Open Access Article
Open Access ArticleSynthesis, characterisation and antibacterial activity of novel Ga(III) polypyridyl catecholate complexes†
Lewis
More O'Ferrall
 ab,
Magdalena
Piatek
ab,
Magdalena
Piatek
 bc,
Brendan
Twamley
d,
Kevin
Kavanagh
bc,
Brendan
Twamley
d,
Kevin
Kavanagh
 bc,
Christine
O'Connor
bc,
Christine
O'Connor
 *a and
Darren M.
Griffith
*a and
Darren M.
Griffith
 *be
*be
aSchool of Food Science & Environmental Health, Technological University Dublin, Dublin 7, Ireland
bSSPC, the Science Foundation Ireland Research Centre for Pharmaceuticals, Ireland. E-mail: dgriffith@rcsi.ie
cDepartment of Biology, Maynooth University, Ireland
dSchool of Chemistry, Trinity College Dublin, University of Dublin, Dublin 2, Ireland
eDepartment of Chemistry, RCSI, 123 St. Stephens Green, Dublin 2, Ireland
First published on 8th August 2023
Abstract
Ga(III) polypyridyl catecholate complexes of type [Ga(bipy)2(O,O)](NO3) or [Ga(phen)2(O,O)](NO3) respectively were readily synthesised on reaction of Ga(NO3)3 in methanol with 1 equivalent of catecholate ligand (2,3-DHBA, 3,4-DHBA, 2,3,4-THBA or CafA) and then 2 equivalents of either bipy or phen. The complexes were characterised in full including by X-ray crystallography, which established that the catecholate ligands coordinate the Ga(III) centres in a bidentate manner via the two deprotonated hydroxy groups. All Ga(III) complexes exhibited good in vitro antibacterial activity against the Gram-negative pathogenic bacteria Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa. The complexes were inactive against the Gram-positive pathogenic bacteria Staphylococcus aureus including against a methicillin-resistant Staphylococcus aureus strain (MRSA). [Ga(bipy)2(2,3-DHBA-2H)](NO3)·1.5H2O (1) was shown to be non toxic in vivo in larvae of Galleria mellonella at doses up to 2000 μg mL−1 and to offer protection at doses of 100 and 250 μg mL−1 at 48 and 96 h to larvae infected with P. aeruginosa.
Introduction
The World Health Organisation clearly states that antibacterial resistance is a major global health and development threat.1 The emergence and spread of drug-resistant bacterial pathogens, so-called superbugs, that have acquired new resistance mechanisms and cause infections that are not treatable with existing medicines is of grave concern. Significantly the pipeline of new innovative antibacterial drugs is remarkably dry.1 There is therefore an urgent need for the development and advancement of new classes of antibacterial drugs.Metal-based drugs play very important and well-established roles in the clinic as both therapeutic and diagnostic agents. They represent a unique class of chemotherapeutics with many metal-based complexes under investigation and development as anticancer and antimicrobial agents for instance.2–4 Though bismuth- and silver-based anti-bacterials are currently in clinical use as antimicrobial agents, there is much recent interest in the role that gallium-based compounds can play in the fight against antibacterial resistance.5–8
Gallium (Ga) in its +3 oxidation state, Ga(III), is an effective Fe(III) mimic given it has a similar size and the same charge.9 Significantly Ga(III) is redox inert under physiological conditions and therefore cannot be reduced. It consequently inhibits critical physiological Fe redox activity and in turn Ga(III)-substituted proteins disrupt important metabolic pathways.9 Fe for example is found as a co-factor in numerous important enzymes such as oxidoreductases, which play roles in electron transfer, DNA synthesis and oxidative stress.5,9
Ga-based compounds are believed to hold promise in targeting antibacterial resistance as Fe is an essential nutrient for bacteria, particularly pathogenic bacteria, and it is difficult for biological systems to distinguish between Fe(III) and Ga(III).5 Bacteria have evolved numerous strategies to acquire iron, including the use of low molecular weight iron sequestering agents, siderophores. It is believed siderophores facilitate the cellular uptake of Ga(III) through Fe-siderophore uptake pathways.5,7,9
Ga(III) is readily hydrolysed under physiological conditions to produce insoluble Ga(III) hydroxides. The bioavailability and antibacterial activity of Ga(III) can therefore be enhanced by developing Ga(III) coordination compounds with increased water solubility.
