Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Persilylation of ferrocene: the ultimate discipline in sterically overcrowded metal complexes†
Susanne M.
Rupf
 a,
Robin
Sievers
a,
Paulin S.
Riemann
a,
Marc
Reimann
a,
Robin
Sievers
a,
Paulin S.
Riemann
a,
Marc
Reimann
 b,
Martin
Kaupp
b,
Martin
Kaupp
 b,
Carlo
Fasting
a and
Moritz
Malischewski
b,
Carlo
Fasting
a and
Moritz
Malischewski
 *a
*a
aFreie Universität Berlin, Fabeckstr. 34-36, 14195 Berlin, Germany. E-mail: moritz.malschewski@fu-berlin.de
bTechnische Universität Berlin, Straße des 17. Juni 135, 10623 Berlin, Germany
First published on 19th April 2023
Abstract
We report the preparation and structural characterization of the first persilylated metallocene via the metalation of decabromoferrocene. Although Grignard conditions turned out to be insufficient due to the steric and electronic effects of silyl groups causing a decreased nucleophilicity of the metalated intermediates, stepwise lithium–halogen exchange yields complex mixtures of polysilylated compounds FeC10DMSnH10−n (n = 10, 9, 8) including the targeted decasilylated ferrocene. These mixtures were successfully separated allowing a systematic study of silylation effects on ferrocene by XRD, CV, NMR and UV/vis spectroscopy supported by DFT calculations. The findings were used to develop a high-yielding and simple preparation method to generate a tenfold substituted overcrowded ferrocene, FeC10DMS8Me2.
Introduction
Given the outstanding variety of possible cyclopentadienyl (Cp) complexes reported so far, cyclopentadienyl ligands with dimethylsilyl (DMS) or trimethylsilyl (TMS) groups have found widespread synthetic applications. As they are superior in steric demand to the corresponding carbon containing analogues, iso-propyl and tert-butyl,1,2 they allow the isolation of reactive f-block3–7 and main group complexes8–12 and improve hydrocarbon solubility.13 Moreover, the introduction of silyl groups allows the generation of sensitive (redox) species due to hyperconjugation effects, allowing the isolation of highly reduced organic compounds14–18 or low-valent actinide and lanthanide compounds, like {[C5H5−n(SiMe3)n]3M}1− (n = 1, 2), containing metal centers with a formal oxidation state of +II as demonstrated by the Evans group.19–24 Although higher degrees of silylation are expected to result in an increased kinetic stability of low-valent species due to steric demand and changed electronic properties of the Cp ligand, the steric overload required and the further changed electronic properties of the Cp ligands pose a formidable synthetic challenge.So far, only one transition metal complex with a pentakis(dimethylsilyl) substituted Cp ligand is known in the literature. Sünkel and Hofmann prepared [Mn(CO)3(C5DMS5)] by iterative lithiation and silylation of [Mn(CO)3(C5Br5)] (Scheme 1, top).25 Although the preparation of [C5DMS5]− by the reaction of C5DMS6 with MeLi has been reported,26 it has never been used for follow-up chemistry. Here, the corresponding C5DMS6 was prepared by the reaction of C5Br6 with Mg and DMSCl. Claims in the Russian literature regarding the preparation of C5TMS6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 27 were not reproducible. In contrast, the synthesis of [C5TMS3H2]− is well established and the ligand is easily coordinated in reactions with metal halides.28–31 However, the synthetic challenge becomes more demanding for metallocenes due to the repulsion of the sterically crowded Cp ligands in the “sandwich” structure. Okuda achieved high silylation degrees by the reaction of iron(II) halides with tris(1,2,4-TMS)cyclopentadienyllithium to yield 1,1′,2,2′,4,4′-hexakis(trimethylsilyl)ferrocene. As a consequence of the steric demand, a distorted structure of the silylated Cp ligands is observed.32 Butler et al. reported an alternative approach for hexakis(trimethylsilyl)ferrocene including a lithium–halogen exchange of 1,1′,2,2′,3,3′-hexabromoferrocene followed by silylation with TMSCl.33 Recently, some of us reported an improved synthetic protocol for decabromoferrocene34 by the bromination of [FeC10(Hg(O2CC3H7)10].35 The reaction pathway emerging from perbrominated ferrocene is supposed to be a promising route to persilylation. In the context of ferrocene as a highly versatile model compound in organometallic chemistry,36 we were interested in synthesizing the first persilylated ferrocene to investigate the resulting steric and electronic effects.
