 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
What do we know about bifunctional cage-like T8 silsesquioxanes? Theory versus lab routine†
Kamila
Fuchs
 a,
Edyta
Nizioł
a,
Edyta
Nizioł
 a,
Jolanta
Ejfler
a,
Jolanta
Ejfler
 a,
Wiktor
Zierkiewicz
a,
Wiktor
Zierkiewicz
 b,
Anna
Władyczyn
b,
Anna
Władyczyn
 a and
Łukasz
John
a and
Łukasz
John
 *a
*a
aFaculty of Chemistry, University of Wrocław, 14 F. Joliot-Curie, 50-383 Wrocław, Poland. E-mail: lukasz.john@uwr.edu.pl
bFaculty of Chemistry, Wrocław University of Science and Technology, 27 Wybrzeże Wyspiańskiego, 50-370 Wrocław, Poland
First published on 29th September 2023
Abstract
In this article, we explore theoretical validations of experimental findings pertaining to the classical corner-capping reactions of a commercially available heptaisobutyltrisilanol cage to mono-substituted phenylhepta(isobutyl)-POSS cages. Additionally, the process of opening a fully condensed cage is tracked to assess the possibility of isolating and separating the resulting isomers. The corner-capping reactions of potential silanotriols, both as monomers and dimers, and the impact of these structural motifs on their closing to bifunctional POSS cages are also investigated. Our studies highlight that analyzing experimental results alone, without incorporating complex theoretical investigations, does not offer a clear understanding of the reactions involving multiple simultaneously reacting substrates, which may also undergo further transformations, potentially complicating the conventional pathways of classic corner-opening/capping reactions.
Introduction
Octafunctional polyhedral oligomeric silsesquioxanes (POSSs) of general formula (RSiO3/2)8 (namely T8R8; T8 is an octahedral cage containing eight silicon atoms; R is the reactive or non-reactive group or hydrogen atom) constitute an attractive group of organosilicons with hybrid architecture.In the case of cubic derivatives, the silsesquioxane core comprises eight silicon atoms connected via oxygen bridges. The siloxane moieties forming an inorganic core impart tremendous thermodynamic, mechanical, and chemical stability. Moreover, various organic groups can modify the vertices of the inner cube. Among the T8-type silsesquioxanes, the most recognized are mono- (Fig. 1A) and octafunctional (Fig. 1B) derivatives routinely obtained from, e.g., octakis(3-aminopropyl)octasilsesquioxane or octakis(vinyl)octasilsesquioxanes.1 The third type of cage-like silsesquioxanes, which have not been widely studied, can be considered a surrogate to cis/trans bifunctional double-decker silsesquioxanes (DDSQs).2 Bifunctional derivatives of silsesquioxanes shown in Fig. 1C are predominantly synthesized using corner-opening and corner-capping strategies.3 Unusually, there are only a small number of literature reports on this topic, considering the potential of bifunctional POSSs as precise nano-building blocks for advanced materials (e.g., polymers, dendrimers, and Janus silsesquioxanes) with peculiar properties in a bottom-up approach.4 On the other hand, accurately positioning functional groups in custom-designed architectures through precise synthesis can be an overwhelming challenge.5–13
 | ||
| Fig. 1 Possible cubic T8-type silsesquioxanes: (A) monofunctional, (B) octafunctional, and (C) bifunctional. | ||
So far, little reliable evidence has been presented for obtaining POSSs with several (less than 8 in the case of octa-substituted derivatives) reactive substituents.14 There is also a lack of information on this type of organosilicon compounds’ crystal structures. The only shreds of evidence of obtaining such structures are low-quality 29Si NMR spectra.15,16 Conclusions drawn from interpreting the number and position of signals in the 29Si NMR spectrum are insufficient to formulate a definite assumption on the structure of caged silsesquioxane, as other analytical methods should support such findings. W.-B. Zhang14 presented the evidence of bifunctional (and also trifunctional) POSS describing the preparation of heterofunctional POSSs by modification of an octa-vinyl-substituted silsesquioxane cage. As a result, some of the vinyl groups are modified under the influence of trifluoroacetic acid to hydroxyethyl groups. The use of a 5-fold excess of acid and a short reaction time (4–5 h) caused the vinyl substituents to react partially – thus, a mixture of POSS substituted with vinyl and hydroxyethyl groups in different proportions and positions was obtained. A relatively short reaction time was necessary in this case because it was observed that the extension of the reaction time destroys the cage structure due to the hydrolysis of Si–O and Si–C bonds. As a result of ‘multi-variant’ column chromatography, it was possible to separate the para (D3d, 4% yield), ortho (C2v, 10%), and meta (C2v, 10%) isomers in a ratio of 1/3/3 and three isomers for the trifunctional cage, which was confirmed by NMR spectroscopy (29Si, 1H, and 13C). In addition, the results described by B. Dudziec, P. Żak, et al.17 on cross-metathesis, where octa-vinyl-substituted POSS was also used as a substrate for further modifications, confirm that such reactions occur non-stoichiometrically, obtaining a mixture of products with varying degrees of substitution.