Gallium compounds such as Ga(III) citrate, Ga(III) maltolate Fig. 1, Ga(III) tartrate, tris(8-quinolinolato) Ga(III) (KP46), and Ga(III) complexes of α-N-heterocyclic thiosemicarbazones, exhibit encouraging antibacterial activity against Gram-negative and Gram-positive bacteria, including Pseudomonas aeruginosa, Acinetobacter baumannii, Mycobacterium tuberculosis, and methicillin-resistant Staphylococcus aureus (MRSA).7 We recently used quantitative proteomics to reveal Ga(III) maltolate induces an iron-limited stress response and reduces quorum-sensing in P. aeruginosa.10 These processes are a fundamental component of bacterial virulence and dissemination and therefore point to a potential role for Ga(III) maltolate in the treatment of P. aeruginosa infection.10
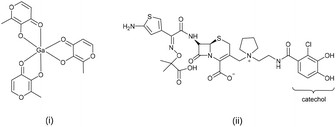
Cefiderocol, Fig. 1 is a recently approved siderophore cephalosporin-based antibiotic, which exhibits antibacterial activity against Gram-negative pathogens including multidrug-resistant (MDR) enterobacteriaceae and non-fermenting organisms.11 Significantly the catechol-type siderophore was incorporated to bind to extracellular free iron and utilise the bacterial active iron transport channels to enhance uptake and antibacterial potency of the antibiotic.
We surmised that Ga(III) complexes of catecholates would represent interesting antibacterial drug candidates. We therefore sought to develop water soluble heteroleptic Ga(III) compounds as antibacterial agents. We describe within the development of a novel class of Ga(III) polypyridyl catecholate complexes of 2,3-dihydroxybenzoic acid (2,3-DHBA), 3,4-dihydroxybenzoic acid (3,4-DHBA), 2,3,4-trihydroxybenzoic acid (2,3,4-THBA) and caffeic acid (CaffA), their activity against a panel of Gram-positive and Gram-negative pathogenic bacteria in vitro and in vivo in larvae of Galleria mellonella infected with P. aeruginosa.
Results and discussion
Synthesis of Ga(III) complexes 1 to 5
Reaction of Ga(NO3)3 hydrate in methanol with 1 equivalent of catecholate ligand (2,3-DHBA, 3,4-DHBA, 2,3,4-THBA or CafA) and then 2 equivalents of either bipy or phen gave the corresponding complexes of general formula [Ga(bipy)2(O,O)](NO3) or [Ga(phen)2(O,O)](NO3) respectively, Fig. 2.All Ga(III) complexes were fully characterised by elemental analysis, 1H NMR, 13C NMR and IR spectroscopy and mass spectrometry and the structures of 1 to 4 elucidated by X-ray crystallography (Fig. S1–S21†).
Briefly with regards to the characterisation of [Ga(bipy)2(2,3-DHBA-2H)](NO3)·1.5H2O (1) as a representative example, the elemental analysis is fully consistent with two bipy ligands, one doubly deprotonated 2,3-DHBA ligand, one nitrate counterion and one and a half water molecules per Ga(III) centre. In the 1H NMR spectrum of 1 (CD3CN, Fig. S1†) the free and uncoordinated carboxylic acid proton signal is found at 13.01 ppm. The sixteen aromatic protons associated with the two bipy ligands are observed across ten signals ranging from 7.63 to 8.98 ppm. The three signals associated with the 2,3-DHBA-2H ligand are found at 6.54, 6.72 and 7.04 ppm. These signals have shifted from 6.77, 7.06 and 7.34 ppm in the free ligand, indicative of coordination of the catecholate group. In the 13C NMR spectrum (Fig. S2†) 25 unique signals are observed for the 27 carbons. In the IR spectrum (Fig. S3†) the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) for the free carboxylic acid is observed at 1701 cm−1.
