Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Interplay between anti–anti and syn–anti conformations of thiourea modulating ON–OFF catalysis†
Renitta
Benny
 and
Soumen
De
and
Soumen
De
 *
*
School of Chemistry, Indian Institute of Science Education and Research Thiruvananthapuram (IISER-TVM), Thiruvananthapuram 695551, India. E-mail: soumende@iisertvm.ac.in
First published on 17th October 2023
Abstract
The design, synthesis and operation of a readily accessible two-state switch are demonstrated. The switch initially exists in an intramolecularly hydrogen-bonded self-locked state, as evidenced by the solution-state NMR and solid-state structure. The switch can be reversibly altered between anti–anti and syn–anti conformations by adding and removing Cu+ ions, as evidenced by the NMR and crystallographic study. The anti–anti form was found to be catalytically active in the Michael addition reaction, whereas the syn–anti form was catalytically inactive.
Introduction
Majority of the enzymatic processes are tuned via allosteric regulation,1 which is a major inspiration behind the development of “smart” synthetic catalysts2 whose efficacy in altering the rate3 or regio/stereo-selectivity4 of diverse chemical transformations can be modulated by external stimuli. Understanding the principles and behaviour of these synthetic switchable catalysts will not only help mimic the biological counterparts where several reactions occur in parallel without interfering with each other but also aid in identifying their potential applications in sustainable chemistry and green synthesis. A traditional catalyst generally can catalyse one reaction; however, switchable catalysts can control multiple reactions from several states,5 and they can selectively react with specific components.6 Moreover, they are the basis of artificial signal transduction.7 This approach minimises waste generation, reduces energy consumption, and improves overall process efficiency by selectively activating catalysts only when needed. Moreover, the capability to control catalytic reactions in real time enables adaptive and responsive chemical processes, paving the way for dynamic and programmable chemistry with promising prospects. Therefore, discovering multiple switchable catalysts is essential. In this regard, several switchable catalysts have been reported that can control one reaction,8 multiple reactions,9 tandem reactions10etc. However, most switchable catalysts synthesised to date involve multi-steps (more than ten steps in general), thus limiting their use. Herein, we report a two-step synthesis of a switchable catalyst that can switch between two states by adding and removing copper ions and regulating Michael addition11 reactions between the 1,3-dicarbonyl compound and trans-beta nitro styrene in an ON/OFF manner.Design
The design of the switch combines thiourea, a known organocatalyst,12 and an unshielded phenanthroline in such a way that the thiourea moiety is locked with the phenanthroline unit via intramolecular hydrogen bonding, as shown in Fig. 1. Thus, we expect the thiourea moiety to be unavailable in the solution for catalysing the Michael addition reaction between the 1,3-dicarbonyl compound and β-nitrostyrene. However, adding one equivalent of copper-loaded shielded phenanthroline would form a HETPHEN-type13 complex that will disrupt the intramolecular hydrogen bond, and N–H will be available in solution for catalysing the Michael addition reaction. Adding a better complexing agent, such as KCN/cyclam,14 will abstract Cu+, resetting the switch to its initial locked state and thus switching off the reaction.Results and discussion
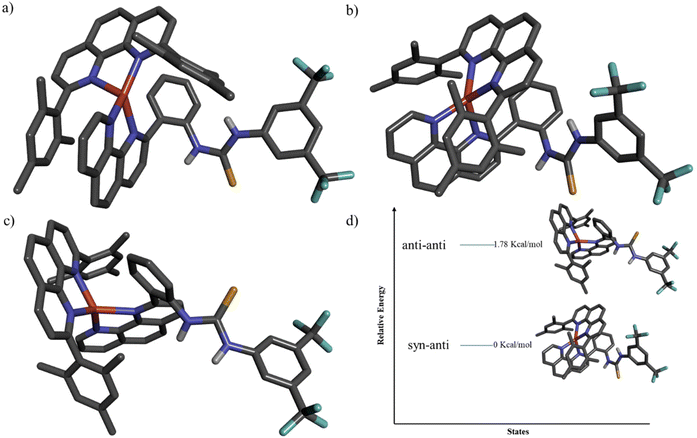