27 were not reproducible. In contrast, the synthesis of [C5TMS3H2]− is well established and the ligand is easily coordinated in reactions with metal halides.28–31 However, the synthetic challenge becomes more demanding for metallocenes due to the repulsion of the sterically crowded Cp ligands in the “sandwich” structure. Okuda achieved high silylation degrees by the reaction of iron(II) halides with tris(1,2,4-TMS)cyclopentadienyllithium to yield 1,1′,2,2′,4,4′-hexakis(trimethylsilyl)ferrocene. As a consequence of the steric demand, a distorted structure of the silylated Cp ligands is observed.32 Butler et al. reported an alternative approach for hexakis(trimethylsilyl)ferrocene including a lithium–halogen exchange of 1,1′,2,2′,3,3′-hexabromoferrocene followed by silylation with TMSCl.33 Recently, some of us reported an improved synthetic protocol for decabromoferrocene34 by the bromination of [FeC10(Hg(O2CC3H7)10].35 The reaction pathway emerging from perbrominated ferrocene is supposed to be a promising route to persilylation. In the context of ferrocene as a highly versatile model compound in organometallic chemistry,36 we were interested in synthesizing the first persilylated ferrocene to investigate the resulting steric and electronic effects.
Results and discussion
Unsubstituted ferrocene
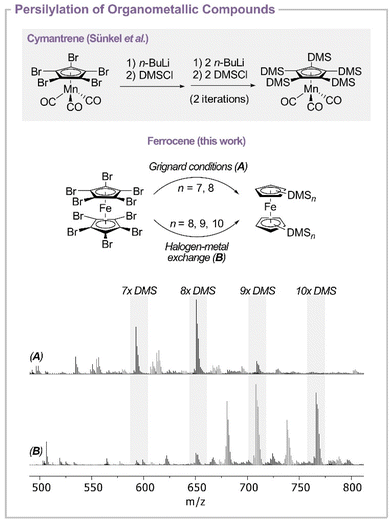
One major challenge in converting [FeC10Br10] effectively into the subsequent silylated ferrocene was to avoid trapping of protons by basic intermediates. Therefore, the reaction itself and especially the used solvents and silanes as well as the glassware had to be made as dry as possible (ESI, S1†). The persilylation of [FeC10Br10] was carried out by a direct reaction with elemental magnesium or by bromine–lithium exchange followed by subsequent trapping with a suitable DMS electrophile (Scheme 1, bottom). Herein, the degree of silylation was followed by mass spectrometry.All reactions including Grignard formation were carried out using DMSCl or DMSOTf in tetrahydrofuran (THF) by varying the conditions like temperature and reaction time (ESI, Table S1†). For all reactions, mass spectrometry revealed that the Grignard formation was complete as no brominated species were observed. However, the silylation step seems to proceed very slowly at high degrees of silylation, as the formation of decasilylferrocene (m/z = 766.2530 for [FeC10DMS10]+) was only observed in traces. Instead, mixtures of [FeC10DMS7H3] (m/z = 592.1711 for [FeC10DMS7H3]+) and [FeC10DMS8H2] (m/z = 650.1946 for [FeC10DMS8H2]+) as main products were obtained (Scheme 1, bottom) in the presence of DMSCl indicating that the nucleophilicity of the Grignard species {FeC10DMSn(MgBr)10−n} (n = 8, 9, 10) is decreased with every DMS group attached to it. Therefore, additional reactions were carried out with DMSOTf as a more electrophilic silylation reagent. In the reactions including DMSOTf, however, silylation of the attached DMS groups takes place rather than the formation of persilylferrocene, causing oligomerization of the attached silyl groups as well as polymerization of the solvent.
