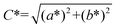Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Deciphering the role of water and a zinc-doping process in a polyol-based approach for obtaining Zn/Co/Al-based spinels: toward “green” mesoporous inorganic pigments†
Maria-Gabriela
Alexandru
 a,
Adelina-Carmen
Ianculescu
*b,
Oana
Carp
c,
Daniela C.
Culita
a,
Adelina-Carmen
Ianculescu
*b,
Oana
Carp
c,
Daniela C.
Culita
 c,
Silviu
Preda
c,
Silviu
Preda
 c,
Cristian D.
Ene
c,
Cristian D.
Ene
 c,
Bogdan Stefan
Vasile
c,
Bogdan Stefan
Vasile
 b,
Vasile-Adrian
Surdu
b,
Vasile-Adrian
Surdu
 b,
Adrian-Ionut
Nicoara
b,
Florentina
Neatu
b,
Adrian-Ionut
Nicoara
b,
Florentina
Neatu
 d,
Ioana
Pintilie
d,
Ioana
Pintilie
 d and
Diana
Visinescu
d and
Diana
Visinescu
 *c
*c
aDepartment of Inorganic Chemistry, Physical Chemistry and Electrochemistry, Faculty of Chemical Engineering and Biotechnologies, University Politehnica of Bucharest, 1-7 Gh. Polizu Street, 011061 Bucharest, Romania
bDepartment of Science and Engineering of Oxide Materials and Nanomaterials, University Politehnica of Bucharest, Bucharest, 060042, Romania. E-mail: adelina.ianculescu@upb.ro
c“Ilie Murgulescu” Institute of Physical Chemistry, Romanian Academy, 202 Splaiul Independentei, 060021 Bucharest, Romania. E-mail: diana.visinescu@gmail.com
dNational Institute of Materials Physics, P.O. Box MG-7, Bucharest-Magurele 077125, Romania
First published on 22nd June 2023
Abstract
Two new families of zinc/cobalt/aluminum-based pigments, with a unique composition, were obtained through the polyol method. The hydrolysis process of a mixture of Co(CH3COO)2, Zn(acac)2 and Al(acac)3 (acac− = acetylacetonate ion) in 1,4-butanediol afforded dark blue gels (wPZnxCo1−xAl), in the presence of a supplementary amount of water, and light green powders (PZnxCo1−xAl), respectively, for the water-free procedure (x = 0, 0.2, 0.4). The calcination of the precursors yielded dark green (wZnxCo1−xAl) and blue (ZnxCo1−xAl) products. XRD measurements and Rietveld refinement indicate the co-existence of three spinel phases, in different proportions: ZnxCo1−xAl2O4, Co3O4 and the defect spinel, γ-Al2.67O4. The Raman scattering and XPS spectra are in agreement with the compositions of the samples. The morphology of wZnxCo1−xAl consists of large and irregular spherical particle aggregates (ca. 5–100 mm). Smaller agglomerates (ca. 1–5 mm) with a unique silkworm cocoon-like hierarchical morphology composed of cobalt aluminate cores covered with flake-like alumina shells are formed for ZnxCo1−xAl. TEM and HR-TEM analyses revealed the formation of crystalline, polyhedral particles of 7–43 nm sizes for wZnxCo1−xAl, while for ZnxCo1−xAl, a duplex-type morphology, with small (7–13 nm) and larger (30–40 nm) particles, was found. BET assessment showed that both series of oxides are mesoporous materials, with different pore structures, with the water-free samples exhibiting the largest surface areas due, most likely, to the high percent of aluminum oxide. A chemical mechanism is proposed to highlight the role of the water amount and the nature of the starting compounds in the hydrolysis reaction products and, further, in the morpho-structural features and composition of the resulting spinel oxides. The CIE L*a*b* and C* colorimetric parameters indicate that the pigments are bright, with a moderate degree of luminosity, presenting an outstanding high blueness.
Introduction
Metal aluminates, MIIAlIII2O4, with a cubic spinel structure, represent a fascinating family of multifunctional ternary oxides, with different applications as (photo)catalysts,1–6 adsorbents,7 magnetic materials,8 humidity and gas sensors9,10 and, more recently, for energy storage.11 In particular, cobalt aluminate (CoAl2O4), known as Thénard's Blue (TB) or cobalt blue pigment, is an old and famous high-quality blue pigment, with significant photo-/thermochemical stability, superior coloring properties and an extraordinary resistance to corrosion. Nowadays, CoAl2O4 is largely used for the coloration of ceramic bodies, plastics, paint, paper, concrete, and fibers, or as a contrast-enhancing luminescent pigment with self-cleaning abilities.12–15 The typical and intense blue color of TB is mainly due to the strong preference of Co2+ ions for the tetrahedral sites within the cubic lattice of the normal spinel-type AIIBIII2O4, whereas its green color is characteristic of an inverse spinel structure, with the Co2+ ions located in octahedral sites.16–18 The color and coloring capabilities of the cobalt aluminates strongly depend on their phase purity, as well as on their morphological (sizes, shapes and textures) and structural (crystallinity degree and inversion degree) characteristics.19,20The ceramic synthesis of the CoAl2O4 pigment, based on a solid-state reaction between individual oxides (CoO or Co3O4 and α-Al2O3, respectively), represents the conventional route in the synthesis of metal aluminate oxides. Such a procedure demands high temperature (ca. 1300 °C), long reaction time and extended grinding process to obtain low-sized pigments with superior hiding properties.21,22 For obtaining high-performance materials a certain degree of control on their morpho-structural features and composition is required. Therefore, the development of soft chemistry approaches for the synthesis of nanomaterials accelerated the transition toward the next generation of inorganic multifunctional oxide-based pigments.23 From the numerous advantages provided by the soft chemistry synthesis, it is worth mentioning the economical aspects (low energy consumption, shorter reaction times, and cheap raw materials) and, also, the large perspectives opened in the design of mixed oxides, containing different metal ions, with various stable oxidation states and preferred geometries that particularly influence the color of the pigments.24–28 Intense efforts have been made to develop efficient synthetic methods of cobalt aluminate that, on one hand, could provide the best pigment characteristics, and, on the other hand, reduce the toxic effect of Co2+ ions from CoAl2O4 on the human health and environment, by partially replacing Co2+ with non-toxic metal ions.29,30 Thus, sol–gel,8,15 hydrothermal,31,32 reverse microemulsion,26 low-temperature thermolysis of heteronuclear coordination compounds,33 combustion,34–36 and co-precipitation methods37 were commonly employed in the synthesis of nano-sized aluminates with improved optical and coating properties. Besides, such synthesis routes opened interesting “green” alternatives by replacing harmful raw materials with eco-friendly ingredients (as polysaccharides).2,38 In the case of TB, the gradual substitution of the toxic cobalt(II) ions with the non-hazardous metal cations (like Zn2+, Ca2+ or Mg2+), by preserving and even improving their chromaticity, represents a step forward toward eco-friendly inorganic pigments.2,38–43
The (poly)alcohol synthesis is one of the most convenient, resourceful, and straightforward soft chemistry routes to obtain well-crystallized metals or metal oxides with high compositional homogeneity, diversity of shapes, assembly motifs, and narrow size distributions.44–46 Synthesis parameters like reaction time and/or temperature, the presence/absence of reaction additives, as well as the concentration of the metal precursor, strongly influence the crystallite nucleation and growth kinetics, as well as the surface chemistry of the resulting oxide particles.44–46 Moreover, many of the polyols are green solvents because they show low to moderate toxicity and are highly biodegradable. Polyol synthesis performed in various (ether)-diols, with different chain lengths, linear or branched, proved to be efficient for obtaining ternary oxides, with well-defined stoichiometry, such as spinel-type oxides, MIIMIII2O4 (MII = Mg, Zn, Co, Ni, Mn; MIII = Fe, Co, Al).47–49 Focusing on TB materials, the nature and concentration of the precursors, as well as the reaction temperature, have a significant effect on the structural and morphological characteristics of the oxides.50,51
Herein, we describe the synthesis and characterization (structural, morphological, and optical) of two new series of Zn/Co/Al oxide-based pigments, obtained through a hydrolysis process, in 1,4-butanediol, followed by the thermolysis of the as-obtained precursors. The morpho-structural investigation outlined the influence of the preparation method (the water amount, in situ generated or intentionally added, and the ZnII cation incorporation process) on the composition of the precursors and, consequently, on the composition, size/shape, and texture of the corresponding oxide particles and, further, on their opto-chromatic properties.