O) for the free carboxylic acid is observed at 1701 cm−1.
HRMS in the positive mode (Fig. S5†) was also used to verify the complex cation of 1, [Ga(bipy)2(2,3DHBA-2H)]+ (533.0743 a.m.u). The analysis outlined for 1 is also supported by X-ray crystallography.
A UV-Vis, HRMS and 1H NMR study was undertaken to demonstrate that 1 is stable in solution over 24 hours (Fig. S22–S24†). In the UV-Vis spectrum of 1 in water at 37 °C over the course of 24 h, there is no reduction in the absorbance at λmax at 283 nm. The HRMS spectrum of the same solution also verifies the presence of the complex cation of 1, [Ga(bipy)2(2,3DHBA-2H)]+ (533.0747 a.m.u). In the 1H NMR study in D2O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CD3CN (50
CD3CN (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50), a solvent system that solubilises 1, bipy and 2,3-DHBA, it is clear that there is no release of bipy or 2,3 DHBA ligands in this timeframe (Fig. S24†).
50), a solvent system that solubilises 1, bipy and 2,3-DHBA, it is clear that there is no release of bipy or 2,3 DHBA ligands in this timeframe (Fig. S24†).
All Ga complexes 1–5 are isolated as racemic mixtures of Λ and Δ enantiomers. Racemic mixtures are optically inactive and no CD spectra of solutions of complexes 1–5 were recorded exhibiting noteworthy signals. Furthermore no rotation of plan polarised light was observed on investigation of the optical properties of solutions of complexes 1–5 by polarimetry.
X-ray crystal structures
1, 2, 3 and 4 were readily converted to [Ga(bipy)2(2,3-DHBA-2H)](PF6)·MeOH (1b), [Ga(bipy)2(3,4-DHBA-2H)](PF6)·MeOH (2b), [Ga(bipy)2(3,4,5-THBA-2H)](PF6)·MeOH (3b) and [Ga(phen)2(2,3-DHBA-2H)](PF6)·EtOH (4b) on stirring parent complexes with 1.5 eq. of KPF6 in water. Slow evaporation of solutions of the resultant precipitates from MeOH (1b, 2b, 3b) or EtOH (4b) produced single crystals suitable for X-ray crystallography, Fig. 3 and Fig. S25–31 (ESI†). In each complex the catecholate ligands 2,3-DHBA, 3,4-DHBA, and 3,4,5-THBA as expected coordinate the Ga(III) centres in a bidentate manner via two deprotonated hydroxy groups. | ||
| Fig. 3 Structure of the complex cation of 1 only with displacement shown at 50% probability. The molecular structure is completed by a PF6 anion and methanol solvate, not shown. See ESI Fig. S22† for the complete asymmetric unit. | ||
Antimicrobial activity
| Complex | E. coli IC50 (μg mL−1) | K. pneumonia IC50 (μg mL−1) | P. aeruginosa IC50 (μg mL−1) |
|---|---|---|---|
| 1 | 37 | 60 | 52 |
| 2 | 53 | 77 | 73 |
| 3 | 62 | 62 | 64 |
| 4 | 12 | 18 | 26 |
| 5 | 65 | 81 | 71 |
| GaN | nd | nd | 100 |
On average the five complexes were slightly more active against E. coli. 4 was most active with 1 being the second most active of the Ga(III) polypyridyl catecholate complexes against all three bacteria. These results support the promising activity previously reported for Ga(III) compounds against the Gram-negative bacteria E. coli, K. pneumoniae and P. aeruginosa.12–14
We are particularly interested in the activity of our complexes against the cystic fibrosis (CF)-related pathogen P. aeruginosa. All five complexes had lower IC50 values as compared to gallium nitrate, Ga(NO3)3 (GaN, 100 μg mL−1, Fig. S34, ESI†) and gallium maltolate, [Ga(Mal−1H)3] (GaM, 62.5 μg mL−1)10 against P. aeruginosa.