As the generated silylated Grignard intermediates seem to be too unreactive for further silylation, to give the target [FeC10DMS10], organolithium compounds were used instead. Herein, lithium is introduced by lithium–halogen exchange with tBuLi and FeC10Br10 followed by the addition of DMSCl. The introduction of lithium in the ortho-position to bromine can cause a subsequent elimination of lithium bromide, which is known for several aryls followed by aryne formation and decomposition.37,38 Therefore, a lithiation agent and an electrophile were added at −100 °C. The stability of the lithiated species varies depending on the solvents. While in n-pentane the compounds only decompose slowly at room temperature, which can be explained by the low solubility in alkanes, in THF and Et2O decomposition was observed above ca. −80 °C. The silylation itself was mainly observed between ca. −80 °C and room temperature in THF and in n-pentane between −50 °C to room temperature indicating that the silylation proceeds faster in THF. However, in Et2O the lithiated intermediates decompose before the silylation process starts. The mass spectra of the reactions in THF and n-pentane still revealed incomplete substitution as after the first lithiation attempt with up to 80 equivalents of tBuLi, in both cases brominated species were still visible at m/z = 672.0876 for [FeC10DMS7BrH2]+, m/z = 730.1196 for [FeC10DMS8BrH]+, m/z = 750.0010 for [FeC10DMS7Br2H]+, m/z = 788.1330 for [FeC10DMS9Br]+ and m/z = 808.0328 for [FeC10DMS8Br2]+ (ESI, Fig. S57†). Here, the compounds [FeC10DMS8Br2] (1) and [FeC10DMS9Br] (2) were isolated and characterized (see the ESI†). Due to the presence of brominated species in the mass spectra, we exclude the formation of [FeC10Li10] as the main intermediate of the reaction. Improved conversion is achieved by multiple metalation cycles. Here, the choice of the solvent becomes more important after multiple lithiation and silylation iterations. While after four iterations in n-pentane brominated species are still visible in the mass spectra, in THF three iterations are sufficient to transform all brominated compounds. However, the formation of side products at m/z = 606.1510 (FeC25H54Si7), m/z = 680.2075 (FeC28H64Si8) and m/zfound = 738.2315 (FeC30H70Si9) is observed (Fig. 1, bottom). As n-pentane still offers sufficient reaction speed it was chosen to be the most suitable solvent for the first lithiation and later changed to THF to reduce side reactions. Nevertheless, via this approach we were able to isolate the desired [FeC10(DMS)10] (6) in milligram amounts.
The persilylated derivative is only poorly soluble in most organic solvents but can consequently be easily separated by crystallization from THF or n-pentane to afford compound 6 as a purple solid. Further purification of the remaining polysilylated metallocene mixtures was accomplished by reverse phase HPLC, using methanol/water as the eluent. Starting from the crude mixture not only were the hepta-, octa-, nona- and decakis(dimethylsilyl)ferrocene separated, but also their corresponding regioisomers in milligram scale. Considering the heptakis- and octakis(dimethylsilyl)ferrocene, in principle four or three possible regioisomers, respectively, exist. However, only four of the seven possible compounds were isolated. The distribution of the observed substitutional pattern reveals that silylation occurs equally at both Cp ligands rather than a consecutive silylation of only one Cp ligand, as metallocenes 3c, 3d and 4c with a tetrakis(dimethylsilyl)cyclopentadienyl ligand were dominantly formed (Fig. 1, purple). This indicates that silylation might have been hampered due to strong steric hindrance.
Single crystals of all isolated compounds were obtained by recrystallization from MeOH/H2O or THF mixtures and analyzed via single-crystal XRD. The steric influence of the polysilylated cyclopentadienyl ligands becomes clear from several features: for the sterically overcrowded metallocenes a staggered conformation is preferred. In the solid state structures extraordinarily large Fe–C bond lengths up to 2.118(1) Å in comparison with that of unsubstituted ferrocene (Fe–C: 2.051(2)–2.063(2) Å (ref. 39)) are observed, indicating repulsion of the parallel cyclopentadienyl ligands. Furthermore, a bent structure for the cyclopentadienyl ligands is observed, as almost all Si atoms are found out of the Cp plane (Table 1, Si–Cpplane). This effect is the most pronounced in the [FeC10DMS10] (6) and [FeC10DMS9H] (5) structures which exhibit even larger angulations in 1,1′,2,2′,4,4′-hexakis(trimethylsilyl)ferrocene (12.8° (ref. 40)). Moreover, the DMS groups on the persilylated ligand prefer to order in a highly symmetric circular manner. As a result, two sets of methyl groups are found pointing towards or away from the iron center while the silicon–hydrogen bond is in the same plane as the Cp ligand. In unsymmetrically silylated ferrocenes (e.g.3a, 4a), the repulsive interaction is compensated by tilting of the respective ferrocene (Table 1, Cp–Cp′tilt), whereupon the parallel orientation of the DMS substituents on the less substituted Cp ligands is repealed (Fig. 2).