The selective synthesis of derivatives with more than one reactive substituent is problematic because the hydrolytic condensation of trihalo/trialkoxy silanes is a complicated equilibrium process. The selectivity of the reaction is influenced not only by the stoichiometry of the reactants but also by the type of solvent, temperature, or catalyst.18 The products of hydrolytic condensation using a mixture of trichloro/trialkoxy silanes differing in substituent have been proven to be a mixture of octa-T8R8, mono-T8R7R′, bifunctional T8R6R′2 (para, ortho and meta isomers) cages, and polysilsesquioxanes.16,19 Among the inconveniences associated with obtaining pure, hetero-functional T8 cages, one can also mention the problem with their separation.20 These derivatives have a similar structure (and thus polarity) and tend to form weak intermolecular interactions. These factors are the basis for the coelution of the components of the mixture of heterosubstituted derivatives and geometric isomers of bifunctional cages. Therefore, POSS derivatives are sought, the synthesis of which would be scalable, reproducible, and relatively inexpensive.
L. Marchese et al.21 developed a method for the synthesis of bifunctional POSSs, in which the monofunctional POSS was used as a starting material, which undergoes hydrolysis in only one corner of the cage (so-called corner-opening reaction). The hydrolysis was carried out in THF in the presence of tetramethylammonium hydroxide (TEAOH) for 4 h under reflux. Next, the opened cage reacts with Ti(OiPr)4, forming a new cage through condensation. The resulting cage possesses six isobutyl groups, one 3-aminopropyl group, and a titanium atom embedded in the cage structure with a coordinated isopropoxy group located in the para position relative to it. The chance of success of this strategy comes from the assumption that the hydrolysis of the monofunctional cage is favored for one of the corners of the cage. According to this report, the Si atom in the para position to the 3-aminopropyl substituent and the Si atom with the 3-aminopropyl substituent undergo hydrolysis, with the efficiency of the first process being as much as 88% and 12% for the second one. The main product of the synthesis was probably identified based on the number of signals in the 29Si NMR spectrum. para-Adduct is the most symmetrical isomer; it has three Si atoms with the same chemical environment, so the number of signals for this isomer is 3; for ortho-adduct and meta-adduct, five signals are expected. Unfortunately, the authors do not explain how the T7R7(OH)3 open cage was separated from the T7R6R′(OH)3 cage. It is also not entirely clear which premise the percentage content of the mixture products was determined. The mentioned concept inspired K. Naka15,21 to apply this strategy to introduce the same silane into the hydrolyzed corner used at the monofunctional cage synthesis stage. First, the authors repeated the procedure by opening the 3-aminopropyl heptaisobutyl cage in the presence of TEAOH. Next, the hydrolysis product was purified by washing with methanol, which probably caused the isolation of T7R7(OH)3 from the reaction mixture. The authors extended the reaction time from 4 to 7 h, obtaining the T7R6R′(OH)3 derivative with a 93% yield. The next step involved the open cage reaction with the 3-aminopropyl substituent with 3-aminopropyltriethoxysilane.22 The reaction was carried out in THF at room temperature for 48 h. The bifunctional cage was obtained after dissolving the reaction mixture in methanol, concentrating the filtrate, and washing it with acetonitrile, with a good yield of 68%. The fact that the para isomer was obtained is supported by the 29Si NMR spectrum with two signals (which is the expected effect for a symmetric para isomer). However, the resolution of the published spectrum is poor, and the signal integration ratio does not agree with the theoretical one. For the para isomer, two signals with 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 integration are expected, whereas this report equals 1
6 integration are expected, whereas this report equals 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. This work is also enriched with DFT calculations, the result of which explains the preference of nucleophilic attack for the silicon atom in the para position.
1. This work is also enriched with DFT calculations, the result of which explains the preference of nucleophilic attack for the silicon atom in the para position.
A similar synthetic strategy was used to prepare bivinylheptaisobutyl-POSS.15 Vinylheptaisobutyl-POSS was used as a precursor for the corner-opening reaction. The reaction was carried out at room temperature and not, as in the case of 3-aminopropylheptaisobutyl-POSS, noticing fewer chemical shifts in the spectrum. The analysis of multinuclear (29Si, 1H, and 13C) NMR and FT-IR spectra proves that carrying out the reaction at a lower temperature leads to the selective formation of an open cage in the para position. After the reaction, an appropriate amount of 2 M HCl solution was introduced to separate the product of its reaction with TEAOH from the post-reaction mixture. The mixture was then concentrated, methanol was added to the residue, and the formed precipitate (probably unreacted substrate) was filtered off. The product was obtained as a precipitate after the concentration of the solution and drying in a vacuum. Then, the dried product was used in the corner-capping procedure. As a second substrate, trichlorovinyl silane was used, which was applied in a slight excess relative to POSS. The reaction was carried out for 1 h at 0 °C and then for 3 h at room temperature.
