Molar excess of coordinating N-heterocyclic carbene ligands triggers kinetic digestion of gold nanocrystals†
Abstract
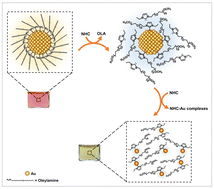
There has been much interest in evaluating the strength of the coordination interactions between N-heterocyclic carbene (NHC) molecules and transition metal ions, nanocolloids and surfaces. We implement a top-down core digestion test of Au nanoparticles (AuNPs) triggered by incubation with a large molar excess of poly(ethylene glycol)-appended NHC molecules, where kinetic dislodging of surface atoms and formation of NHC–Au complexes progressively take place. We characterize the structure and chemical nature of the generated PEG-NHC–Au complexes using 1D and 2D 1H–13C NMR spectroscopy, supplemented with matrix assisted laser desorption/ionization mass spectrometry, and transmission electron microscopy. We further apply the same test using thiol-modified molecules and find that though etching can be measured the kinetics are substantially slower. We discuss our findings within the classic digestion of transition metal ores and colloids induced by interactions with sodium cyanide, which provides an insight into the strength of coordination between the strong σ-donating (soft Lewis base) NHC and Au surfaces (having a soft Lewis acid character), as compared to gold-to-gold covalent binding.


 Please wait while we load your content...
Please wait while we load your content...