| Compound | Distances/Å | Angles/° | |
|---|---|---|---|
| Fe–C | Cp–Cp′tilt | Si–Cpplane | |
| a Multiple metallocene molecules are found within the asymmetric unit. | |||
| FeC10DMS10 (6) | 2.000(8)–2.118(2) | 0.0(1) | 15.1(2)–13.2(1) |
| FeC10DMS9H (5)a | 2.044(17)–2.115(3) | 3.0(4), 3.5(1) | 31.7(3)–9.5(3) |
| FeC10DMS8H2 (4a) | 2.055(5)–2.111(6) | 8.0(2) | 20.4(2)–5.4(2) |
| FeC10DMS8H2 (4b)a | 2.051(3)–2.115(3) | 2.5(1), 3.8(1) | 10.1(1)–3.6(1) |
| FeC10DMS7H3 (3a) | 2.056(2)–2.097(2) | 5.0(1) | 10.4(1)–1.3(1) |
| FeC10DMS7H3 (3b) | 2.057(2)–2.099(2) | 3.1(1) | 11.4(1)–3.3(1) |
The repulsive interactions cause signs of rotational hindrance regarding the DMS groups in the 1H and 13C{1H} NMR spectra. Considering rotatable silyl groups, the compound [FeC10DMS10] (6) should show one signal for the magnetically equivalent DMS methyl protons in the 1H and 13C{1H} NMR spectra, respectively. However, two signals at δ = 0.76 and 0.09 ppm with an integral ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in the 1H spectrum and at δ = 2.41 and −0.18 ppm in the 13C{1H} spectrum are observed instead, as the pairs of methyl groups do not resolve together and are consequently chemically inequivalent. A similar behavior is observed for compounds 5 and 4b with [C5(DMS)4H]− ligands. The metallocenes with threefold silylated cyclopentadienyl ligands exhibit lower rotational barriers for the silyl groups next to the unsubstituted position. Hence, heptasilyl isomers 3a and 3b do not show the expected four methyl signals in their 13C{1H} NMR spectra, but instead six signals, while the octasilyl compound 4a exhibits four differentiated 13C{1H} signals instead of three expected in the absence of rotational hindrance.
1 in the 1H spectrum and at δ = 2.41 and −0.18 ppm in the 13C{1H} spectrum are observed instead, as the pairs of methyl groups do not resolve together and are consequently chemically inequivalent. A similar behavior is observed for compounds 5 and 4b with [C5(DMS)4H]− ligands. The metallocenes with threefold silylated cyclopentadienyl ligands exhibit lower rotational barriers for the silyl groups next to the unsubstituted position. Hence, heptasilyl isomers 3a and 3b do not show the expected four methyl signals in their 13C{1H} NMR spectra, but instead six signals, while the octasilyl compound 4a exhibits four differentiated 13C{1H} signals instead of three expected in the absence of rotational hindrance.
Substitution of the cyclopentadienyl protons typically results in significant electronic and electrochemical changes in metallocenes as HOMO and LUMO energies are shifted. In classical acceptor-substituted compounds, e.g. haloferrocenes, the substituents mainly stabilize the HOMO while weakly destabilizing the LUMO,41 resulting in high redox potentials for the reversible redox couple Fe+II/+III.42 In silylated ferrocenes, e.g. [Fe(C5(TMS)H4)2] (0.01 V vs. FcH), similar redox potentials to those of unsubstituted ferrocene are observed.43 The electrochemical properties of the polysilylated metallocenes 3 to 6 were determined by cyclic voltammetry in THF and referenced against the FcH/FcH+ redox couple. All compounds exhibit a reversible one-electron oxidation with half-wave potentials between E1/2 = 0.161 V and 0.172 V, indicating that the silyl groups do not affect the HOMO energies significantly.