Interestingly, the authors did not use the addition of a tertiary amine (such as TEA or DIPEA) in this reaction, which is typically used in corner-capping reactions to neutralize HCl released as a byproduct. Adding an amine increases the yield of the reaction for two reasons. Firstly, it shifts the reaction equilibrium towards product formation. Secondly, it neutralizes HCl, which can cause cage hydrolysis. The reaction mixture was concentrated, and the pure product was purified by size exclusion chromatography, leading to bi(vinyl)hexaisobutyl-POSS. It is worth mentioning that the postulated efficiency of obtaining a bifunctional cage after purification by this method is only 20%. The purification method by fractional crystallization using propane-2-ol and acetonitrile reported a yield of 50%; however, further optimization of the synthesis and purification is needed.
In this paper, the quest for ortho, meta, and para isomers, as a part of the enigmatic synthesis of bifunctional T8R6R′2 POSS, from theoretical and experimental standpoints, is deeply analyzed and discussed.
Experimental section
Materials and methods
All commercially available chemicals were used without further purification: phenyltrimethoxysilane (>97%, Sigma Aldrich), isobutyltrisilanol POSS (Hybrid Plastics Inc.), tetraethyl–ammonium hydroxide (35% w/w aq. soln., Alfa Aesar), hydrochloric acid solution (2 M, Sigma Aldrich). Solvents for standard workup (tetrahydrofuran, ethanol, methanol, acetonitrile) were used as received and were purchased from Chempur and VWR International. Elemental analyses were measured on an Elementar's Vario EL Cube analyzer. The NMR spectra were recorded with the Bruker Avance II 500 MHz spectrometer and Bruker Avance III 600 MHz spectrometer. The solvent used during the measurements was chloroform. Spectra were calibrated based on the residual solvent signal, taking the following values: 7.26 ppm for 1H NMR spectra and 77.00 ppm for 13C NMR spectra. FTIR spectra were recorded on Bruker Vertex 70 spectrometer. The measured samples were prepared by making a KBr pellet. MALDI-MS spectra were recorded using JEOL JMS-S3000 SpiralTOF™-plus Ultra-High Mass Resolution MALDI-TOFMS Mass Spectrometer.Synthetic procedures
Diphenylhexa(isobutyl)-POSS T8(i-Bu)6(Ph)2 synthesis attempt
0.5 g of 2 (0.6 mmol) was dissolved in 20 mL of THF, and phenyltrimethoxysilane (0.122 g, 0.6 mmol) was added when stirring. The solution was stirred at room temperature for 48 h, and then THF was evaporated on the rotary evaporator. The residue was dispersed in 30 mL of acetonitrile and dried under vacuum. The identified reaction product was partially condensed trisilanol T7(i-Bu)7(OH)3 (3) instead of T8(i-Bu)6(Ph)2, which was obtained with 83% yield. 1H NMR (500 MHz, CDCl3), δ (ppm): 6.80 (s, 3H), 1.84 (m, 7H), 0.93 (dd, 42H), 0.55 (m, 14H). 13C NMR (126 MHz, CDCl3), δ (ppm): 25.78 (d, 14C) 23.91 (s, 7C), 23.27 (s, 4C), 22.86 (s, 3C). 29Si NMR (99 MHz, CDCl3), δ (ppm): 59.00, 67.48, 68.74. MALDI-MS m/z: 813.26 {calcd for [M + Na]+ 813.28}.Theoretical calculations
Optimizations and molecular orbitals for all structures presented in this article were calculated at the B3LYP-D3/6-31g(d) (hybrid density functional with D3 damping function to include dispersion) level of theory in the gas phase.24–27 The optimizations were conducted without any geometrical constraints, and the vibrational frequencies were calculated to confirm that all obtained geometries are true minima, as there were no imaginary frequencies. All optimizations were carried out in Gaussian16, Rev.C.O1 set of codes.28 The Chemcraft29 software was applied to visualize molecular orbitals.Theoretical diffusion coefficients (D) have been calculated for DFT optimized structures following the Einstein–Stokes equation, corrected by a factor derived from micro-frictional theory30 and semi-empirically improved by Chen31eqn (1), where k is Boltzmann constant, T is the temperature, and η is the viscosity of deuterated benzene.32 Spherical equivalent radii (Req.) have been calculated from volumes limited by the van der Waals surface (V) eqn (2). van der Waals volumes have been calculated using MultiWFN software33,34 and visualized in the VMD program.35
 | (1) |
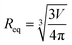 | (2) |
Results and discussion
The strategic synthesis of bifunctional POSS cages (T8R6R′2) is highly significant in developing novel hybrid compounds with exceptional properties. Achieving selectivity in the placement of sidearms at the ortho (o), meta (m), or para (p) positions is crucial. The traditional approach involves opening the T8 octa-functional (T8R8) cage, followed by closing the resulting T7R7(OH)3 silanotriol to form the monofunctional POSS (T8R7R′) cage. The closing step is typically uncomplicated, and the reaction efficiency and product yield depend on the characteristics of the substituents on the cage core and the optimization of the corner-capping reaction. However, reopening the cage lacks selectivity, resulting in a mixture of ortho/meta/para silanotriols of formula T7R6R′(OH)3 (Fig. 2). The separation of this mixture or the selective closure of a monofunctional derivative holds great significance for the synthesis of bifunctional T8R6R′2 cages.Finding a solution to this problem remains one of the most intriguing synthetic strategies in silsesquioxane cage chemistry. Consequently, a controlled process necessitates selective reactions involving the closing and opening of monofunctional T8R7R′ cages (Fig. 2).