Larvae of G. mellonella were administered doses of 250, 500, 1000 and 2000 μg mL−1 of 1 to 5 in order to measure toxicity in the host. Larvae administered 1, 2, 3 and 5 showed no reduction in viability (Fig. S35†) whereas larvae administered 4 did experience toxic effects with a loss of viability observed at 1000 but in particular 2000 μg mL−1 (Fig. S35†).
Experimental
Materials and instrumentation
GaNO3·xH2O, 1,10 phenanthroline (phen), 2,2 bipyridine (bipy), 2,3-dihydroxy benzoic acid (2,3-DHBA), 3,4-dihydroxy benzoic acid (3,4-DHBA), 3,4,5-trihydroxy benzoic acid (3,4,5-THBA) and caffeic acid (CafA) were used as received from Sigma Aldrich (Sigma Aldrich Ireland Ltd, Arklow, Co Wicklow). All other commercially available reagents and solvents, including deuterated solvents, were also purchased from Sigma-Aldrich and used without further purification. 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400 NMR spectrometer. The spectra were analysed using MestReNova software. The residual undeuterated solvent signals were used as internal references.17 Mass spectrometry experiments were performed on an Advion Expression Compact Mass Spectrometer where 10 μL of the samples were injected in 300 μL of methanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropanol
isopropanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water
water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) formic acid (80
formic acid (80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v). The mass spectrometry data were acquired in positive ion mode and the spectra analysed using the Advion Mass Express software programme. Elemental analysis (C, H, N) was performed at the Microanalytical Laboratory, School of Chemistry and Chemical Biology, University College Dublin, Ireland.
1 v/v). The mass spectrometry data were acquired in positive ion mode and the spectra analysed using the Advion Mass Express software programme. Elemental analysis (C, H, N) was performed at the Microanalytical Laboratory, School of Chemistry and Chemical Biology, University College Dublin, Ireland.
Synthetic procedures
IR (cm−1): 1698, 1655, 1604, 1570, 1476, 1444, 1311 (NO3−), 1270, 1201, 1109, 1081, 941, 886, 874, 851. Anal. required for C27H20GaN5O8·H2O: C, 51.46, H, 3.52, N, 11.11%. Found: C, 51.34, H, 3.12, N, 11.00%. MS (ESI, positive ion): m/z: 550.2 [Ga(bipy)2(3,4,5THBA-2H)]+.