In contrast to this, the UV/VIS spectra of isolated silylferrocenes 3 to 6 indicate decreasing HOMO–LUMO energy gaps. Those spectra were recorded in n-hexane (Fig. 3). Unsubstituted ferrocene exhibits an absorption band at λ = 441 nm in the visible regime corresponding to the unresolved 1A1 → 1E1 and 1A1 → 1E2 spin-allowed d–d transitions, explaining the orange color of this compound.41,44 Incorporation of dimethylsilyl groups causes a bathochromic shift yielding a color transition from red to purple from heptakis- to decakis(dimethylsilyl)ferrocene. This shift has been observed in previously reported polysilylated ferrocenes2,45,46 as well. However, in the spectra of silylferrocenes 3 to 6 this band is accompanied by a shoulder which is most pronounced in the spectrum of [FeC10DMS10] (6).
 | ||
| Fig. 3 Normalized UV/VIS spectra of compounds [FeC10DMS10] (6), [FeC10DMS9H] (5), [FeC10DMS8H2] (4b), [FeC10DMS7H3] (3b) and ferrocene in n-hexane. | ||
To explain these findings, density functional theory (DFT) calculations were performed, comparing unsubstituted ferrocene, [FeCp2], with the persilylated derivative (6) (ωB97X-D/def2-TZVPP(D)//r2SCAN-3c level, see the ESI† for details). For [FeCp2], the expected D5h symmetric minimum is found, with the usual D5d transition state for the rotation of the Cp ligands (at a free energy of about 5 kJ mol−1, consistent with the well-known free rotation in solution47). Time-dependent DFT calculations of the lowest excitation energies give a band at λ = 461 nm, for a 1A1 → 1E1 transition (in D5 notation) and two additional bands at λ = 351 nm and 512 nm, arising from the second 1A1 → 1E1 and the formally forbidden 1A1 → 1E2 transitions, respectively (ESI, Fig. S78†). The overall spectrum agrees very well with the experimentally determined spectrum. It is likely that the closeness of the two lowest excitation energies and the similar intensities (when also considering contributions from the D5d transition state) lead to a broadening of the peak, without a clearly discernible shoulder.
Replacing the Cp–hydrogen atoms with DMS groups so that the fivefold axis of the Cp ligand is retained results in two possible conformers (of D5 and S10 symmetry, respectively) which differ in the relative orientation of the Si–H bonds on the two rings. The electronic energy difference between the two conformers vanishes, consistent with the experimentally observed disorder in the crystal structure of 6 (ESI, Fig. S79†). Based on our calculations, these two conformers lead to qualitatively different spectra (ESI, Fig. S78†). A low-energy shoulder is found for the S10 structure but not for the D5 structure. However, the D5 conformer exhibits much larger intensities, as the 1A1 → 1E1 transition becomes dipole-allowed. We suspect that the shoulder observed experimentally (Fig. 3) may be due to a significant contribution to these low-energy regions of the spectrum from further areas on the conformational energy surface that are accessed dynamically at experimental temperatures. Contributions from different, energetically similar structures may be expected also for the hepta-, octa- and nonasilylated compounds, explaining their comparable band shapes.
Most notably, the TDDFT calculations clearly confirm the overall bathochromic shift of the spectrum upon silylation. NPA charges (ESI, Table S78†) become more positive from [FeCp2] to 6. Most of this substituent effect seems to be structural, caused by the substantially longer Fe–C distances in 6. Computations on [FeCp2] with the carbon positions of 6 do already provide most of the changes in charges and band positions, while finally adding the DMS substituents has a relatively small effect.
Closer analysis of the MOs and of the character of the electronic excitations shows that the degenerate HOMO in 6 is genuinely nonbonding (with dxy and dx2−y2 character) and thus also changes very little (by less than 0.15 eV) upon expanding the Fe–C distances and upon finally adding the DMS substituents. This is consistent with the small effect of the silyl substituents on the redox potentials (see above). The low-lying excitations are out of this HOMO and out of another high-lying occupied MO with predominant metal dz2 character. This MO, which is very slightly σ-antibonding, drops in energy by about 0.25 eV upon bond expansion but goes up by 0.17 eV when adding the DMS substituents, thus staying also almost unchanged overall compared to [FeCp2]. The changes in the band gap and low-lying excitation energies from [FeCp2] to 6 are thus largely due to changes in the virtual MO energies. In particular, the (twofold degenerate) LUMO comes down by 0.47 eV upon bond lengthening and by another 0.20 eV upon adding the DMS substituents. The LUMO is clearly π-antibonding and becomes less antibonding for the silyl-substituted ferrocenes, thus explaining the bathochromic shifts.