Experimental
Materials and synthesis
Cobalt acetate [Co(CH3COO)2·4H2O, Sigma-Aldrich], zinc acetylacetonate [Zn(C5H7O2)2·H2O, Merck], aluminum acetylacetonate [Al(C5H7O2)3, Sigma-Aldrich] and 1,4-butanediol [HO(CH2)4OH, 1,4-BD, >99.5%] of analytical grade were used without further purification.Thermal investigations
The thermal analysis has been carried out in alumina crucibles under static air, with a sample mass of ca. 10 mg at a heating rate of 5 K min−1 on a NetzchSTA 409 PC/PG thermo-balance.Spectral investigations
The IR spectra (KBr pellets) were recorded in the 4000–400 cm−1 range, with a FTIR Brucker Tensor V-37 spectrophotometer. NIR-UV-Vis spectra (diffuse reflectance technique) were recorded on a 200–1800 nm domain with a JASCO V-670 spectrophotometer, using MgO as the standard. The diffuse reflectance of the samples was measured with a PerkinElmer Lambda 35 spectrophotometer, in the 300–800 nm range, using standard D65 illumination. The CIE-L*a*b* colorimetric method, recommended by the Commission Internationale de l'Eclairage (CIE), was followed: L* is the lightness axis [black (0) → white (100)], b* is the blue (−) → yellow (+) axis, and a* is the green (−) → red (+) axis. The parameter C* (chroma) represents the saturation of the colour, being defined as:Raman spectroscopy analyses were carried out at room temperature to investigate the local order in the spinel powders. The spectra were recorded using a LabRaman HR Evolution spectrometer (Horiba, Kyoto, Japan) with a 514 nm line of an argon ion laser, by focusing a 125 mW beam of a few micrometer sized spots on the samples under investigation. An acquisition time of 10 s was established for each of the 80 accumulations during the investigation.
Structural and morphological investigations
Results and discussion
Synthesis and characterization of wPZnxCo1−xAl and PZnxCo1−xAl precursors (x = 0, 0.2, 0.4)
The reaction of zinc(II) acetylacetonate, cobalt(II) acetate and aluminum(III) acetylacetonate with an [xZn2+ + (1 − x)Co2+]:2Al3+ stoichiometry in 1,4-butanediol afforded two types of products, depending on the water amount in the reaction: dark blue gels (wPZnxCo1−xAl) in water-assisted synthesis and light green powders (PZnxCo1−xAl) in reactions carried out without the addition of an extra amount of water (x = 0, 0.2, 0.4). Generally, metal substituted cobalt aluminates were obtained by a gradual incorporation of cobalt(II) ions into a non-hazardous MIIAlIII2O4 host lattice (M = Zn, Mg).25,38,40–42 Herein, we investigated a reversed incorporation procedure, in which the cobalt(II) ions are progressively replaced by zinc(II) cations within the CoAl2O4 host spinel matrix.A preliminary characterization of the water-added/free precursors has been carried out through FTIR and NIR-UV-Vis spectroscopy as well as through thermal analysis. FTIR measurements were performed on the wPZnxCo1−xAl and PZnxCo1−xAl precursors to reveal the nature of the formed compounds by detecting the functional groups through the specific vibrations of C–H/C–OH/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/O–H bonds (3500–1000 cm−1 range) and, also of the M–O bonds (covering the 900–400 cm−1 region). Thus, the FTIR spectra of the wPZnxCo1−xAl and PZnxCo1−xAl precursors show the formation of two different types of compounds (Fig. S1 and S2 and detailed discussion in the ESI†), suggesting the formation of different intermediates, with bands at 1400–1720 and 1000–1100 cm−1 specific for hydroxide and/or hydroxyacetate, diol/acac complexes,52,53 as well as between 400 and 900 cm−1, characteristic of M–O bonds.54 For the PZnxCo1−xAl precursor, typical absorptions of boehmite, γ-AlO(OH), occurred: two intense bands at 1107 and 1036 cm−1 stand for symmetric (δs) and asymmetric (δas) bending vibrations of Al–O–H, while torsional, stretching and bending modes of Al–O bonds occur at 776, 636 and 485 cm−1 as medium/weak intensity bands.55 Also, NIR-UV-Vis spectroscopy measurements were carried out on the two types of precursors to probe the electronic transition of the metal ions, especially for the tetrahedral Co(II) cations that exhibit typical d–d electronic transitions in visible and near-infrared regions. The NIR-UV-Vis spectra for both water-assisted, wPZnxCo1−xAl, and water-free, PZnxCo1−xAl, precursors show absorption bands attributed to d–d transitions of tetrahedral cobalt(II) ions (Fig. S3 and S4 of ESI†).38 The PXRD patterns confirm the resemblances of the water-free precursors, independent of the Co2+/Zn2+ ratio (Fig. S5 of ESI†), the peaks at (020), (120), (031) and (200) being attributed to the orthorhombic γ-AlO(OH) and/or incipient spinel phases. Besides, boehmite exhibits blue emission under visible excitation wavelength, due to oxygen vacancies and hydroxyl groups bound to surface aluminum (AlOH) centers.56 The photoluminescence spectra of the PZnxCo1−xAl precursors also exhibit typical boehmite broad emission centered at ca. 460 nm (λexc = 400 nm, Fig. S6 of ESI†),56 whereas the EDX analysis of the richest zinc(II) precursor (x = 0.4) shows the predominance of the Al element in the cocoon-like aggregates (Fig. S7–S9 of ESI†).