The initial step in the ‘transformation’ process involves opening the octafunctional T8R8 cage, which is straightforward, and the resulting product, the silanotriol T7R7(OH)3, is readily available commercially. Similarly, closing a silanotriol to form a mono-POSS cage (T8R7R′) is not problematic as long as there is no condensation reaction between the hydroxyl groups of the open cages. Such condensation could lead to contamination with the T8R8 cage or several other difficult-to-identify byproducts. Another factor that disrupts corner-capping reactions is the formation of the [T7R7(OH)3]2 dimer through intermolecular hydrogen bonds among the T7R7(OH)3 silanotriols. The contamination of the main product with the T8R8 cage can be detected using 29Si NMR spectroscopy or mass spectrometry. However, identifying the [T7R7(OH)3]2 dimer and controlling its reactivity is neither straightforward nor trivial. Similar challenges arise with monofunctional open cages, namely o/m/p T7R6R′(OH)3, where the statistical opening of the T8R7R′ cage increases the number of potential combinations, including the possibility of dimer formation. In this case, none of the T8R7R′ silicon atoms is favored in the hydrolysis reaction, resulting in a mixture of monomeric regioisomers o/m/p-T7R6R′(OH)3, as well as homo(o–o, p–p, m–m) and hetero(o–p, o–m, p–m) combinations. Unusual rearrangements of cages or unintended closures can be confirmed when these compounds crystallize, providing an opportunity to assess the potential synthesis strategy.
This study focuses on the hindrance of further cage corner-capping reactions caused by the crystallization of a stable dimer. Consequently, computational methods were employed to analyze this issue and determine the crucial factors in evaluating the reactivity of POSS mono-cages in both closed T8R7R′, T8R6R2′, and open states. The open cages, specifically silanotriols, exist as monomers and dimers denoted as T7R6R′(OH)3 and [T7R6R′(OH)3]2, respectively. In these structures, R represents i-Bu or Ph, while R′ represents 3-aminopropyl (C3H6NH2), vinyl (C2H3), or phenyl (Ph). The closed derivatives (T8R6R′2) exhibit ortho, meta, and para isomers. Similarly, open forms, both as monomeric and dimeric silanotriols, are mixtures of o/m/p isomers (Fig. 3).
 | ||
| Fig. 3 Examples of monomers and dimers in opened versions as ortho, meta, and para isomers: T7R6(C3H6NH2)(OH)3, and [T7R6(C3H6NH2(OH)3)]2, and closed bifunctional T8R6(C3H6NH2)2 cages. | ||
In order to gain insight into the reactivity of closed POSS forms in corner-capping reactions and open forms in corner-opening reactions, DFT calculations were performed. The optimized structures of closed POSS cages were analyzed to examine the patterns of the Lowest Unoccupied Molecular Orbital (LUMO) and its neighboring unoccupied MOs, which play a role in nucleophilic attacks initiating corner-opening reactions. The LUMO pattern was also calculated for open forms and bifunctional POSS cages (Table S1 and Fig. S19†).
