A closed-shell phenalenyl-based dinuclear iron(iii) complex as a robust cathode for a one-compartment H2O2 fuel cell†
Abstract
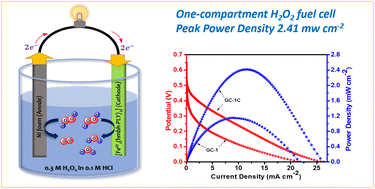
Closed-shell phenalenyl (PLY) systems are increasingly becoming more attractive as building blocks for developing promising catalysts and electroactive cathode materials, as they have tremendous potential to accept electrons and participate in redox reactions. Herein, we report a PLY-based dinuclear [FeIII2(hmbh-PLY)3] complex, 1, and its utility as a cathode material in a H2O2 fuel cell. Complex 1 was synthesized from a new Schiff base ligand, (E)-9-(2-(2-hydroxy-3-methoxybenzylidene)hydrazineyl)-1H-phenalen-1-one, hmbh-PLYH2, designed using a PLY precursor, Hz-PLY. The newly derived ligand and complex 1 were characterized by various analytical techniques, including single-crystal X-ray diffraction (SCXRD). The cyclic voltammetry (CV) study revealed that complex 1 undergoes five electron reductions under an applied electric potential. When the electroactive complex 1 was employed as a cathode in a membrane-less one-compartment H2O2 fuel cell, with Ni foam as the corresponding anode, the designed fuel cell exhibited an exceptionally high peak power density (PPD) of 2.41 mW cm−2, in comparison with those of all the previously reported Fe-based molecular complexes. DFT studies were performed to gain reasonable insights into the two-electron catalytic reduction (pathway I) of H2O2 by the Fe-center of complex 1 and to explore the geometries, energetics of the electrocatalyst, reactive intermediates and transition states.


 Please wait while we load your content...
Please wait while we load your content...