O/O–H bonds (3500–1000 cm−1 range) and, also of the M–O bonds (covering the 900–400 cm−1 region). Thus, the FTIR spectra of the wPZnxCo1−xAl and PZnxCo1−xAl precursors show the formation of two different types of compounds (Fig. S1 and S2 and detailed discussion in the ESI†), suggesting the formation of different intermediates, with bands at 1400–1720 and 1000–1100 cm−1 specific for hydroxide and/or hydroxyacetate, diol/acac complexes,52,53 as well as between 400 and 900 cm−1, characteristic of M–O bonds.54 For the PZnxCo1−xAl precursor, typical absorptions of boehmite, γ-AlO(OH), occurred: two intense bands at 1107 and 1036 cm−1 stand for symmetric (δs) and asymmetric (δas) bending vibrations of Al–O–H, while torsional, stretching and bending modes of Al–O bonds occur at 776, 636 and 485 cm−1 as medium/weak intensity bands.55 Also, NIR-UV-Vis spectroscopy measurements were carried out on the two types of precursors to probe the electronic transition of the metal ions, especially for the tetrahedral Co(II) cations that exhibit typical d–d electronic transitions in visible and near-infrared regions. The NIR-UV-Vis spectra for both water-assisted, wPZnxCo1−xAl, and water-free, PZnxCo1−xAl, precursors show absorption bands attributed to d–d transitions of tetrahedral cobalt(II) ions (Fig. S3 and S4 of ESI†).38 The PXRD patterns confirm the resemblances of the water-free precursors, independent of the Co2+/Zn2+ ratio (Fig. S5 of ESI†), the peaks at (020), (120), (031) and (200) being attributed to the orthorhombic γ-AlO(OH) and/or incipient spinel phases. Besides, boehmite exhibits blue emission under visible excitation wavelength, due to oxygen vacancies and hydroxyl groups bound to surface aluminum (AlOH) centers.56 The photoluminescence spectra of the PZnxCo1−xAl precursors also exhibit typical boehmite broad emission centered at ca. 460 nm (λexc = 400 nm, Fig. S6 of ESI†),56 whereas the EDX analysis of the richest zinc(II) precursor (x = 0.4) shows the predominance of the Al element in the cocoon-like aggregates (Fig. S7–S9 of ESI†).
 | ||
| Fig. 1 Thermal curves (TG, DTG and DSC) for: water-assisted wPZnxCo1−xAl (top) and water-free PZnxCo1−xAl (bottom) samples (x = 0). | ||
The mass loss for water-free PZnxCo1−xAl compounds is significantly higher than that for the water-assisted analogues (e.g. 51.3 vs. 18.3% for x = 0), due to a higher content of the organic residue. For both types of precursors, no resolved phase transformation is spotted at high temperatures. However, the broad and low intensity peak in the DSC curves, in the temperature range of 500–900 °C, could be attributed to the crystallization of the spinels. The thermal plots of the zinc-incorporated water-assisted samples have similar profiles to the zinc-free one, indicating a resembling thermal behavior (Fig. S10 and S12 of ESI†). Nevertheless, for x = 0.2, the second stage corresponding to the two overlapped processes of dehydration and degradation/combustion of organic materials is well-delimited, with a high weight loss (ca. 61%) occurring in the temperature range of 130–215 °C. This could be caused by a higher water content and/or hydroxy species that most likely are eliminated in this step. For PZnxCo1−xAl precursors, the zinc doping process does not significantly affect the thermal behavior of the precursors, but the thermal effects are more delimited due to a higher compositional inhomogeneity induced by the zinc incorporation (Fig. S11 and S13 in the ESI†).
Characterization of the wZnxCo1−xAl and ZnxCo1−xAl oxides (x = 0, 0.2, 0.4)
X-ray diffraction. In the XRD patterns of the zinc/cobalt aluminate samples, wZnxCo1−xAl and ZnxCo1−xAl oxides (x = 0, 0.2, 0.4), typical peaks of the spinel phase were identified (Fig. 2(a) and (b)). Nevertheless, a significant difference between the two groups of oxides was noticed.
 | ||
| Fig. 2 PXRD patterns of the mixed oxides obtained after a heating treatment at 800 °C/1 h: (a) wZnxCo1−xAl and (b) ZnxCo1−xAl. | ||
Thus, well-delimited and sharp peaks, roughly assigned to the cobalt aluminate spinel (ICDD Card. No. 00-038-0814) for water-added samples, wZnxCo1−xAl, vs. broadened peaks for water-free samples, were detected.57–60 The larger peaks for the ZnxCo1−xAl samples could be the result of small sizes of the oxide particles and/or the presence of secondary crystalline phases, most likely also with a spinel structure. Indeed, the analysis of the XRD data indicates for the water-free oxides a high amount of alumina spinel (Al2.67O4), which becomes the major phase in the Zn-substituted samples (Table 1). From the results of the Rietveld refinement carried out on both wZnxCo1−xAl and ZnxCo1−xAl oxides (Table 1 and Fig. S14–S19 in the ESI†), several conclusions should be outlined:
(i) For all samples, the best fit corresponds to a mixture of three spinel phases, i.e. iso-structural CoAl2O4, Co3O4 – guite (ICDD Card No. 00-043-1003), and the defect spinel, Al2.67O4 (ICDD Card No. 04-005-4662), except for ZnxCo1−xAl (x = 0.2) oxide in which only CoAl2O4 and Al2.67O4 crystalline phases were identified.
(ii) The highest proportion of crystalline CoAl2O4 was found for the water-assisted wZnxCo1−xAl sample (x = 0.2), 83.3%, whereas, the water-free analogue, ZnxCo1−xAl (x = 0.2), contains the smallest amount of cobalt aluminate crystalline phase (3.9%). However, in the last case, the very low concentration of CoAl2O4 is most likely a result of the amorphous degree of the sample (crystallinity degree has the lowest value, 54.11%, Fig. 3 and Table 1).
