef.
- S. Spiridonov, A. Perevolotskii, T. Perevolotskaya, R. Aleksakhin and E. Spirin, At. Energy, 2017, 123, 122–126 CrossRef.
- A. Paulillo, J. M. Dodds, S. J. Palethorpe and P. Lettieri, Sustainable Mater. Technol., 2021, 28, e00278 CrossRef CAS.
- D. Mallants, K. Travis, N. Chapman, P. V. Brady and H. Griffiths, Energies, 2020, 13(4), 833 CrossRef.
- J. Plášil, J. Geosci., 2014, 59, 99–114 CrossRef.
- J. Janeczek and R. C. Ewing, J. Nucl. Mater., 1992, 190, 128–132 CrossRef CAS.
- R. J. Finch, R. Haddal and G. T. Baldwin, Safeguards Implications for Deep Borehole Disposal of Spent Fuel, SAND2016-4591 report, 2016, Sandia National Laboratories, USA.
- R. C. Ewing, Nat. Mater., 2015, 14, 252–257 CrossRef CAS PubMed.
- D. J. Wronkiewicz, J. K. Bates, S. F. Wolf and E. C. Buck, J. Nucl. Mater., 1996, 238, 78–95 CrossRef CAS.
- R. J. Finch and R. C. Ewing, J. Nucl. Mater., 1992, 190, 133–156 CrossRef CAS.
- J. Janeczek and R. Ewing, J. Nucl. Mater., 1992, 190, 157–173 CrossRef CAS.
- J. Plášil, Eur. J. Mineral., 2017, 29, 1–15 CrossRef.
- P. C. Burns, Hydrated uranium oxides, in Comprehensive Nuclear Materials, ed. R. Konings and R. Stoller, Elsevier, 2nd edn, 2020 Search PubMed.
- Y. Zhang, K. T. Lu and R. Zheng, Dalton Trans., 2022, 51, 2158–2169 RSC.
- T. A. Olds, J. Plášil, A. R. Kampf, T. Spano, P. Haynes, S. M. Carlson, P. C. Burns, A. Simonetti and O. P. Mills, Am. Mineral., 2018, 103, 143–150 CrossRef.
- P. C. Burns, Can. Mineral., 1998, 36, 1061–1067 CAS.
- C. L. Cahill and P. C. Burns, Am. Mineral., 2000, 85, 1294–1297 CrossRef CAS.
- J. Plášil, R. Škoda, J. Čejka, V. Bourgoin and J.-C. Boulliard, Eur. J. Mineral., 2016, 28, 959–967 CrossRef.
- K. A. Hughes, P. C. Burns and U. Kolitsch, Can. Mineral., 2003, 41, 677–685 CrossRef CAS.
- J. Plášil, Eur. J. Mineral., 2018, 30, 237–251 CrossRef.
- M. L. Miller, R. J. Finch, P. C. Burns and R. C. Ewing, J. Mater. Res., 1996, 11(12), 3048–3056 CrossRef CAS.
- P. C. Burns and F. C. Hill, Can. Mineral., 2000, 38, 163–173 CrossRef CAS.
- F. C. Hill and P. C. Burns, Can. Mineral., 1999, 37, 1283–1288 CAS.
- R. E. Glatz, Y. Li, K.-A. Hughes, C. L. Cahill and P. C. Burns, Can. Mineral., 2002, 40, 217–224 CrossRef CAS.
- P. C. Burns and F. C. Hill, Can. Mineral., 2000, 38, 175–181 CrossRef CAS.
- K. T. Lu, Y. Zhang, T. Wei, T. A. Ablott, T. H. Nguyen and R. Zheng, New J. Chem., 2022, 46, 1371–1380 RSC.
- K. T. Lu, Y. Zhang, T. Wei, T. A. Ablott, J. Plasil, I. Karatchevtseva and R. Zheng, New J. Chem., 2023, 47, 13286–13296 RSC.
- Y. Li and P. C. Burns, Can. Mineral., 2000, 38, 1433–1441 CrossRef CAS.
- M. Rivenet, N. Vigier, P. Roussel and F. Abraham, J. Solid State Chem., 2009, 182, 905–912 CrossRef CAS.
- N. G. Chernorukov, O. V. Nipruk, K. A. Klinshova, O. N. Tumaeva and D. V. Sokolov, New J. Chem., 2021, 45, 9922–9935 RSC.
- T. A. Ablott, K. T. Lu, T. Wei and Y. Zhang, Dalton Trans., 2023, 52, 6629–6640 RSC.
- Y. Zhang, J. Čejka, G. R. Lumpkin, T. T. Tran, I. Aharonovich, I. Karatchevtseva, J. R. Price, N. Scales and K. Lu, New J. Chem., 2016, 40, 5357–5357 RSC.
- Y. Zhang, R. Aughterson, I. Karatchevtseva, L. Kong, T. T. Tran, J. Čejka, I. Aharonovich and G. R. Lumpkin, New J. Chem., 2018, 42, 12386–12393 RSC.
- Y. Zhang, R. D. Aughterson, Z. Zhang, T. Wei, K. Lu, J. Čejka and I. Karatchevtseva, Inorg. Chem., 2019, 58, 10812–10821 CrossRef CAS PubMed.
- G. L. Murphy, P. Kegler, M. Klinkenberg, A. Wilden, M. Henkes, D. Schneider and E. V. Alekseev, Dalton Trans., 2021, 50, 17257–17264 RSC.
- K.-A. Kubatko and P. C. Burns, Inorg. Chem., 2006, 45, 10277–10281 CrossRef CAS PubMed.
- T. A. Ablott, K. T. Lu, R. D. Aughterson and Y. Zhang, Dalton Trans., 2022, 51, 15965–15973 RSC.