1,1-Substituted ferrocenes
Although we were able to isolate the persilylated derivative [FeC10DMS10] (6) the demonstrated synthetic approach is inconvenient for further synthetic applications in terms of selectivity and experimental effort. In order to generate highly overcrowded metallocenes in a selective manner we modified the substitutional pattern to 1,1′-disubstituted ferrocenes. Here the presence of methyl groups should in part compensate the electron-withdrawing and steric effects of the silyl substituents. The polybrominated compound [FeC10Br8Me2] (7) was synthesized by eightfold mercuration of 1,1′-dimethylferrocene followed by bromination with KBr3, analogous to the synthetic protocol for decabromoferrocene.34 The silylation of the corresponding metallocene was carried out with elemental magnesium and DMSCl in anhydrous THF. In contrast to the analogous reaction of decabromoferrocene the perfunctionalized derivative was obtained selectively in high yields (88%). Due to the high selectivity of the reaction step the purification of the substrates is accomplished easily by recrystallization instead of complex chromatographic methods. The obtained compound [FeC10DMS8Me2] (8) was analyzed by NMR, UV/VIS and single-crystal XRD. The polysilylated metallocenes show similar features as [FeC10DMS10] (6). In the 1H and 13C{1H} NMR spectra the signals which were assigned to the methyl groups attached to silicon split into a set of four signals indicating again a hindered rotation along the Si–CCp axis. In the UV/VIS spectra the observed absorption maximum in the visible region at λmax = 490 nm is again red-shifted, indicating decreased HOMO–LUMO energy gaps. The observed wavelengths are similar to those of [FeC10DMS8H2] (4b: λmax = 495 nm) with tetrasilylated Cp ligands. Single crystals of compound 8 were obtained upon crystallization from MeOH/THF mixtures. The molecular structure shows again elongated metal–Cp bonds of 2.082(4)–2.099(4) Å compared to those of unsubstituted ferrocene (Fe–C: 2.051(2)–2.063(2) Å 39). The Cp rings deviate from a parallel arrangement by only 4.6(2)° due to the lower steric demand of the methyl groups. The silyl groups are found out of the Cp plane (5.3–20.9°) in spite of the reduced steric demand induced by the methyl groups.Conclusions
The focus of this work was to find appropriate methods for the generation of highly silylated ferrocenes to investigate the effects of steric overload and charge stabilization by the silyl groups.48 Reactions were carried out by metalation of bromoferrocenes [FeC10Br10] and [FeC10Br8Me2] followed by quenching with DMSCl. We observed that the introduction of silyl groups inhibits further silylation yielding only a mixture of polysilylated compounds [FeC10DMSnH10−n] (n = 10, 9, 8) containing the targeted decasilylated ferrocene in low yields. The reactivity of the polymetalated intermediates and the selectivity of the products drastically improved when electron donating groups were applied and the corresponding octasilylated metallocenes [FeC10DMS8Me2] were isolated purely in good yields despite the sterically demanding substituents.Mixtures of polysilylated metallocenes [FeC10DMSnH10−n] (n = 10, 9, 8, 7) were successfully separated by HPLC allowing a systematic study on silylation effects on ferrocene by XRD, CV, NMR and UV/VIS spectroscopy. Here, the steric overcrowding is reflected by significant rotational hindrance of the tetra- and pentasilylated cyclopentadienyl ligands regarding the DMS groups and greater ordering. Furthermore, the cyclopentadienyl ligands are found to exhibit distortions from planarity within the molecular structure.49 The maximum UV/VIS absorbances are systematically red-shifted with further silylation indicating decreased energy gaps between occupied and virtual MOs. DFT calculations show that this is largely due to the lengthening of the Fe–C bonds caused by the steric crowding induced by the silyl substituents. The occupied frontier MOs are largely nonbonding in nature and thus not affected much by the substitution, in agreement with small changes in the redox potentials. But the metal–ligand antibonding character of the lowest-lying virtual MOs is reduced compared to that of the parent ferrocene, giving rise to the observed red shifts.
The approach of exploiting sterically demanding and electron donating groups to force high degrees of silylation allows the isolation of sterically overcrowded cyclopentadienyl compounds on multigram scale. Even a subsequent methylation to obtain the trimethylsilyl substituted analogues should be feasible to obtain chemically more inert and increasingly overloaded compounds. In the future, the syntheses of other main group, transition metal or lanthanide or actinide complexes with persilylated cyclopentadienyl ligands could be of great interest.