| wZnxCo1−xAl | ZnxCo1−xAl | |||||
|---|---|---|---|---|---|---|
| x = 0 | x = 0.2 | x = 0.4 | x = 0 | x = 0.2 | x = 0.4 | |
| a ICDD cards used for indexing: CoAl2O4 – ICDD Card No. 00-038-0814; Co3O4 – ICDD Card No. 00-043-1003; and Al2.67O4 – ICDD card no. 04-005-4662. | ||||||
| Phase composition | CoAl2O4 (81.6%) | CoAl2O4 (83.3%) | CoAl2O4 (73.3%) | CoAl2O4 (48.6%) | CoAl2O4 (3.9%) | CoAl2O4 (38.9%) |
| Co3O4 (3.9%) | Co3O4 (16.0%) | Co3O4 (19.8%) | Co3O4 (4.6%) | Co3O4 (0%) | Co3O4 (3.4%) | |
| Al2.67O4 (14.5%) | Al2.67O4 (0.7%) | Al2.67O4 (6.9%) | Al2.67O4 (46.8%) | Al2.67O4 (96.1%) | Al2.67O4 (57.7%) | |
| Symmetry | Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
Cubic, Fd![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m m |
| Unit cell parameters (a = b = c) (Å) | 8.0928 ± 0.0037 (CoAl2O4) | 8.0949 ± 0.0010 (CoAl2O4) | 8.0959 ± 0.0025 (CoAl2O4) | 8.0835 ± 0.0049 (CoAl2O4) | 8.1376 ± 0.0101 (CoAl2O4) | 8.0885 ± 0.0194 (CoAl2O4) |
| 8.0845 ± 0.0054 (Co3O4) | 8.0978 ± 0.0054 (Co3O4) | 8.0885 ± 0.0019 (Co3O4) | 8.0899 ± 0.0051 (Co3O4) | — (Co3O4) | 8.0211 ± 0.0612 (Co3O4) | |
| 8.0210 ± 0.0185 (Al2.67O4) | 8.0760 ± 0.0011 (Al2.67O4) | 8.0276 ± 0.0011 (Al2.67O4) | 8.0553 ± 0.0135 (Al2.67O4) | 7.9807 ± 0.0064 (Al2.67O4) | 7.9489 ± 0.0109 (Al2.67O4) | |
| Unit cell volume (Å3) | 530.0298 (CoAl2O4) | 530.4326 (CoAl2O4) | 530.6428 (CoAl2O4) | 528.2087 (CoAl2O4) | 538.87 (CoAl2O4) | 529.1732 (CoAl2O4) |
| 528.492 (Co3O4) | 530.9946 (Co3O4) | 529.1827 (Co3O4) | 529.457 (Co3O4) | — (Co3O4) | 516.0634 (Co3O4) | |
| 516.0363 (Al2.67O4) | 526.7281 (Al2.67O4) | 517.289 (Al2.67O4) | 522.6867 (Al2.67O4) | 508.2983 (Al2.67O4) | 502.2574 (Al2.67O4) | |
| R expected, Rexp | 6.46054 | 6.65252 | 6.89274 | 6.43947 | 6.44366 | 7.08213 |
| R profile, Rp | 6.04426 | 6.12248 | 6.3433 | 5.27937 | 4.69992 | 5.18676 |
| Weighted R profile, Rwp | 7.63774 | 7.9653 | 8.38335 | 6.70315 | 5.98996 | 6.72176 |
| Goodness of fit, χ2 | 1.39793 | 1.43362 | 1.47928 | 1.08357 | 0.86441 | 0.90082 |
| Crystallinity | 69.12 | 87.19 | 76.15 | 57.22 | 54.11 | 66.46 |
| Crystallite sizes, <D> nm | 9.83 ± 1.06 (CoAl2O4) | 10.91 ± 7.69 (CoAl2O4) | 7.94 ± 1.62 (CoAl2O4) | 26.44 ± 5.51 (CoAl2O4) | 7.23 ± 2.38 (CoAl2O4) | 4.90 ± 1.21 (CoAl2O4) |
| 56.96 ± 3.93 (Co3O4) | 28.48 ± 3.63 (Co3O4) | 48.05 ± 6.23 (Co3O4) | 7.36 ± 0.31 (Co3O4) | — (Co3O4) | 5.17 ± 1.53 (Co3O4) | |
| 10.76 ± 1.15(Al2.67O4) | 17.42 ± 10.65 (Al2.67O4) | 24.60 ± 18.32 (Al2.67O4) | 3.73 ± 4.16 (Al2.67O4) | 3.22 ± 1.75 (Al2.67O4) | 4.44 ± 0.43 (Al2.67O4) | |
| Internal strains, <S> (%) | 1.02 ± 0.58 (CoAl2O4) | 0.59 ± 0.75 (CoAl2O4) | 1.31 ± 0.88 (CoAl2O4) | 0.39 ± 0.31 (CoAl2O4) | 0.81 ± 0.88 (CoAl2O4) | 2.08 ± 1.30 (CoAl2O4) |
| 0.17 ± 0.07 (Co3O4) | 0.17 ± 0.22 (Co3O4) | 0.21 ± 0.11 (Co3O4) | 1.32 ± 0.57 (Co3O4) | — (Co3O4) | 2.14 ± 1.31 (Co3O4) | |
| 0.86 ± 0.29 (Al2.67O4) | 0.78± 0.63 (Al2.67O4) | 0.55± 0.56 (Al2.67O4) | 3.18 ± 1.62 (Al2.67O4) | 4.43 ± 2.22 (Al2.67O4) | 2.25 ± 1.24 (Al2.67O4) | |
 | ||
| Fig. 3 Chart diagrams showing the variation of the phase composition depending on the zinc substitution degree (x) for: (a) water-assisted wZnxCo1−xAl and (b) water-free ZnxCo1−xAl oxides. | ||
(iii) The Zn-doping process has different effects on the oxide phase composition: for the water-assisted samples, wZnxCo1−xAl, the gradual insertion of Zn(II) ions determines an increase of the Co3O4 concentration and a corresponding diminishment of Al2.67O4 content. Conversely, for the water-free ZnxCo1−xAl oxides, a reversed evolution of the alumina proportion occurred, increasing dramatically for x = 0.2 and x = 0.4, correlated with the low amount of Co3O4, down to 0 for x = 0.2 (Fig. 3).
(iv) The particle sizes are in the nanometer range and, for the water-assisted samples, they are generally higher than those of the water-free analogues.
(v) For the water-added oxides, wZnxCo1−xAl, the Zn-doping process determines an increasing trend only for the aluminum oxide crystallites (from ca. 11 up to 25 nm), whereas Co3O4 has the highest crystallite size (ca. 57 nm). Conversely, for the water free ZnxCo1−xAl spinels, the Zn(II) cation incorporation process led to a decrease of the crystallite sizes of Zn/Co aluminates (from 26 down to 4.9 nm), whereas the aluminum oxide crystallite dimensions were not influenced by the doping process.