The starting point for optimization of dimer structures was based on the known crystal structures of the silanotriol dimers [T7(i-Bu)7(OH)3]2, which crystallize in the monoclinic crystallographic system in the space group P21/n.36–39 Two open cage molecules are connected by three hydrogen bonds between the silanol groups (the distances between adjacent oxygen atoms of the hydroxyl groups are between 2.64–2.68 Å); molecules creating dimer are symmetry-related by inversion center (Fig. S16†). The dimer reported by F. Feher et al.39 was observed to crystallize during the condensation reaction of cyclohexyltrichlorosilane in aqueous acetone, with the crystallization process lasting from several months to 3 years (!). The dimer crystallized in the mixture containing T8R8 (R = i-Bu) cages and an unusually open T8R8(OH)2. On the other hand, a dimer with phenyl substituents could be obtained within a few minutes by reacting the T7R7(ONa)3 derivative with an aqueous HCl solution, as reported by Liu et al.37 The same group also described the equilibrium between these cages and another dimer structure.40 In each case, confirming the structure of the main dimer product required a lengthy crystallization period. Additionally, the products of unusual transformations crystallized only after isolating the dimer, and it was through long-term crystallization and determination of the crystal structure that these structural motifs could be diagnosed. X-ray data confirmed the synthesis of cages with unique structures, but they did not offer clear guidelines for planning effective synthesis strategies or proposing reaction equations with absolute certainty.
The structure in the solid state may or may not be consistent with that in the solution after the crystals have dissolved. In the most straightforward approach, dimer, monomer, or monomer-dimer dynamics may be in solution. In order to verify whether the dimer motif is maintained in solution, the experimental diffusion coefficient (Dexp) determined from the DOSY spectra and the theoretical one were compared using the methodology presented in our previous work.41 The diffusion coefficient (D) for trisilanol (3) from the experimental studies is close to that calculated for the monomer (Fig. 4 and Table 1). Similarly, for silanols (2), the Dexp is consistent with DDFT for monomeric form. The careful analysis of experimental data and their validation through computational methods suggest that the dimeric motif is prevalent in the crystalline form. At the same time, monomers are the dominant species in solution.
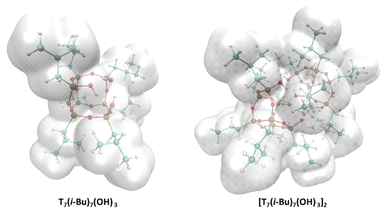 | ||
| Fig. 4 van der Waals surfaces generated around DFT optimized T7(i-Bu)7(OH)3 and [T7(i-Bu)7(OH)3]2 structures. | ||
| POSS | V [Å] | D DFT [m2 s−1] | D exp [m2 s−1] |
|---|---|---|---|
| T7(i-Bu)7(OH)3 | 979.800 | 6.7 × 10−10 | 6.1 × 10−10 |
| [T7(i-Bu)7(OH)3]2 | 1945.593 | 5.1 × 10−10 | — |
| ortho-T7(i-Bu)6(Ph)(OH)3 | 982.897 | 6.7 × 10−10 | 6.8 × 10−10 |
| [ortho-T7(i-Bu)6(Ph)(OH)3]2 | 1955.177 | 5.1 × 10−10 | — |
| meta-T7(i-Bu)6(Ph)(OH)3 | 982.688 | 6.7 × 10−10 | 6.8 × 10−10 |
| [meta-T7(i-Bu)6(Ph)(OH)3]2 | 1955.478 | 5.1 × 10−10 | — |
| para-T7(i-Bu)6(Ph)(OH)3 | 983.848 | 6.7 × 10−10 | 6.8 × 10−10 |
| [para-T7(i-Bu)6(Ph)(OH)3]2 | 1947.774 | 5.1 × 10−10 | — |
To gain a deeper understanding of the factors influencing the transition of silanotriol structures from monomers to dimers, theoretical investigations were conducted on T8R8 cage structures featuring phenolic and isopropyl substituents. The computational results for open cages, both monomers and dimers, with i-Bu substituents and closed bifunctionalized cages possessing 3-aminopropyl sidearms are presented in Fig. 5. Additionally, Fig. S18 and Table S2† exhibit other results for cages of this type but containing vinyl and phenyl arms.
 | ||
| Fig. 5 Relative Gibbs free energies (kcal mol−1) for DFT optimized POSS structures with a 3-aminopropyl sidearm. | ||
Theoretical and experimental studies confirm that, in each instance, dimers exhibit greater stability. This is evident from the crystallization of open cages in their dimeric form from concentrated solutions. However, when the solutions are more dilute, dissociation occurs, leading to the dominance of open cages in the form of monomers. There are notable distinctions between cages with 3-aminopropyl and vinyl sidearms. Regarding cages with a core featuring i-Bu and Ph substituents and a 3-aminopropyl substituent, dimers exhibit lower reactivity than monomers (Fig. 5, Fig. S18†). Corner-capping reactions for dimers require higher energies, with the meta form being the least reactive.
A contrasting scenario arises for cages with a vinyl arm, as depicted in Fig. 6 and Fig. S18.† In these cases, both monomers and dimers undergo spontaneous corner-capping reactions. Similarly, the meta form is less reactive. However, these differences are not substantial enough to significantly impact the regioselectivity of the reaction.