The desired switch was synthesised in two steps from commercially available compounds. First, 2-bromo[1,10]phenanthroline was subjected to a Suzuki coupling reaction with 2-aminophenyl boronic acid in the presence of Pd(0) as a catalyst to yield compound 2 which upon further treatment with 3,5-bistrifluoromethylphenylthiocyanate afforded switch 1 (Fig. 2a). The intermediate and switch 1 were unequivocally characterised by 1H (Fig. S2 and S6†) and 13C NMR (Fig. S3 and S8†), as well as ESI-MS. For example, the formation of switch 1 was confirmed by the appearance of a peak centred at m/z 543.1072 (Fig. S10†) in the ESI mass spectrum, which corresponds to [1 + H]+ with a matching isotopic distribution pattern between experimental and theoretical spectra. The formation of 1 was further confirmed by NMR spectroscopy. The appearance of the sharp N–H protons of the switch at a very downfield region (16.6 and 10.92 ppm) in the 1H NMR spectrum indicates hydrogen bonding with the phenanthroline nitrogen (Fig. S6†). The concentration-independent 1H NMR spectrum further supports intramolecular hydrogen bonding (Fig. S21†). The correlation of c-H (10.92 ppm) with o-H (8.38 ppm) and b-H (8.04 ppm) in the NOESY spectrum further supports the self-locked conformation (Fig. S12†). The conformation of switch 1 has also been unveiled by single-crystal X-ray crystallography, which shows that the switch exists in a self-locked state (Fig. 2c) where the N–Hs are intramolecularly hydrogen bonded with phenanthroline nitrogen (1.898 (d-H and N1) and 2.148 (c-H and N10) Å). In summary, all the experimental data demonstrate 1 to be intramolecularly hydrogen-bonded as expected.With 1 in our hand, we first focused on switching from state A to state B by adding [Cu(CH3CN)4]PF6 as a stimulus. Indeed, when copper-loaded phenanthroline was added to a solution of switch 1, the yellow colour turned immediately red (MLCT), indicating the formation of the copper complex. The formation of the HETPHEN-type complex in solution was further established from its 1H NMR spectrum, which showed a significant upfield shift of the mesityl protons from 6.96 ppm to 6.13 and 6.27 ppm (Fig. 4) due to shielding by the ring currents from the switch. Moreover, the ESI mass spectrometry of the complex revealed the expected peak for the heteroleptic complex at m/z 1021.2537 Da (Fig. S17†) with a isotopic splitting pattern matching with the theoretical one. The HETPHEN type complex was further established by the appearance of the MLCT band at 433 nm in UV-vis spectroscopy (Fig. S23†).
The 1H NMR spectroscopy results supported the coordination-induced conformational change of the switch. The upfield shifts of N–H protons of the switch from 16.6 and 10.92 ppm to 8.11 and 7.31 ppm (Fig. S13†) signify the disruption of intramolecular hydrogen bonding, probably due to the formation of state B. Moreover, the NOESY spectrum of the complex does not show the correlation between c-H (7.96 ppm) and o-H (8.63 ppm) (Fig. 4a), signifying the change of conformation. The switch can be brought back to its initial state by treating the complex with either KCN or cyclam. As anticipated, adding KCN extracted Cu+ from [Cu(1)(3)]PF6, thereby regenerating the switch in its initial state as evidenced by the appearance of N–H protons at 16.6 and 10.92 ppm and the disappearance of mesityl protons from 6.13 and 6.27 ppm (Fig. 4). Reversible switching between the two states of the switch by adding and removing Cu+ was performed for up to three cycles without any noticeable degradation (Fig. S20†).
Having established the reversible switching between the two states of the switch by addition and removal of copper ions, we investigated the efficacy of the switch and the copper complex of the switch to promote Michael addition of 1,3-dicarbonyl nucleophiles to trans-β-nitrostyrene (4). We first established that the Michael addition reaction between 4 and 5a does not proceed without the catalyst (Fig. S24†). We then carried out the Michael addition reaction in the presence of switch 1. To our surprise, when a mixture of 4, 5a, and Et3N (in a 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000
1000![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio) was stirred in the presence of five mol% of switch 1 in CDCl3 at room temperature, the Michael addition product 6 was obtained (∼40% conversion after 25 h), as evidenced by 1H NMR spectroscopy (Fig. S25†). In contrast, the use of the copper complex of the switch does not induce the formation of any product under similar conditions (Fig. S26†). Similarly, switch 1 can catalyse the Michael addition reactions of 4 with various Michael donors (5b, 5c, 5d and 5e), whereas the above reactions did not proceed in the presence of [Cu(1)(3)]PF6 (Scheme S4 and Fig. S31–S38†).