Raman spectra. Raman scattering provides additional information about the crystal structure and types of spinels in our samples. Thus, the Raman spectra, recorded on the water-assisted samples, wZnxCo1−xAl (Fig. 4), revealed the presence of five main bands corresponding to the expected Raman active modes of a cubic spinel structure in the Fd
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m spatial group: A1g at 755 (vw) and 681/685 (s) cm−1, F2g(3) at 613/619 cm−1, F2g(2) at 516/520 cm−1, Eg at 406 cm−1 and F2g(1) at ca. 191/194/197 cm−1.61,62
m spatial group: A1g at 755 (vw) and 681/685 (s) cm−1, F2g(3) at 613/619 cm−1, F2g(2) at 516/520 cm−1, Eg at 406 cm−1 and F2g(1) at ca. 191/194/197 cm−1.61,62
The high energy phonon modes, A1g and F2g(3), are generally correlated with M3+–O stretching (symmetric and asymmetric) and bending vibrations from the octahedral MO6 units of the spinel structure.63,64 The occurrence of the two shoulders at 701 and 581 cm−1 in zinc-doped oxides could be related to the presence of a significant amount of Co3+ ions in the octahedral sites of the spinel lattice. The band located at 476 − 481 cm−1 and whose intensity increases for the zinc containing samples is generally attributed to the Eg vibration mode of Co3O4.63,64 The lowest wavenumber band located between 191 and 197 cm−1 corresponds to the F2g(1) vibration and is attributed to the tetrahedral CoIIO4 units.64
The zinc incorporation has multiple effects on the phonon vibrations due to the increase of the Co3O4 spinel content (see Table 1): a lower cobalt concentration determines a red shift of the translational motion of the entire tetrahedral AO4 unit within the lattice [F2g(1) mode] corroborated with the decrease of band intensity. Besides, for x = 0.2 and 0.4, a significant increase of the Eg mode band intensity, which depends on the octahedral sites, is also observed. The occurrence of a shoulder at ca. 580 cm−1 due to the vibration of Al–O bonds and F2g(3) symmetry indicates a change of the octahedral MO6 units and this could also be correlated with a higher concentration of the octahedral Co3+ sites. However, many factors could interfere and deform/shift the Raman vibrations, like cation disorder, non-stoichiometry, or defects as well as the hierarchical arrangement of spinel nanoparticles.64,65 The water-free ZnxCo1−xAl oxides show very weak Raman bands, and only for x = 0 all the vibrations corresponding to the spinel structure (190, 482, 518, 610, 682 and 740 cm−1 respectively; see Fig. S20 of the ESI†) are identified. The gradual incorporation of Zn2+ ions cancels the Raman phonon vibrations, most likely because of the Raman inactive Al2.67O4 formation.66
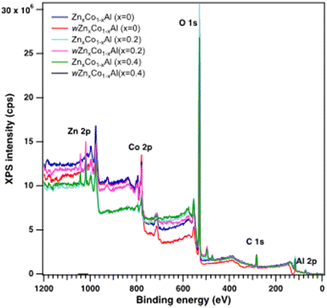 | ||
| Fig. 5 Wide-scan X-ray photoelectron spectra of wZnxCo1−xAl and ZnxCo1−xAl oxides (x = 0, 0.2, 0.4). | ||
 | ||
| Fig. 6 High-resolution Co 2p XPS of the water-assisted wZnxCo1−xAl and water-free ZnxCo1−xAl oxides (x = 0, 0.2 and 0.4). | ||
For Co 2p high resolution data, the multiplet splitting and background subtraction, full width at half maximum (FWHM), relative shifts, relative intensities and component shape were considered.69 Two multiplets were identified (Fig. 6), one corresponding to Co3O4 and the second one corresponding to Co2+ ions.70 A significant concentration of Co3O4 oxide occurred on the surface of the water-added samples, wCo1−xZnxAl, and the Co 2p XPS spectra showed an increased amount of spinel-type cobalt(II/III) oxide (Fig. 6, left). Conversely, only a small amount of Co3O4 was identified on the surface of the materials, in the case of the water-free samples, ZnxCo1−xAl (Fig. 6, right).
The high-resolution O 1s spectra reveal the presence of four O-containing species in all samples (Fig. 7). The first two components observed at low binding energies ∼530.6 eV and ∼531.5 eV can be assigned to the O2− from the Co/Zn–O bond and O2− from the aluminum oxide bond, respectively.71 The third (ca. 532.3 eV) and the fourth (ca. 533.3 eV) components are assigned to the hydroxyl group and H2O adsorbed surface, respectively.71 The zinc-enriched ZnxCo1−xAl (x = 0.2, 0.4) oxides have the highest number of Al–O bonds at the surface. The Al 2p XPS spectra (Fig. 8, left) are shifted up to 1 eV, depending on the type of aluminum-containing phase. Thus, Al3+ ions from the spinel aluminum oxide are usually identified at high values of binding energies (74.3 eV) and have a wider FWHM (∼2.1 eV), while aluminum(III) ions from CoAl2O4 are found at lower values of binding energies (73.4 eV) and have a lower FWHM (with 0.2–0.3 eV).72
 | ||
| Fig. 7 High-resolution O 1s XPS of the water-assisted wZnxCo1−xAl and water-free ZnxCo1−xAl oxides (x = 0, 0.2 and 0.4). | ||
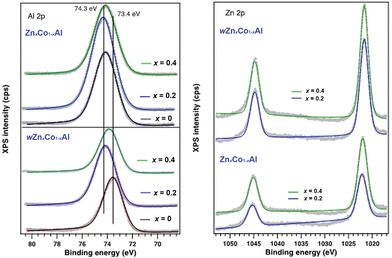 | ||
| Fig. 8 High-resolution: Al 2p (left) and Zn 2p (right) XPS of the water-assisted wZnxCo1−xAl and water-free ZnxCo1−xAl oxides (x = 0, 0.2 and 0.4). | ||
One can observe that stoichiometric wZnxCo1−xAl (x = 0) has the largest amount of CoAl2O4 on the surface, while for the Zn-doped water-free oxide, ZnxCo1−xAl (x = 0.2), the main material on the surface is aluminum oxide. For wZnxCo1−xAl (x = 0.2, 0.4) and ZnxCo1−xAl (x = 0, 0.4) an intermediate ratio of the two aluminum-based phases is established. The Zn 2p high resolution spectra (Fig. 8, right) indicate the presence of Zn2+ found at ∼1021.9 eV (Zn 2p3/2).72,73 No significant changes are observed in the binding energies of both series of the oxides, confirming that the oxidation state is preserved.
The XPS results show that the surface composition of wZnxCo1−xAl, and water free ZnxCo1−xAl oxides (x = 0, 0.2 and 0.4) follows the same trend observed in XRD analysis (on the bulk sample): a predominance of Co-based spinels for water-assisted samples and, for ZnxCo1−xAl oxides, of aluminum oxide.
SEM analysis. The amount of water added to the reaction had a strong impact on the morphology of the calcination products. Thus, the SEM micrographs of the wZnxCo1−xAl oxides revealed the formation of large, irregular micro-aggregates of nanoparticles whose dimensions vary from 5 up to 100 μm. For x = 0 (Fig. 9a–c), the sizes of the aggregates range between 5 and 50 μm and consist of small, spherical nanoparticles. The incorporation of zinc ions into the spinel lattice led to larger, more compact micro-aggregates (Fig. 9d, e, g and h) of nanoparticles (Fig. 9f and i). A rough estimation indicates that the particle size ranges between 8 and 30 nm.