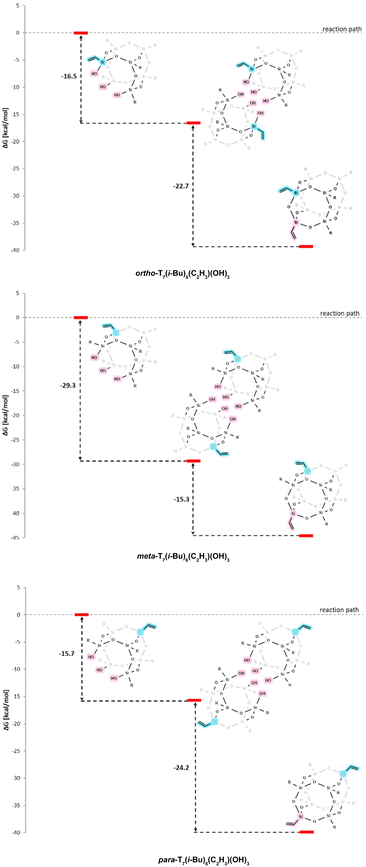 | ||
| Fig. 6 Relative Gibbs free energies (kcal mol−1) for DFT optimized POSS structures with a vinyl arm. | ||
Subsequent HOMO/LUMO investigations reveal the possibility of distinguishing the reactivity of individual forms (Fig. 7, Fig. S19 and Table S1†). Among cages featuring an i-Bu substituent in the core, the most negligible energy difference is observed for mono-POSS with a phenyl arm. This suggests its heightened susceptibility to corner-opening reactions; however, the differences are insignificant compared to cages with a vinyl or 3-aminopropyl sidearm. The open forms of silanotriols exhibit comparable reactivity, with a slight decrease in activity observed for the meta form (Fig. 8).
 | ||
| Fig. 7 Calculated HOMO (orange) and LUMO (blue) energy levels and their visualization, and HOMO–LUMO energy gaps (red) of optimized mono-POSS models with different arms: i-Bu, vinyl, amine, phenyl. | ||
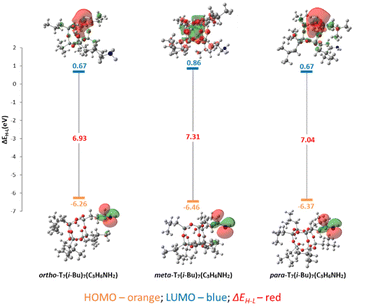 | ||
| Fig. 8 Calculated HOMO (orange) and LUMO (blue) energy levels and their visualization, and HOMO–LUMO energy gaps (red) of optimized open-POSS models with amine arms. | ||
Conclusions
In conclusion, the strategic synthesis of bifunctional POSS cages (T8R6R′2) seems significant for developing novel hybrid compounds with exceptional properties. However, achieving selectivity in placing sidearms at different positions (ortho, meta, and para) is demanding, if at all possible, with reasonable yield. The traditional approach involves opening the T8 octa-functional cage (T8R8) and then closing the resulting silanotriol T7R7(OH)3 to form the monofunctional POSS (T8R7R′) cage. While the closing step is typically uncomplicated, reopening the cage lacks selectivity, resulting in a mixture of different silanotriol isomers. Our findings proved that this mixture poses challenges in synthesizing bifunctional T8R6R′2 cages. The performed study highlights the hindrance caused by the crystallization of a stable dimer in further cage corner-capping reactions. Computational methods were used to analyze this issue and evaluate mono-cages’ reactivity in both open and closed states. It was observed that dimers exhibit greater stability and are prevalent in the crystalline form, while monomers dominate the solution. The theoretical investigations provided insights into the factors influencing the transition of silanotriol structures from monomers to dimers. Moreover, cages with different substituents and side reactive arms showed varying reactivity in corner-capping reactions.In silsesquioxane cage chemistry, finding solutions to control selective reactions involving the closing and opening of monofunctional cages remains challenging and intriguing. The study lays the groundwork for designing effective synthesis strategies and understanding the reactivity of POSS cages with different sidearms. At the moment, this will require further research, optimization of synthesis conditions, supported by quantum calculations.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Science Centre, Poland (Grant No. 2020/39/B/ST4/00910) and the Wrocław Centre for Networking and Supercomputing (https://www.wcss.wroc.pl) by providing computational time and facilities.References
- C. Hartmann-Thompson, Applications of Polyhedral Oligomeric Silsesquioxanes, Springer, New York, 2011 Search PubMed.
- A. Władyczyn, A. Gągor, K. Ślepokura and Ł. John, Hydroxyalkyl-substituted double-decker silsesquioxanes: effective separation of cis and trans isomers, Inorg. Chem. Front., 2022, 9, 3999–4008 RSC.