[Ga(phen)2(2,3DHBA-2H)](NO3) (4)
2,3,-DHBA (0.186 g, 1.37 mmol) dissolved in methanol (10 mL) was added to Ga(NO3)3 hydrate (0.35 g, 1.37 mmol) in methanol (15 mL). After 30 min stirring at RT, the reaction was brought to reflux and a solution of 1,10′-phenantroline (0.49 g, 2.74 mmol) in methanol (10 mL) of was added dropwise. The yellow reaction mixture was stirred at reflux for 8 hours, cooled, filtered. The filtrate was reduced in vacuo to ca. 15 mL and subsequently stored at 4 °C for 24 h. The yellow solid that precipitated was collected via filtration and washed with small amounts of isopropanol and diethyl ether before being recrystallized from MeOH/H2O to give 5 as an orange solid. Yield: 0.59 g (67%). 1H NMR (400 MHz, CD3CN): δ/ppm = 13.07 (s, 1H, COOH) 9.36 (d, 1H, ArH), 9.30 (d, 1H, ArH), 9.06 (m, 2H, ArH), 8.82 (dd, 2H, ArH), 8.36 (m, 2H, ArH), 8.31–8.26 (m, 4H, ArH), 8.01 (d, 1H, ArH), 7.93 (d, 1H, ArH), 7.80–7.75(m, 2H, ArH), 7.06 (dd, 1H, ArH), 6.76 (dd, 1H, ArH). 6.55 (t, 1H, ArH). 13C NMR (100 MHz, CD3CN, 25 °C): δC 168.97, 154.77, 154.28, 150.27, 150.22, 148.65, 148.41, 143.73, 143.28, 138.56, 138.32, 128.95, 128.93, 128.22, 128.14, 127.48, 118.78, 118.58, 115.32. IR (cm−1): 1726, (s, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) 1586, 1566, 1471, 1428, 1279 (NO3−), 1206, 1142, 1107, 1031, 851, 720. Anal. required for C31H20GaN5O7: C, 57.79, H, 3.13, N, 10.87%. Found: C, 57.41, H, 3.24, N, 10.77%. MS (ESI, positive ion): m/z: 581.2 [Ga(phen)2(2,3DHBA-2H)]+.
O) 1586, 1566, 1471, 1428, 1279 (NO3−), 1206, 1142, 1107, 1031, 851, 720. Anal. required for C31H20GaN5O7: C, 57.79, H, 3.13, N, 10.87%. Found: C, 57.41, H, 3.24, N, 10.77%. MS (ESI, positive ion): m/z: 581.2 [Ga(phen)2(2,3DHBA-2H)]+.
The orange solid that precipitated was collected via filtration and washed with small amounts of isopropanol and diethyl ether before being recrystallized from MeOH/H2O to yield 4 as a yellow solid. Yield: (0.47 g, 53%). 1H NMR (400 MHz, DMSO-d6): δ/ppm = 11.77 (s, 1H, COOH) 8.85 (m, 4H, ArH), 8.68 (d, 2H, ArH), 8.48 (t, 2H, ArH), 8.38 (d, 2H, ArH), 7.99–7.93 (m, 4H, ArH), 7.47–7.44(m, 2H, ArH), 7.35 (dd, 1H, ArH), 6.88 (s, 1H, ArH), 6.65 (d, 1H, ArH), 6.52 (d, 1H, ArH), 5.98 (d, 1H, ArH) ppm. 13C NMR (100 MHz, DMSO-d6, 25 °C): δC 168.45, 157.68, 155.23, 153.74, 149.29, 146.97, 146.60, 144.89, 143.07, 137.32, 128.12, 124.20, 123.11, 122.46, 120.43, 112.28, 111.25, 109.87.
IR (cm−1): 3067, 1615, 1604, 1578, 1555, 1478, 1446, 1398, 1313, 1260, 1159, 1118, 1032, 769, 732, 661, 540. Anal. required for C29H22GaN5O7·0.5H2O: C, 55.18, H, 3.67, N, 11.09%. Found: C, 54.78, H, 3.60, N, 11.52%. MS (ESI, positive ion): m/z: 559.2 a.m.u. [Ga(bipy)2(CafA-2H)]+.