Author contributions
S. M. R. designed the project and wrote the manuscript. Furthermore, she performed the synthesis, recorded cyclic voltammetry and UV/VIS data as well as prepared, measured and solved the single-crystal XRD data. R. S. and P. S. R. performed experiments and analyzed the data. Both authors were supervised by S. M. R. M. R. and M. K. performed the quantum chemical calculations and revised the manuscript. C. F. designed the HPLC experiments. M. M. supervised the project and revised the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was funded by Deutsche Forschungsgemeinschaft (DFG) – project number: 387284271 – SFB 1349. We would like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG.References
- J. Okuda, Chem. Ber., 1989, 122, 1075–1077 CrossRef CAS.
- J. Okuda and E. Herdtweck, J. Organomet. Chem., 1989, 373, 99–105 CrossRef CAS.
- M. P. Plesniak, X. Just-Baringo, F. Ortu, D. P. Mills and D. J. Procter, Chem. Commun., 2016, 52, 13503–13506 RSC.
- D. Cui, O. Tardif and Z. Hou, J. Am. Chem. Soc., 2004, 126, 1312–1313 CrossRef CAS PubMed.
- F. Ortu, J. Lui, M. Burton, M. Fowler, A. Formanuik, M.-E. Boulon, N. F. Chilton and D. P. Mills, Inorg. Chem., 2017, 56, 2496–2505 CrossRef CAS PubMed.
- D. Cui, M. Nishiura and Z. Hou, Angew. Chem., Int. Ed., 2005, 44, 959–962 CrossRef CAS PubMed.
- W.-X. Zhang, Z. Wang, M. Nishiura, Z. Xi and Z. Hou, J. Am. Chem. Soc., 2011, 133, 5712–5715 CrossRef CAS PubMed.
- L. M. Engelhardt, P. C. Junk, C. L. Raston and A. H. White, J. Chem. Soc., Chem. Commun., 1988, 1500–1501 RSC.
- A. H. Cowley, P. Jutzi, F. X. Kohl, J. G. Lasch, N. C. Norman and E. Schlüter, Angew. Chem., 1984, 96, 603–604 CrossRef CAS.
- M. J. Harvey, T. P. Hanusa and M. Pink, J. Chem. Soc., Dalton Trans., 2001, 1128–1130 RSC.
- P. Jutzi, E. Schlüter, M. B. Hursthouse, A. M. Arif and R. L. Short, J. Organomet. Chem., 1986, 299, 285–295 CrossRef CAS.
- M. Westerhausen, S. Schneiderbauer, N. Makropoulos, M. Warchhold, H. Nöth, H. Piotrowski and K. Karaghiosoff, Organometallics, 2002, 21, 4335–4341 CrossRef CAS.
- C. H. Winter, X. X. Zhou and M. J. Heeg, Inorg. Chem., 1992, 31, 1808–1815 CrossRef CAS.
- A. Sekiguchi, T. Nakanishi, C. Kabuto and H. Sakurai, J. Am. Chem. Soc., 1989, 111, 3748–3750 CrossRef CAS.
- A. Sekiguchi, M. Ichinohe, C. Kabuto and H. Sakurai, Organometallics, 1995, 14, 1092–1094 CrossRef CAS.
- A. Sekiguchi, T. Matsuo, K. Ebata and H. Sakurai, Chem. Lett., 1996, 25, 1133–1134 CrossRef.
- A. Sekiguchi, T. Matsuo and H. Watanabe, J. Am. Chem. Soc., 2000, 122, 5652–5653 CrossRef CAS.
- A. Sekiguchi, K. Ebata, C. Kabuto and H. Sakurai, J. Am. Chem. Soc., 1991, 113, 1465–1466 CrossRef.
- J. C. Wedal, J. M. Barlow, J. W. Ziller, J. Y. Yang and W. J. Evans, Chem. Sci., 2021, 12, 8501–8511 RSC.
- M. R. Trinh, J. C. Wedal and W. J. Evans, Dalton Trans., 2021, 50, 14384–14389 RSC.
- R. R. Langeslay, M. E. Fieser, J. W. Ziller, F. Furche and W. J. Evans, Chem. Sci., 2015, 6, 517–521 RSC.
- R. R. Langeslay, M. E. Fieser, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2016, 138, 4036–4045 CrossRef CAS PubMed.
- M. R. MacDonald, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2013, 135, 9857–9868 CrossRef CAS PubMed.
- M. E. Fieser, M. R. MacDonald, B. T. Krull, J. E. Bates, J. W. Ziller, F. Furche and W. J. Evans, J. Am. Chem. Soc., 2015, 137, 369–382 CrossRef CAS PubMed.
