Reaction of a Co(iii)-peroxo complex with nitric oxide: putative formation of a peroxynitrite intermediate†
Abstract
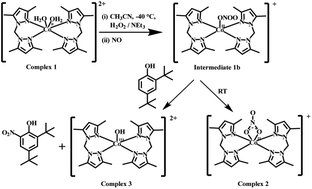
A Co(II) complex, [CoII(L)2(H2O)2](ClO4)2, 1, having a bidentate ligand L [L = bis(3,5-dimethylpyrazolyl)methane] has been synthesized. Complex 1 in acetonitrile solution at −40 °C, in the presence of H2O2 and NEt3, afforded the corresponding Co(III)-peroxo species, [CoIII(L)2(O22−)]+, as the transient intermediate 1a. Thermal instability precluded its isolation and further characterization. The addition of nitric oxide (NO) gas into the freshly prepared [CoIII(L)2(O22−)]+ in acetonitrile at −40 °C resulted in the corresponding Co(II)-nitrato complex, [CoII(L)2(NO3)](ClO4) (2). The reaction is proposed to proceed through a putative Co(II)-peroxynitrite intermediate 1b. It was evidenced by the characteristic phenol ring nitration reaction.


 Please wait while we load your content...
Please wait while we load your content...