X-Ray crystallography
Data for 1, 3 and 4 were measured on a Bruker D8 Quest ECO, using Mo Kα radiation (λ = 0.71073 Å) and data for 2 was measured on a Bruker APEX DUO. Each sample was mounted on a MiTeGen cryoloop and data collected at 100(2) K using and Oxford Cryosystems Cobra or Cryostream low temperature device. Bruker APEX18 software was used to collect and reduce data. Absorption corrections were applied using SADABS.19 Structures were solved with the SHELXT structure solution program20 using Intrinsic Phasing. All were refined using Least Squares method on F2 with SHELXL.21 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were assigned to calculated positions using a riding model with appropriately fixed isotropic thermal parameters. Molecular graphics were generated using OLEX2.22 Crystal data, details of data collection and refinement are given in Table S1.†In 1, donor O–H hydrogen atoms were located and refined semi-free using restraints (DFIX). In 2, disorder in one Ga bipy was modelled in two locations Ga1, N13–N24 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 35% with restraints (SIMU, DFIX). Disorder in the other Ga2-3,4-DHBA moiety was modelled in two locations, O60–O70, 52
35% with restraints (SIMU, DFIX). Disorder in the other Ga2-3,4-DHBA moiety was modelled in two locations, O60–O70, 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 48% with restraints (SADI, SIMU). Both PF6 anions were modelled disordered over two locations using rigid groups, P1 83
48% with restraints (SADI, SIMU). Both PF6 anions were modelled disordered over two locations using rigid groups, P1 83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 17%, with restraints (SIMU) and P2, 53
17%, with restraints (SIMU) and P2, 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 47% with restraints also (SIMU).
47% with restraints also (SIMU).
In 3, a weak high angle data lead to a high R(int). There was also mixed anion with disorder, with the PF6 anion only 50% occupied and the remaining Cl anion modelled over three locations (22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8%) and both modelled with displacement restraints (ISOR, RIGU, SUMP) and constraints (EADP). Donor hydrogen atoms on the Ga complex were refined ‘semi-free’ with geometric restraints (DFIX). There are water and MeOH solvent molecules in the void. There are a total of 2.08 water molecules in 4 locations (100
8%) and both modelled with displacement restraints (ISOR, RIGU, SUMP) and constraints (EADP). Donor hydrogen atoms on the Ga complex were refined ‘semi-free’ with geometric restraints (DFIX). There are water and MeOH solvent molecules in the void. There are a total of 2.08 water molecules in 4 locations (100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 33
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25%) and refined with displacement restraints (ISOR). Hydrogen bonding networks were arranged manually to provide optimum contacts. The MeOH molecule is 20% occupied and modelled with geometric (DFIX) and displacement (ISOR) restraints. Methyl torsion angles did not refine and the CH3 group was restrained in place (AFIX3). One water molecule hydrogen (O3s–H3sb) does not have any optimal hydrogen bonding contacts.
25%) and refined with displacement restraints (ISOR). Hydrogen bonding networks were arranged manually to provide optimum contacts. The MeOH molecule is 20% occupied and modelled with geometric (DFIX) and displacement (ISOR) restraints. Methyl torsion angles did not refine and the CH3 group was restrained in place (AFIX3). One water molecule hydrogen (O3s–H3sb) does not have any optimal hydrogen bonding contacts.
In 4, the 2,3-DHBA was disordered (90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10%) and modelled in two locations using a rigid group, restraints (SIMU) and constraints (EADP). The partially occupied (75%) EtOH was modelled in two locations with a rigid group with restraints (SIMU).
10%) and modelled in two locations using a rigid group, restraints (SIMU) and constraints (EADP). The partially occupied (75%) EtOH was modelled in two locations with a rigid group with restraints (SIMU).