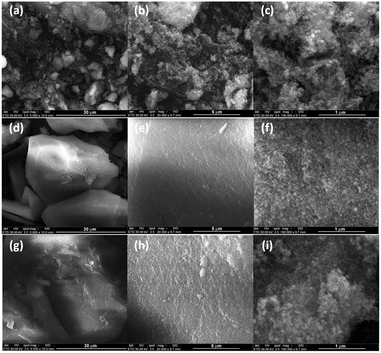 | ||
| Fig. 9 SEM images at different magnifications of water-added wZnxCo1−xAl oxides: (a–c) x = 0; (d–f) x = 0.2 and (g–i) x = 0.4. | ||
The ZnxCo1−xAl oxides exhibit a completely different morphology compared to their water-assisted analogues. Therefore, the zinc-free powder exhibits smaller micro-sized aggregates (from 1 up to ca. 5 μm) with a narrower size distribution, showing silkworm hierarchical cocoon-like shapes composed of very small, spherical particles of Co3O4/CoAl2O4, covered with large flakes of Al2.67O4 (Fig. 10a–f). Generally, the cocoon-type morphology is quite rare for metal oxides74–78 and, to our knowledge, this is the first report of spinel structures with such a hierarchical architecture. For the zinc-containing samples, the aluminum oxide amount shows a significant increase, and the flakes completely cover the zinc/cobalt aluminate cores (Fig. 10d–i). However, FE-SEM investigation is not powerful enough to reveal the structure of these flakes.
 | ||
| Fig. 10 SEM images at different magnifications of water-free ZnxCo1−xAl oxides: (a–c) x = 0; (d–f) x = 0.2, and (g–i) x = 0.4. | ||
The EDX spectra of both wZnxCo1−xAl and ZnxCo1−xAl oxides confirm the incorporation of zinc ions for the powders with x = 0.2 and x = 0.4 (Fig. 11, 12 and Table S2 in the ESI†). The exclusive presence of the Co, Zn, Al and O species that make up the spinel phases rules out any contamination during the synthesis process.
TEM, HRTEM and SAED analyses. In order to have a more realistic image of the morphological details, such as: (i) the size and shape of particles of the different spinel phases obtained by both synthesis paths, (ii) interdistribution of these phases in the powders and (iii) the flake structure for the water-free ZnxCo1−xAl oxides, TEM/HRTEM analyses coupled with EDX mapping in STEM mode are required.
The TEM image of the water-added wZnxCo1−xAl powder reveals the presence of polyhedral spinel particles, with well-defined boundaries and sizes ranging between 7 and 43 nm (Fig. 13a). The Zn2+ addition seems to determine a slight particle size decrease, a higher homogeneity in the size of particles, as well as a gradual change of their shape toward a more rounded morphology as the zinc content increases (Fig. 13d and g). The HRTEM images (Fig. 13b, e and h) of the wZnxCo1−xAl particles show long-range ordered fringes which prove their high crystallinity degree. The aspect of the concentric dashed diffraction rings, consisting of well-defined bright spots in the corresponding SAED patterns (Fig. 13c, f and i), confirms the observations from the HRTEM images regarding the crystallinity of these samples.
 | ||
| Fig. 13 (a), (d) and (g) TEM images; (b), (e) and (h) HRTEM images and (c), (f) and (i) SAED patterns of the water-added wZn1−xCoxAl powders: (a)–(c) x = 0; (d)–(f) x = 0.2 and (g)–(i) x = 0.4. | ||
Significant changes in the morphology and crystallinity of the particles were noticed for the water-free ZnxCo1−xAl powders (Fig. 14a, d and g). Thus, for the zinc-free sample (x = 0), the TEM image of Fig. 11a shows a duplex-type morphology, due to the coexistence of small particles (7–13 nm) and larger ones (30–40 nm), belonging, most likely, to different spinel phases.
 | ||
| Fig. 14 (a), (d) and (g) TEM images; (b), (e) and (h) HRTEM images and (c), (f) and (i) SAED patterns of the water-free Zn1−xCoxAl powders: (a)–(c) x = 0; (d)–(f) x = 0.2 and (g)–(i) x = 0.4. | ||
Indeed, for the sample with Zn2+ content corresponding to the substitution degree of x = 0.2 for which, according to the XRD results, the formation of cobalt-containing spinel phases (CoAl2O4 and Co3O4) is suppressed, so that almost only aluminum-based spinel was identified (see Table 1). The corresponding TEM image of Fig. 14d reveals the exclusive presence of small particles (≤13 nm), which allowed us to conclude that they belong to the Al2.67O4 phase. It is worth mentioning that these small Al2.67O4 particles are agglomerated comprising the thin and transparent flakes noticed in the related FE-SEM image (see Fig. 10f). For the sample with the highest Zn2+ content (x = 0.4), where the amount of Al2.67O4 spinel still prevails over the amounts of the secondary CoAl2O4 and Co3O4 phases, the powder morphology is somewhat similar to the one corresponding to the sample ZnxCo1−xAl (x = 0.2) already discussed above (Fig. 14g). Unlike the water-added samples, in this case, the significantly lower size of the particles strongly affects the crystallinity degree, especially for the Zn2+-containing powders, as the HRTEM images of Fig. 14b, e and h as well as the more diffuse diffraction rings from the SAED patterns of Fig. 14c, f and i suggest.
The STEM images together with the overall and elemental EDX maps for the water-added wZnxCo1−xAl (x = 0, Fig. 15 and x = 0.2, Fig. 16) samples show the presence of all the three spinel phases: ZnxCo1−xAl2O4 (A), Co3O4 (B) and Al2.67O4 (C), in agreement with the results concerning the phase composition obtained by the Rietveld analysis presented in Table 1.
 | ||
| Fig. 15 STEM image and the overall and elemental EDX maps of the water-added wZnxCo1−xAl powder (x = 0, A – CoAl2O4; B – Co3O4 and C – Al2.67O4). | ||
 | ||
| Fig. 16 STEM image and the overall and elemental EDX maps of the water-added wZnxCo1−xAl powder (x = 0.2, A – Zn1−xCoxAl2O4; B – Co3O4 and C – Al2.67O4). | ||
EDX investigations were also performed on the water-free ZnxCo1−xAl (x = 0, Fig. 17 and x = 0.2, Fig. 18) powders. For the Zn2+-free powder, it is obvious that the thin flakes denoted as C in Fig. 17 are exclusively built up of very small Al2.67O4 particles, while larger Co3O4 particles (B) seem to grow on the particles belonging to the major CoAl2O4 phase (A).
 | ||
| Fig. 17 STEM image and the overall and elemental EDX maps of the water-free ZnxCo1−xAl powder (x = 0, A – Zn1−xCoxAl2O4; B – Co3O4 and C – Al2.67O4). | ||
 | ||
| Fig. 18 STEM image and the overall and elemental EDX maps of the ZnxCo1−xAl powder (x = 0.2, A – ZnxCo1−xAl2O4; B – Co3O4 and C – Al2.67O4). | ||
The EDX mapping of the region displayed in the STEM image of Fig. 18 reveals that the ZnxCo1−xAl (x = 0.2) powder consists almost entirely of rows of nanoparticles assembled in thin, planar Al2.67O4 flakes. All these results are also in good agreement with the XRD data and SEM/TEM observations presented above.