- K. Sünkel and J. Hofmann, Organometallics, 1992, 11, 3923–3925 CrossRef.
- A. Sekiguchi, Y. Sugai, K. Ebata, C. Kabuto and H. Sakurai, J. Am. Chem. Soc., 1993, 115, 1144–1146 CrossRef CAS.
- M. S. Miftakhov, G. A. Tolstikov and S. I. Lomakina, Zh. Obshch. Khim., 1976, 46, 2754–2754 CAS.
- P. Jutzi, E. Schlüter, C. Krüger and S. Pohl, Angew. Chem., Int. Ed. Engl., 1983, 22, 944–944 Search PubMed.
- M. A. Edelman, P. B. Hitchcock, M. F. Lappert, D.-S. Liu and S. Tian, J. Organomet. Chem., 1998, 550, 397–808 CrossRef CAS.
- H. Chen, P. Jutzi, W. Leffers, M. M. Olmstead and P. P. Power, Organometallics, 1991, 10, 1282–1286 CrossRef CAS.
- C. P. Morley and P. Jutzi, Organometallics, 1987, 6, 1084–1090 CrossRef CAS.
- J. Okuda and E. Herdtweck, Chem. Ber., 1988, 121, 1899–1905 CrossRef CAS.
- I. R. Butler, D. M. Evans, P. N. Horton, S. J. Coles and P. J. Murphy, Organometallics, 2021, 40, 600–605 CrossRef CAS.
- S. M. Rupf, I. S. Dimitrova, G. Schröder and M. Malischewski, Organometallics, 2022, 41, 1261–1267 CrossRef CAS.
- S. M. Rupf, G. Schröder, R. Sievers and M. Malischewski, Chem. – Eur. J., 2021, 27, 5125–5129 CrossRef CAS PubMed.
- P. Štěpnička, Dalton Trans., 2022, 51, 8085–8102 RSC.
- C. H. Winter and K. N. Seneviratne, Comments Inorg. Chem., 1996, 19, 1–23 CrossRef CAS.
- J. R. Baran Jr., C. Hendrickson, D. A. Laude Jr. and R. J. Lagow, J. Org. Chem., 1992, 57, 3759–3760 CrossRef.
- P. Seiler and J. D. Dunitz, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1982, 38, 1741–1745 CrossRef.
- J. Okuda, J. Organomet. Chem., 1989, 376, C1–C4 CrossRef.
- M. S. Inkpen, S. Du, M. Hildebrand, A. J. P. White, N. M. Harrison, T. Albrecht and N. J. Long, Organometallics, 2015, 34, 5461–5469 CrossRef CAS.
- D. A. Khobragade, S. G. Mahamulkar, L. Pospíšil, I. Cíšarová, L. Rulísek and U. Jahn, Chem. – Eur. J., 2012, 18, 12267–12277 CrossRef CAS PubMed.
- A. Abri and A. abbaszad Rafe, J. Chin. Chem. Soc., 2015, 62, 273–279 CrossRef CAS.
- Y. S. Sohn, D. N. Hendrickson and H. B. Gray, J. Am. Chem. Soc., 1971, 93, 3603–3612 CrossRef CAS.
- O. J. Curnow, G. M. Fern, S. Klaib, U. Böhme, H. Lang and R. Holze, J. Electroanal. Chem., 2005, 585, 167–171 CrossRef CAS.
- J. Okuda, R. W. Albach and E. Herdtweck, Polyhedron, 1991, 10, 1741–1748 CrossRef CAS.
- M. Rosenblum and R. B. Woodward, J. Am. Chem. Soc., 1958, 80, 5443–5449 CrossRef CAS.
- A previous version of the manuscript has been deposited on a preprint server: S. M. Rupf, R. Sievers, P. S. Riemann, M. Reimann, K. Kaupp, C. Fasting and M. Malischewski, ChemRxiv, 2023, DOI:10.26434/chemrxiv-2023-538rg.
- Deposition numbers CCDC 2248053–2248061 contain the supplementary crystallographic data for this paper.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2248053 (1), 2248054 (2), 2248060 (3a), 2248059 (3b), 2248057 (4a), 2248058 (4b), 2248056 (5) and 2248055 (6). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt01133j |
| This journal is © The Royal Society of Chemistry 2023 |