- D. B. Cordes, P. D. Lickiss and F. Rataboul, Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes, Chem. Rev., 2010, 110, 2081–2173 CrossRef CAS PubMed.
- D. A. Tomalia, J. B. Christensen and U. Boas, Dendrimers, Dendrons, and Dendritic Polymers: Discovery, Application, and the Future, Cambridge University Press, Cambridge, UK, 2012 Search PubMed.
- G. M. Whitesides and B. Grzybowski, Self-Assembly at all Scales, Science, 2002, 295, 2418–2421 CrossRef CAS PubMed.
- M. Laird, N. Herrmann, N. Ramsahye, C. Totée, C. Carcel, M. Unno, J. R. Bartlett and M. Wong Chi Man, Large Polyhedral Oligomeric Silsesquioxane Cages: The Isolation of Functionalized POSS with Unprecedented Si18O27 Core, Angew. Chem., Int. Ed., 2021, 60, 3022–3027 CrossRef CAS PubMed.
- B. Dudziec and B. Marciniec, Double-decker Silsesquioxanes: Current Chemistry and Applications, Curr. Org. Chem., 2017, 21, 2794–2813 CAS.
- A. Mrzygłód, M. Kubicki and B. Dudziec, Vinyl- and chloromethyl-substituted mono-T8 and double-decker silsesquioxanes as specific cores to low generation dendritic systems, Dalton Trans., 2022, 51, 1144–1149 RSC.
- K. Naka and Y. Irie, Synthesis of single component element-block materials based on siloxane-based cage frameworks, Polym. Int., 2017, 66, 187–194 CrossRef CAS.
- M. Wang, H. Chi, K. S. Joshy and F. Wang, Progress in the Synthesis of Bifunctionalized Polyhedral Oligomeric Silsesquioxane, Polymers, 2019, 11, 2098 CrossRef CAS.
- J. Kaźmierczak, D. Lewandowski and G. Hreczycho, B(C6F5)3-Catalyzed Dehydrocoupling of POSS Silanols with Hydrosilanes: A Metal-Free Strategy for Effecting Functionalization of Silsesquioxanes, Inorg. Chem., 2020, 59, 9206–9214 CrossRef PubMed.
- K. Kuciński and G. Hreczycho, A Highly Effective Route to Si-O-Si Moieties through O-Silylation of silanols and Polyhedral Oligomeric Silsesquioxane Silanols with Disilazanes, ChemSusChem, 2019, 12, 1043–1048 CrossRef PubMed.
- A. Mrzygłód, R. Januszewski, J. Duszczak, M. Dutkiewicz, M. Kubicki and B. Dudziec, Tricky but repeatable synthetic approach to branched, multifunctional silsesquioxane denrimer derivatives, Inorg. Chem. Front., 2023, 10, 4587–4596 RSC.
- X.-M. Wang, Q.-Y. Guo, S.-Y. Han, J.-Y. Wang, D. Han, Q. Fu and W.-B. Zhang, Stochastic/Controlled Symmetry Breaking of the T8-POSS Cages toward Multifunctional Regioisomeric Nanobuilding Blocks, Chem. – Eur. J., 2015, 21, 15246–15255 CrossRef CAS.
- T. Maegawa, Y. Irie, H. Imoto, H. Fueno, K. Tanaka and K. Naka, para-Bisvinylhexaisobutyl-substituted T8 caged monomer: synthesis and hydrosilylation polymerization, Polym. Chem., 2015, 6, 7500–7504 RSC.
- B. J. Hendan and H. C. Marsmann, Semipräparative Trennung gemischt substituierter Octa-(organylsilsesquioxane) mittels Normal-Phase-HPLC und ihre 29Si-NMR-spektroskopische Unters, J. Organomet. Chem., 1994, 483, 33–38 CrossRef CAS.
- M. Bołt, P. Żak, B. Dudziec, A. Schulmann and B. Marciniec, Formation of Bifunctional Octasilsesquioxanes via Silylative Coupling and Cross-Metathesis Reaction, Materials, 2020, 13, 3966 CrossRef PubMed.
- M. G. Voronkov and V. I. Lavrent'yev, Polyhedral oligosilsesquioxanes and their homo derivatives, in Inorganic Ring Systems, Topics in Current Chemistry, Springer, Berlin, Heidelberg, 1982, pp. 199–236 Search PubMed.
- T. N. Martynova and T. I. Chupakhina, Heterofunctional oligoorganylsilsesquioxanes, J. Organomet. Chem., 1988, 345, 10–18 CrossRef.
- M. Z. Asuncion and R. M. Laine, Fluoride Rearrangement Reactions of Polyphenyl- and Polyvinylsilsesquioxanes as a Facile Route to Mixed Functional Phenyl, Vinyl T10 and T12 Silsesquioxanes, J. Am. Chem. Soc., 2010, 132, 3723–3736 CrossRef CAS PubMed.