1 ratio) was stirred in the presence of five mol% of switch 1 in CDCl3 at room temperature, the Michael addition product 6 was obtained (∼40% conversion after 25 h), as evidenced by 1H NMR spectroscopy (Fig. S25†). In contrast, the use of the copper complex of the switch does not induce the formation of any product under similar conditions (Fig. S26†). Similarly, switch 1 can catalyse the Michael addition reactions of 4 with various Michael donors (5b, 5c, 5d and 5e), whereas the above reactions did not proceed in the presence of [Cu(1)(3)]PF6 (Scheme S4 and Fig. S31–S38†).
Initially, we expected that the catalytic activity in the closed state would be less compared to that in the open state. To investigate this unexpected OFF state of the complex, we tried to grow the crystals of the copper complex to get complete confirmation. Fortunately, after several trials, we could grow the crystals of the copper complex. The complex's crystal structure showed that the thiourea conformation is syn–anti instead of anti–anti (Fig. 3a). A literature survey also confirmed that thiourea can exist in the syn–anti conformation.15 Therefore, state-B will not be able to sufficiently increase the electrophilicity of β-nitrostyrene (due to the availability of only one N–H) and does not catalyse the Michael addition reaction. In contrast, the closed state is probably broken in the presence of β-nitrostyrene, a competitive hydrogen bond acceptor. As a result, the electrophilicity of β-nitrostyrene and the reaction rate increase. To understand the solution state structure of the complex, we carried out geometry optimisation of both anti–anti and syn–anti conformations using 6-311G(d,p) as the basis set. It was found that the syn–anti-conformation is more stable than the anti–anti-conformation.
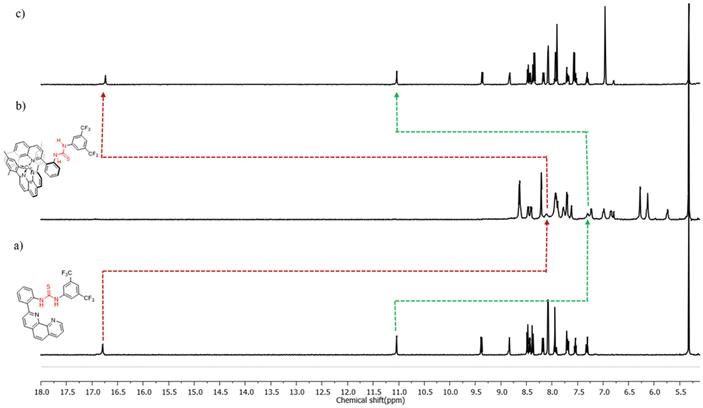 | ||
| Fig. 4 1H NMR (CD2Cl2, 298 K, 500 MHz) spectra of (a) switch 1 (10 mM); (b) [Cu(1)(3)]PF6; and (c) [Cu(1)(3)]PF6 + excess aqueous KCN. | ||
Additionally, we synthesised a model catalyst (1a) resembling compound 1 but lacking a phenanthroline moiety (Fig. 2c). Under analogous conditions, treating 4 (33.5 μmol) with 5a (335 μmol) in the presence of 1a and triethylamine resulted in a lower yield of the Michael addition product compared to switch 1 (Fig. S29 and S30†). The crystal structure analysis of 1a indicated a prevailing syn–anti conformation in the solid state, akin to that observed in [Cu(1)(3)]PF6. This observation accounts for the reduced reactivity of the model catalyst. Importantly, these data indirectly imply that the OFF state of [Cu(1)(3)]PF6 is attributed to its syn–anti conformation.
Furthermore, we evaluated the effectiveness of the switch to catalyse Michael addition reactions by in situ alteration between the catalytic states. Hence, the treatment of 4 (33.5 μmol), 5a (335 μmol) and Et3N (one mol%) in the presence of five mol% of copper-complex, [Cu (1)(3)]PF6, under similar conditions did not give any products. However, when the same mixture was stirred for 8 h after adding KCN, the product was obtained in 18% yield. In contrast, Michael addition was shut down when Cu+ was added to the above mixture. The ON–OFF in situ switching of catalysis was performed for up to three cycles (Fig. 5c).