- Y. Li, C. L. Cahill and P. C. Burns, Chem. Mater., 2001, 13, 4026–4031 CrossRef CAS.
- K. T. Lu, Y. Zhang, R. D. Aughterson and R. Zheng, Dalton Trans., 2020, 49, 15854–15863 RSC.
- K. T. Lu, Y. Zhang, T. Wei, Z. Wang, D. T. Oldfield and R. Zheng, Inorg. Chem., 2021, 60, 13233–13241 CrossRef CAS PubMed.
- Y. Zhang, T. Wei, T. T. Tran, K. T. Lu, Z. Zhang, J. R. Price, I. Aharonovich and R. Zheng, Inorg. Chem., 2020, 59, 12166–12175 CrossRef CAS PubMed.
- Magnesium, National Minerals Information Center, USGS, https://www.usgs.gov/centers/national-minerals-information-center/magnesium-statistics-and-information.
- H. Steinfink and F. J. Sans, Am. Mineral., 1959, 44, 679–682 CAS.
- Search Minerals By Chemistry (mindat.org).
- P. C. Burns, Can. Mineral., 1998, 36, 187–199 CAS.
- R. Vochten, L. Van Haverbeke and R. Sobry, J. Mater. Chem., 1991, 1(4), 637–642 RSC.
- N. G. Chernorukov, O. V. Nipruk, G. N. Chernorukov and O. S. Sedelkina, Radiochemistry, 2015, 57(4), 378–380 CrossRef CAS.
- L. N. Belova, B. I. Ryzhov, O. V. Fedorov and G. V. Lyubomilova, Izv. Akad. Nauk SSSR, Ser. Geol., 1985, 2, 65–72 Search PubMed.
- R. J. Finch and R. C. Ewing, Am. Mineral., 1997, 82, 607–619 CAS.
- D. Aragão, J. Aishima, H. Cherukuvada, R. Clarken, M. Clift, N. P. Cowieson, D. J. Ericsson, C. L. Gee, S. Macedo, N. Mudie, S. Panjikar, J. R. Price, A. Riboldi-Tunnicliffe, R. Rostan, R. Williamson and T. T. Caradoc-Davies, J. Synchrotron Radiat., 2018, 25, 885–891 CrossRef PubMed.
- W. Kabsch, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2010, 66, 133–144 CrossRef CAS PubMed.
- G. M. Sheldrick, SADABS, Empirical Absorption and Correction Software, University of Göttingen, Göttingen, 1996 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- S. Wu, J. Ling, S. Wang, S. Skanthakumar, L. Soderholm, T. E. Albrecht-Schmitt, E. V. Alekseev, S. V. Krivovichev and W. Depmeier, Ber. Dtsch. Chem. Ges., 2009, 27, 4039–4042 Search PubMed.
- Z. Weng, S. Wang, J. Ling, J. M. Morrison and P. C. Burns, Inorg. Chem., 2012, 51, 7185–7191 CrossRef CAS PubMed.
- I. D. Brown, Chem. Rev., 2009, 109(12), 6858–6919 CrossRef CAS PubMed.
- P. C. Burns, P. C. Burns, R. C. Ewing and F. C. Hawthorne, Can. Mineral., 1997, 35, 1551–1570 CAS.
- K. T. Lu, Y. Zhang, T. Wei, J. Čejka and R. Zheng, Dalton Trans., 2020, 49, 5832–5841 RSC.
- O. C. Gagné, Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater., 2018, 74, 49–62 CrossRef.
- Y. Zhang, D. J. Fanna, N. D. Shepherd, I. Karatchevtseva, K. Lu, L. Kong and J. R. Price, RSC Adv., 2016, 6, 75045–75053 RSC.
- N. D. Shepherd, Y. Zhang, I. Karatchevtseva, J. R. Price, L. Kong, N. Scales and G. R. Lumpkin, Polyhedron, 2016, 113, 88–95 CrossRef CAS.
- E. R. Vance, Y. Zhang and Z. Zhang, J. Nucl. Mater., 2010, 400(1), 8–14 CrossRef CAS.
- Y. Zhang, T. Wei, Z. Zhang, L. Kong, P. Dayal and D. J. Gregg, J. Am. Ceram. Soc., 2019, 102(12), 7699–7709 CrossRef CAS.
- Y. Zhang, L. Kong, I. Karatchevtseva, R. D. Aughterson, D. J. Gregg and G. Triani, J. Am. Ceram. Soc., 2017, 100(9), 4341–4351 CrossRef CAS.
- Y. Zhang, L. Kong, R. D. Aughterson, I. Karatchevtseva and R. Zheng, J. Am. Ceram. Soc., 2017, 100(11), 5335–5346 CrossRef CAS.
- R. L. Frost, J. Čejka and M. L. Weier, J. Raman Spectrosc., 2007, 38(4), 460–466 CrossRef CAS.
- Y. Zhang, J. Čejka, I. Karatchevseva, M. Qin, L. Kong, K. Short, S. C. Middleburg and G. R. Lumpkin, J. Nucl. Mater., 2014, 446, 68–72 CrossRef CAS.
- Y. Zhang, I. Karatchevtseva, M. Qin, S. C. Middleburgh and G. R. Lumpkin, J. Nucl. Mater., 2013, 437, 149–153 CrossRef CAS.
- J. R. Bartlett and R. P. Cooney, J. Mol. Struct., 1989, 193, 295–300 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: SEM-EDS and supporting tables. CCDC 2289968 (U-Mg1), 2289969 (U-Mg2p), and 2289970 (U-Mg2n). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt03078d |
| This journal is © The Royal Society of Chemistry 2023 |