Crystallographic data for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication numbers 2253969–2253972.†
Ga complex stock solutions
Stock solutions of complexes 1–5 were dissolved (1 mg mL−1) in sterile deionised water.Bacterial culture conditions
Escherichia coli (clinical isolate), Methicillin-resistant Staphylococcus aureus (MRSA, clinical isolate), Staphylococcus aureus ATCC 33591, Klebsiella pneumoniae NCTC 13443 NDM-1 and Pseudomonas aeruginosa PAO1 were grown in nutrient broth (Oxoid) at 37 °C in an orbital shaker at 200 rpm. Bacterial stocks were maintained on nutrient agar at 4 °C.In vitro susceptibility assays
Sterile nutrient broth (100 μl) was added to all wells in a 96-well plate. For MRSA and K. pneumoniae susceptibility assays, nutrient broth was supplemented with ampicillin 25 μg mL−1 and 50 μg mL−1, respectively, to maintain resistance. GaS stock solutions were added and serially diluted to obtain final concentrations of 0.98–250 μg mL−1. Bacterial cultures grown overnight were measured by obtaining the optical density at 600 nm (OD600) and adjusted to OD 0.1. Cell suspensions (100 μl) were added to serially diluted volumes of the tested complexes and plates were incubated in a static incubator at 37 °C for 24 h. Growth was measured at OD600.Galleria mellonella toxicity evaluation
Sixth instar larvae of the greater wax moth, Galleria mellonella, (Livefoods Direct Ltd, Sheffield, UK) displaying no signs of discolouration (melanisation) were each weighed (200–300 mg) and stored in 9 cm Petri dishes containing wood shavings. Ten healthy larvae were selected per sample group (total n = 30). Larvae were stored at 15 °C in the dark to prevent pupation between experiments.Solutions of 1–5 (250–2000 μg mL−1) were prepared in sterile deionised water. Larvae were injected via the last-left proleg using a U-100 insulin syringe (Terumo Europe, N.V., Belgium) and 25G × 5/8′′ needle (BD Microlance™) to administer 20 μL. Larvae were incubated at 37 °C and monitored every 24 h for 96 h. Survival was determined based on the level of melanisation and response to touch.
Galleria mellonella viability evaluation
P. aeruginosa cells grown overnight were washed twice by centrifugation at 2500 rpm for 10 min and pellets were resuspended in sterile PBS. Bacterial cells were diluted in PBS to obtain a cell density of 1.5 × 101 CFUs mL−1. Suspensions were injected into the last-left proleg as previously described and larvae were incubated at 37 °C for 1 h. Solutions of 1 were prepared in sterile deionised water and administered (20 μL) via the last-right proleg. Control samples were injected with PBS. Larvae were incubated at 37 °C and monitored up to 96 h to determine percentage survival.Data analysis
All experiments were carried out independently in triplicate and error bars denote mean ± S.E. Graphs were constructed using GraphPad Prism Software v.9.5.1 (GraphPad Software Inc., San Diego, CA, USA).Conclusions
There is increasing interest in the development of Ga-based antimicrobial drug candidates. We present a novel class of Ga(III) polypyridyl catecholate complexes of type [Ga(bipy)2(O,O)](NO3) or [Ga(phen)2(O,O)](NO3) respectively The complexes were fully characterised including by X-ray crystallography, which established that the catecholate ligands coordinate the Ga(III) centres in a bidentate manner via the two deprotonated hydroxy groups. All Ga(III) complexes exhibited good in vitro antibacterial activity against the Gram-negative pathogenic bacteria E. coli, K. pneumoniae and P. aeruginosa. This study builds on previous reports, which associated Ga-based compounds with good antibacterial activity against P. aeruginosa. Specifically our lead Ga(III) polypyridyl catecholate complexes, [Ga(bipy)2(2,3-DHBA-2H)](NO3) (1), was shown to be non-toxic in vivo in larvae of G. mellonella at doses up to 2000 μg mL−1 and to offer protection at doses of 100 and 250 μg mL−1 at 48 and 96 h to larvae infected with P. aeruginosa. P. aeruginosa is a major cause of lung infections in people living with cystic fibrosis (CF). The activity of Ga-based compounds should potentially be investigated against other CF-related microbial pathogens.Author contributions
Lewis More O'Ferrall: conceptualisation, methodology, investigation, writing – original draft preparation, funding acquisition. Magdalena Piatek: methodology, investigation, writing – original draft preparation. Brendan TwamLey: methodology, resources, writing – original draft preparation. Kevin Kavanagh: conceptualisation, methodology, resources, writing – original draft preparation. Christine O'Connor: conceptualisation, resources, methodology, writing – original draft preparation, supervision, project administration, funding acquisition. Darren M. Griffith: conceptualisation, resources, methodology, writing – original draft preparation, supervision, project administration, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
LMOF and COC sincerely thank TU Dublin Research Scholarship Fund for financial support. DMG, LMOF, KK and MP acknowledge funding received from the SSPC, the Science Foundation Ireland Research Centre for Pharmaceuticals, financed by a research grant from Science Foundation Ireland (SFI) and co-funded under the European Regional Development Fund under Grant Number 12/RC/2275_P2.References
- W. H. O. (WHO), Antimicrobial resistance, https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance, (accessed 21/11/2022, 2022).