Conclusion
In conclusion, we have demonstrated a very short synthesis of a switchable catalyst fully characterised by NMR spectroscopy, ESI-MS and X-ray crystallography. Both 1H NMR spectra and crystal structure analysis showed that the initial state is self-locked with intramolecular hydrogen bonding, whereas the copper complex existed in the syn–anti conformation. The anti–anti conformation of the switch can catalyse the Michael addition reaction by increasing the Michael acceptor's electrophilicity via hydrogen bonding, whereas the syn–anti conformation obtained by the addition of copper-loaded phenanthroline was catalytically inactive as only one N–H is available for hydrogen-bonding with a Michael acceptor. This interplay of thiorurea's conformation between syn–syn and syn–anti by allosteric regulation for ON–OFF catalysis can open a new direction for switchable catalysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. D. acknowledges the financial assistance from IISER Thiruvananthapuram and SERB, DST India (SRG/2020/001486). R. B. acknowledges the support from DST INSPIRE (DST/INSPIRE/03/2019/001025 (IF190401)). The authors thank Alex Andrews P. (Indian Institute of Science Education and Research Thiruvananthapuram, Kerala 695551, India) for collecting and solving X-ray data of 1, [Cu(1)(3)]PF6, and 1a. The authors also acknowledge the computational facilities of IISER Thiruvananthapuram.References
- (a) N. M. Goodey and S. J. Benkovic, Nat. Chem. Biol., 2008, 4, 474–482 CrossRef CAS PubMed; (b) K. Reiss, V. S. Batista and J. P. Loria, Biochemistry, 2020, 59, 1711–1712 CrossRef CAS PubMed.
- (a) U. Lüning, Angew. Chem., Int. Ed., 2012, 51, 8163–8165 CrossRef PubMed; (b) V. Blanco, D. A. Leigh and V. Marcos, Chem. Soc. Rev., 2015, 44, 5341–5370 RSC; (c) R. Dorel and B. L. Feringa, Chem. Commun., 2019, 5, 6477–6486 RSC; (d) J. Choudhury, Tetrahedron Lett., 2018, 59, 487–495 CrossRef CAS; (e) A. Ghorbani-Choghamarani and Z. Taherinia, RSC Adv., 2022, 12, 23595–23617 RSC.
- (a) C. M. McGuirk, C. L. Stern and C. A. Mirkin, J. Am. Chem. Soc., 2014, 136, 4689–4696 CrossRef CAS PubMed; (b) T. Imahori, R. Yamaguchi and S. Kurihara, Chem. – Eur. J., 2012, 18, 10802–10807 CrossRef CAS PubMed; (c) V. Blanco, A. Carlone, K. D. Hänni, D. A. Leigh and B. Lewandowski, Angew. Chem., Int. Ed., 2012, 51, 5166–5169 CrossRef CAS PubMed; (d) E. Deck, H. E. Wagner, J. Paradies and F. Breher, Chem. Commun., 2019, 55, 5323–5326 RSC; (e) S. Semwal and J. Choudhury, ACS Catal., 2016, 6, 2424–2428 CrossRef CAS.
- (a) J. Wang and B. L. Feringa, Science, 2011, 331, 1429–1432 CrossRef CAS PubMed; (b) Y. Cakmak, S. Erbas-Cakmak and D. A. Leigh, J. Am. Chem. Soc., 2016, 138, 1749–1751 CrossRef CAS PubMed.
- (a) S. De, S. Pramanik and M. Schmittel, Angew. Chem., Int. Ed., 2014, 53, 14255–14259 CrossRef CAS PubMed; (b) K. Eichstaedt, J. Jaramillo-Garcia, D. A. Leigh, V. Marcos, S. Pisano and T. A. Singleton, J. Am. Chem. Soc., 2017, 139, 9376–9381 CrossRef CAS PubMed; (c) A. Goswami, T. Paululat and M. Schmittel, J. Am. Chem. Soc., 2019, 141, 15656–15663 CrossRef CAS PubMed; (d) S. Kundu, D. Mondal, V. V. Rajasekaran, A. Goswami and M. Schmittel, Inorg. Chem., 2022, 61, 17007–17011 CrossRef CAS PubMed; (e) S. Semwal and J. Choudhury, Angew. Chem., Int. Ed., 2017, 56, 5556–5560 CrossRef CAS PubMed.
- Y. Shen, S. M. Shepard, C. J. Reed and P. L. Diaconescu, Chem. Commun., 2019, 55, 5587–5590 RSC.