Scheme 1 shows the possible reaction pathways for the two series of oxides. The reaction conditions for obtaining the water-assisted wPZnxCo1−xAl and water-free PZnxCo1−xAl precursors are similar (temperature/time of reaction and metal salt/complex concentrations), the water amount from the reaction being the only variable. The synthesis starts with the dissolution of the cobalt/zinc and aluminum salts/complexes, followed by the in situ formation of intermediates that further undergo hydrolysis reaction (H).
The chemical conversion of the cobalt/zinc and aluminum sources is strongly influenced by the different dissociation rates of the metal/salt complexes, and the reaction kinetics is expected to decrease in the following order: Co(CH3COO)2 > Zn(acac)2 > Al(acac)3. The cobalt acetate, with the highest dissociation constant,85 will firstly release CH3COO− anions, whereas Al(acac)3 will be the last to dissociate, being much less labile due to the strong Lewis acid character of Al3+ cations and the three chelating (acac)− ligands.81–85 At the same time, 1,4-BD could act as a ligand toward the metal ions to form complexes. Most likely, the resulting polyol-derived and/or cobalt alkoxide complexes will subsequently evolve into layered cobalt hydroxyacetate species.83 Conversely, the metal acetylacetonates (Zn/Al) undergo hydrolysis via metal enolate complexes that are further hydrolyzed to form M-OH chemical species, with the release of acetone.79,80
The hydrolysis ratio (h) represents the critical factor that causes the differences between the two series of precursors, wPZnxCo1−xAl and PZnxCo1−xAl. In the case of water-assisted synthesis (h = 1.2), the hydrolysis process in 1,4-BD is driven by water in a similar way to the aqueous sol–gel processes,86–88 affording dark-blue gels of wPZnxCo1−xAl (pathway 1 in Scheme 1).
As indicated by the FTIR spectra, in the water-assisted precursors multiple chemical species co-exist: hydroxyacetate, alkoxide and hydroxide intermediates and, also, Zn/Co–O–Al based units resulting from the alcohol condensation reactions (C, see Scheme 1, pathway 1). The thermal treatment gave rise to aggregates of nanoparticles of three-component spinel oxides, Znx/Co1−xAl2O4, Co3O4 and Al2.67O4. Interestingly, the increase of the x value determines a predominance of zinc/cobalt-aluminate spinels (up to 83.3%, see Table 1). This trend in the composition of the material is likely to result from the strong preference of cobalt(II) and zinc(II) for the tetrahedral sites that favors the formation of the spinel aluminate. It is worth mentioning that Co3O4 oxides are rarely obtained through the polyol method.86,90,91
For the PZnxCo1−xAl precursors, the necessary water for the hydrolysis processes results from two sources: crystallization water molecules from hydrated metal salts/complexes (cobalt(II) acetate and zinc(II) acetylacetonate), as well as from the esterification reactions of 1,4-BD (pathway 2 in Scheme 1). Nevertheless, the amount of water generated is insufficient to produce gels, as in water-added reactions, the final products being in the form of light-green powders precipitating from dark-blue solutions. Under such conditions, the nature of the starting materials becomes important, and the different dissociation constants of the reactants (Al(acac)3, Zn(acac)2 and Co(CH3COO)2) explain the simultaneous formation of two phases, Zn/Co-containing intermediates52 and boehmite (see Fig. S2 of the ESI†). Thus, the formation of the PZnxCo1−xAl precursors is likely to follow a three-step process, taking into account the stability of the starting materials: (1) the formation of CoII/ZnII intermediate complexes, followed by the Co(II) hydroxyacetate and Zn–OH species (H1); (2) the subsequent formation of the Al(III) complexes that will be layered upon the Co(II)/Zn(II) intermediates and (3) the preferential hydrolysis of the outer Al(III) complexes (H2). However, the dark-blue colored solutions indicate that a part of the cobalt cations remains in solution, most likely as soluble, stable complexes of cobalt(II) ions and, therefore, the initial stoichiometries (molar ratios) of the zinc/cobalt/aluminum metals will not be the same as those corresponding to PZnxCo1−xAl precursors. The hydrolysis process of the aluminum(III) intermediates yields γ-AlO(OH),89 the flakes of boehmite being attached to the already formed zinc/cobalt intermediates to give rise to silkworm-cocoon-like aggregates (Scheme 1). The thermolysis of the PZnxCo1−xAl precursors at 800 °C generates the defect spinel Al2.67O4 through dehydration of γ-AlO(OH), preserving the flake-like morphology, via a topotactic decomposition.92 Due to the strong preference of the Al3+ for the octahedral environment, during the thermal processing, the ZnII/CoII cations could diffuse and easily occupy the tetrahedral sites within the Al2.67O4 spinel lattice to form simultaneously the isostructural zinc/cobalt aluminate phase,93 affording heterostructures with a unique core–shell arrangement: cobalt aluminate aggregate cores within flake-like aluminum oxide shells. The zinc(II)-incorporation process is accompanied by an increase of Al2.67O4 quantity for x = 0.2, 0.4 which completely embeds the zinc/cobalt cores.
In conclusion, the two reaction pathways and, consequently, the nature of the precursors and the composition of their calcination products depend on the water amount in the reaction, the zinc-incorporation process and the stereochemical preference of the metal ions for the tetrahedral/octahedral sites of the spinel lattice:
- for the water-added synthesis, the Zn/Co–O–Al intermediate units (resulting from the alcohol polycondensation reaction) acted as centers of nucleation for Zn/Co-based spinels. The homogeneous distribution of the Zn/Co centers of nucleation in gels together with the strong preference of Zn(II)/Co(II) to occupy the tetrahedral sites of the spinel lattice favor, after the post-synthesis calcination treatment, the formation and predominance of the Co-based spinel crystalline phases.