- D. Gaynor and D. M. Griffith, Dalton Trans., 2012, 41, 13239–13257 RSC.
- E. J. Anthony, E. M. Bolitho, H. E. Bridgewater, O. W. L. Carter, J. M. Donnelly, C. Imberti, E. C. Lant, F. Lermyte, R. J. Needham, M. Palau, P. J. Sadler, H. Shi, F.-X. Wang, W.-Y. Zhang and Z. Zhang, Chem. Sci., 2020, 11, 12888–12917 RSC.
- E. Boros, P. J. Dyson and G. Gasser, Chem, 2020, 6, 41–60 CAS.
- F. Li, F. Liu, K. Huang and S. Yang, Front. Bioeng. Biotechnol., 2022, 10, 87960 Search PubMed.
- V. Vinuesa and M. J. McConnell, Int. J. Mol. Sci., 2021, 22, 2876 CrossRef CAS PubMed.
- N. Kircheva and T. Dudev, J. Inorg. Biochem., 2021, 214, 111309 CrossRef CAS PubMed.
- A. Frei, J. Zuegg, A. G. Elliott, M. Baker, S. Braese, C. Brown, F. Chen, G. D. C. G. Dujardin, N. Jung, A. P. King, A. M. Mansour, M. Massi, J. Moat, H. A. Mohamed, A. K. Renfrew, P. J. Rutledge, P. J. Sadler, M. H. Todd, C. E. Willans, J. J. Wilson, M. A. Cooper and M. A. T. Blaskovich, Chem. Sci., 2020, 11, 2627–2639 RSC.
- G. Centola, F. Xue and A. Wilks, Metallomics, 2020, 12, 1863–1877 CrossRef CAS PubMed.
- M. Piatek, D. M. Griffith and K. Kavanagh, J. Biol. Inorg. Chem., 2020, 25, 1153–1165 CrossRef CAS PubMed.
- J. C. Abdul-Mutakabbir, S. Alosaimy, T. Morrisette, R. Kebriaei and M. J. Rybak, Pharmacotherapy, 2020, 40, 1228–1247 CrossRef CAS PubMed.
- D. Kang, A. V. Revtovich, A. E. Deyanov and N. V. Kirienko, mSphere, 2021, 6, e0040121 CrossRef PubMed.
- M. Mosina, C. Siverino, L. Stipniece, A. Sceglovs, R. Vasiljevs, T. F. Moriarty and J. Locs, J. Funct. Biomater., 2023, 14, 51 CrossRef CAS PubMed.
- L. Rossato, J. P. Arantes, S. M. Ribeiro and S. Simionatto, Diagn. Microbiol. Infect. Dis., 2022, 102, 115569 CrossRef CAS PubMed.
- N. Browne and K. Kavanagh, Virulence, 2013, 4, 271–272 CrossRef PubMed.
- K. Kavanagh and G. Sheehan, Fungi, 2018, 4, 113 CrossRef CAS PubMed.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512–7515 CrossRef CAS PubMed.
- Bruker (2021), APEX4, Bruker AXS Inc., Madison, WI, USA Search PubMed.
- L. Krause, R. Herbst-Irmer, G. M. Sheldrick and D. Stalke, J. Appl. Crystallogr., 2015, 48, 3–10 CrossRef CAS PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2253969–2253972. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt01761c |
| This journal is © The Royal Society of Chemistry 2023 |