- (a) H. Kai, S. Nara, K. Kinbara and T. Aida, J. Am. Chem. Soc., 2008, 130, 6725–6727 CrossRef CAS PubMed; (b) T. Muraoka, K. Kinbara and T. Aida, Nature, 2006, 440, 512–515 CrossRef CAS PubMed; (c) S. Pramanik, S. De and M. Schmittel, Chem. Commun., 2014, 50, 13254–13257 RSC; (d) P. Remon, D. González, M. A. Romero, N. Basílio and U. Pischel, Chem. Commun., 2020, 56, 3737–3740 RSC; (e) P. Remón and U. Pischel, ChemPhysChem, 2017, 5, 1667–1677 CrossRef PubMed.
- (a) M. Schmittel, S. De and S. Pramanik, Angew. Chem., Int. Ed., 2012, 51, 3832–3836 CrossRef CAS PubMed; (b) M. Schmittel, S. Pramanik and S. De, Chem. Commun., 2012, 48, 11730–11732 RSC; (c) S. De, S. Pramanik and M. Schmittel, Dalton Trans., 2014, 43, 10977–10982 RSC; (d) M. V. Peters, R. S. Stoll, A. Kühn and S. Hecht, Angew. Chem., Int. Ed., 2008, 47, 5968–5972 CrossRef CAS PubMed; (e) B. Majia and J. Choudhury, Chem. Commun., 2019, 55, 4574–4577 RSC; (f) M. Szewczyk, G. Sobczak and V. Sashuk, ACS Catal., 2018, 8, 2810–2814 CrossRef CAS; (g) V. Sashuk and O. Danylyuk, Chem. – Eur. J., 2016, 22, 6528–6531 CrossRef CAS PubMed.
- (a) K. Eichstaedt, J. Jaramillo-Garcia, D. A. Leigh, V. Marcos, S. Pisano and T. A. Singleton, J. Am. Chem. Soc., 2017, 139, 9376–9381 CrossRef CAS PubMed; (b) A. Goswami, T. Paululat and M. Schmittel, J. Am. Chem. Soc., 2019, 141, 15656–15663 CrossRef CAS PubMed.
- S. Gaikwad, A. Goswami, S. De and M. Schmittel, Angew. Chem., Int. Ed., 2016, 55, 10512–10517 CrossRef CAS PubMed.
- (a) T. Inokuma, Y. Hoashi and Y. Takemoto, J. Am. Chem. Soc., 2006, 128, 9413–9419 CrossRef CAS PubMed; (b) S. Hoekman, M. O. Kitching, D. A. Leigh, M. Papmeyer and D. Roke, J. Am. Chem. Soc., 2015, 137, 7656–7659 CrossRef CAS PubMed.
- (a) P. R. Schreiner, Chem. Soc. Rev., 2003, 32, 289–296 RSC; (b) T. Parvin, R. Yadav and L. H. Choudhury, Org. Biomol. Chem., 2020, 18, 5513–5532 RSC; (c) S. J. Connon, Chem. – Eur. J., 2006, 12, 5418–5427 CrossRef PubMed.
- (a) M. Schmittel and A. Ganz, Chem. Commun., 1997, 999–1000 RSC; (b) S. De, K. Mahata and M. Schittel, Chem. Soc. Rev., 2010, 39, 1555–1575 RSC.
- (a) C. O. Dietrich-Buchecker, J.-P. Sauvage and J. M. Kern, J. Am. Chem. Soc., 1984, 106, 3043–3045 CrossRef CAS; (b) J. D. Megiatto Jr. and D. I. Schuster, Org. Lett., 2011, 13, 1808–1811 CrossRef PubMed.
- (a) S. T. Tuncel, S. E. Gunal, M. Ekizoglu, N. G. Kelekci, S. S. Erdem, E. Bulak, W. Frey and I. Dogan, J. Mol. Struct., 2019, 1179, 40–56 CrossRef CAS; (b) A. Tarai and J. B. Baruah, ACS Omega, 2017, 2, 6991–7001 CrossRef CAS PubMed; (c) A. A. Ehrhard, L. Gunkel, S. Jäger, A. C. Sell, Y. Nagata and J. Hunger, ACS Catal., 2022, 12, 12689–12700 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2282963, 2282951 and 2297048. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt02398b |
| This journal is © The Royal Society of Chemistry 2023 |