- F. Carniato, E. Boccaleri and L. Marchese, A versatile route to bifunctionalized silsesquioxane (POSS): synthesis and characterisation of Ti-containing aminopropylisobutyl-POSS, Dalton Trans., 2007, 36–39 Search PubMed.
- T. Maegawa, Y. Irie, H. Fueno, K. Tanaka and K. Naka, Synthesis and Polymerization of a para-Disubstituted T8-caged Hexaisobutyl-POSS Monomer, Chem. Lett., 2014, 43, 1532–1534 CrossRef CAS.
- I. Blanco, L. Abate, F. A. Bottino and P. Bottino, Hepta isobutyl polyhedral oligomeric silsesquioxanes (hib-POSS), J. Therm. Anal. Calorim., 2012, 108, 807–815 CrossRef CAS.
- A. D. Becke, Density-Functional Thermochemistry., III. The Role of Exact Exchange, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- C. Lee, W. Yang and R. G. Parr, Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron-Density, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS PubMed.
- S. Grimme, A. Hansen, J. G. Brandenburg and C. Bannwarth, Dispersion-Corrected Mean-Field Electronic Structure Methods, Chem. Rev., 2016, 116, 5105–5154 CrossRef CAS PubMed.
- R. Ditchfield, W. J. Hehre and J. A. Pople, Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules, J. Chem. Phys., 1971, 54, 724–728 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian, Inc., Wallingford CT, 2016.
- G. A. Zhurko, Chemcraft - graphical software for visualization of quantum chemistry computations. Version 1.8, https://www.chemcraftprog.com Search PubMed.
- A. Gierer and K. Z. Wirtz, Molekulare Theorie der Mikroreibung, Z. Naturforsch., A: Astrophys., Phys. Phys. Chem., 1953, 8, 532–538 CrossRef.
- H.-C. Chen and S.-H. Chen, Diffusion of Crown Ethers In Alcohols, J. Phys. Chem., 1984, 88, 5118–5121 CrossRef CAS.
- S. H. Chen and S. K.-H. Wei, Modification of the Stokes-Einstein Equation with a Semiempirical Microfriction Factor for Correlation of Tracer Diffusivities in Organic Solvents, Ind. Eng. Chem. Res., 2011, 50, 12304–12310 CrossRef CAS.
- T. Lu and F. Chen, Quantitative analysis of molecular surface based on improved Marching Tetrahedra algorithm, J. Mol. Graphics Modell., 2012, 38, 314–323 CrossRef CAS PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 CrossRef CAS PubMed.
- W. Humphrey, A. Dalke and K. Schulten, VMD: Visual molecular dynamics, J. Mol. Graphics Modell., 1966, 14, 33–38 CrossRef PubMed.
- F. J. Feher, R. Terroba and J. W. Ziller, A new route to incompletely-condensed silsesquioxanes: base-mediated cleavage of polyhedral oligosilsesquioxanes, Chem. Commun., 1999, 2309–2310 RSC.
- H. Liu, S. Kondo, R. Tanaka, H. Oku and M. Unno, A spectroscopic investigation of incompletely condensed polyhedral oligomeric silsesquioxanes (POSS-mono-ol, POSS-diol and POSS-triol): Hydrogen-bonded interaction and host-guest complex, J. Organomet. Chem., 2008, 693, 1301–1308 CrossRef CAS.
- M. D. Jones, C. G. Keir, A. L. Johnson and M. F. Mahon, Crystallographic characterization of novel Zn(II) silsesquioxane complexes and their application as initiators for the production of polylactide, Polyhedron, 2010, 29, 312–316 CrossRef CAS.
- F. J. Feher, D. A. Newman and J. F. Walzer, Silsesquioxanes as Models for Silica Surfaces, J. Am. Chem. Soc., 1989, 111, 1741–1748 CrossRef CAS.
- S. Spirk, M. Nieger, F. Belaj and R. Pietschnig, Formation and hydrogen bonding of a novel POSS-trisilanol, Dalton Trans., 2009, 163–167 RSC.
- E. Nizioł, D. Jędrzkiewicz, A. Wiencierz, W. Paś, D. Trybuła, W. Zierkiewicz, A. Marszałek-Harych and J. Ejfler, Sodium complexes as precise tools for cutting polymer chains. Exploration of PLA degradation by unique cooperation of sodium centers, Inorg. Chem. Front., 2023, 10, 1076–1090 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: 1H, 13C, 29Si spectra, FT-IR spectra, MALDI-MS spectra, quantum calculations data. See DOI: https://doi.org/10.1039/d3dt02638h |
| This journal is © The Royal Society of Chemistry 2023 |