- for the water free synthesis, the formation of PZnxCo1−xAl precursors (light green powders) is influenced by the dissociation constant of the starting materials. Thus, the slow hydrolysis of Al(acac)3 with the formation of flake-like γ-AlOOH (boehmite) embedded the incipient Zn/Co–Al intermediates. Surprisingly, the addition of Zn and the corresponding diminishing of Co amount has a reversed effect compared to the water-assisted analogues and led to Zn/Co-cation deficient precursors PZnxCo1−xAl whose thermolysis process led to spinel mixtures in which the aluminum oxide prevailed.
| Sample | Total pore volume (cm3 g−1) | Specific surface area, SBET (m2 g−1) |
|---|---|---|
| wZnxCo1−xAl | ||
| x = 0 | 0.37 | 141.7 |
| x = 0.2 | 0.21 | 60.3 |
| x = 0.4 | 0.13 | 39.8 |
| ZnxCo1−xAl | ||
| x = 0 | 0.37 | 167.3 |
| x = 0.2 | 0.46 | 199.2 |
| x = 0.4 | 0.57 | 208.4 |
The N2 adsorption–desorption isotherms are of type IV according to IUPAC classification and indicate mesoporous materials.94 Depending on the amount of water in the reaction mixture (water-added/water-free), the isotherms show two different types of hysteresis loops that could be further correlated with different pore structures. Thus, for wZnxCo1−xAl oxides, the isotherms exhibit relatively wide and asymmetrical hysteresis loops of type H2 with an adsorption branch characteristic of a broad pore size distribution (PSD) and a steeper evaporation branch (Fig. S22–S24 in the ESI†). This type of hysteresis loop is specific for materials with complex pore networks,94 and their asymmetry suggests an interaction mechanism between pores during the desorption process.95 The zinc(II) incorporation determines an enlargement of PSD (∼4–20 nm vs. 3 to 15 nm) and a shift toward higher values of the peak maxima (∼9 nm vs. 14 nm). For ZnxCo1−xAl oxides, the hysteresis loops are of type H3, specific to slit-like pore shapes, in aggregates of plate-like particles that lose their assemblage (Fig. S25–S27 in the ESI†).94 In this case, the incorporation of zinc ions into the spinel lattice seems to induce less disorder and inhomogeneity of the pore system. Thus, for x = 0, the PSD is wide, ranging from 4 to 30 nm, while for zinc-containing oxides it is narrower, ranging from 3 to 12 nm for x = 0.2 and from 3 to 20 nm for x = 0.4. The textural properties of the two types of cobalt aluminate samples correspond well to the morphological analysis results.
The ZnxCo1−xAl mixed oxides exhibit considerably larger areas compared with water-assisted analogues, the surface increasing with the zinc(II) concentration due to the increased concentration of the defect spinel Al2.67O4. Examples of cobalt aluminate-based oxides with high specific areas are very rare and were obtained via different soft chemistry synthesis (sol–gel,96,97 thermal decomposition of Co–Al hydrotalcite,98 and evaporation induced self-assemble methods99) or supporting the mixed oxides on γ-Al2O3.100 The water-added analogues, wZnxCo1−xAl, exhibit higher SBET values than most of the reported examples of metal aluminates. Besides, the gradual zinc-incorporation causes a reversed evolution compared to the case of water-free derivatives, with the SBET values decreasing with the incorporation degree, probably due to a more compact assembly of oxide nanoparticles and increased crystallite sizes.
| Sample | L* | a* | b* | C* |
|---|---|---|---|---|
| wZnxCo1−xAl | ||||
| x = 0 | 6 | 3 | −28 | 28.16 |
| x = 0.2 | 6 | 3 | −27 | 27.16 |
| x = 0.4 | 6 | 3 | −27 | 27.16 |
| ZnxCo1−xAl | ||||
| x = 0 | 12 | −10 | −43 | 44.14 |
| x = 0.2 | 14 | 11 | −94 | 94.64 |
| x = 0.4 | 14 | 7 | −88 | 88.27 |
The water-assisted oxides have closed values of the colorimetric parameters, with no significant influence of the zinc incorporation within the spinel lattice. All chromatic coordinates of wZnxCo1−xAl oxides are lower compared to those of ZnxCo1−xAl samples, meaning that the water-assisted samples are lighter and less blue and have a lower degree of color saturation than the water-free oxides. Nevertheless, considering the large negative values of b* parameters, the samples are blue shifted.
Conclusions
Two new series of mesoporous spinel-type oxides were obtained through a two-step approach: the hydrolysis of ZnIIx/CoII1−x and AlIII-based salts/complexes in 1,4-butanediol followed by a calcination treatment (x = 0, 0.2, 0.4). For all oxide samples, the XRD analysis Raman and XPS spectroscopy revealed the co-existence of three isostructural spinel crystalline phases: ZnxCo1−xAl2O4, Co3O4 and Al2.67O4. The composition and morpho-structural features of the spinel oxides strongly depend on the water amount added for the polyol-assisted synthesis, as well as on the zinc-doping process. Thus, a reaction in which a small amount of water was added led to gels and further, after the thermal treatment, to irregular aggregates of oxide nanoparticles. For the zinc-enriched samples, Znx/Co1−xAl2O4 phases predominate. Conversely, in a similar water-free synthesis, light green powders were obtained, whose thermolysis gave rise to intense blue spinel oxide nanoparticles, with very small sizes and with a unique silkworm cocoon morphology. For x = 0.2 and 0.4 a significant increase of the aluminum oxide phase content occurred. Our results opened new and interesting perspectives in designing “green” inorganic pigments with a beneficial impact on health, environment, and economy which are outlined here: (i) new compositions for spinel-type pigments; (ii) best coloring properties for a composition with the lowest content of cobalt; (iii) water amount representing the main element of control in polyol-assisted synthesis of spinels; and (iv) high SBET values for water-free oxides due to the prevalence of the alumina phase which could help extend the area of applications toward other domains, like catalysis. But no conclusion can be more beautiful, motivating, and suitable as Vincent van Gogh words: “Cobalt is a divine color and there is nothing so beautiful to represent the atmosphere” (in a letter to his brother Theo).Author contributions
M.-G. A.: investigation and writing – review & editing; A.-C. I.: methodology, writing – review & editing, and supervision; D. C. C., S. P., C. D. E., B. S. V., V. A. S., A.-I. N., F. N. and I. P.: investigation, formal analysis, and validation; D. V. and O. C.: methodology, conceptualization, writing – original draft/review & editing, resources, funding acquisition, project administration, and supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by a grant of the Romanian Ministry of Education and Research, CNCS – UEFISCDI, project number PN-III-P4-ID-PCE-2020-2324, within PNCDI III. This work was carried out within the research program “Green chemistry for the synthesis of materials” of “Ilie Murgulescu” Institute of Physical Chemistry, Romanian Academy.References
- L. Gavilà, A. Lähde, J. Jokiniemi, M. Constanti, F. Medina, E. del Río, D. Tichit and M. G. Álvarez, ChemCatChem, 2019, 11, 4944 CrossRef.
- Y. Liu, L. Jia, B. Hou, D. Sun and D. Li, Appl. Catal., A, 2017, 530, 30 CrossRef CAS.
- A. J. Reynoso, J. L. Ayastuy, U. Iriarte-Velasco and M. A. Gutiérrez-Ortiza, Appl. Catal., B, 2018, 239, 86 CrossRef CAS.
- M. Mosleh, J. Mater. Sci.: Mater. Electron., 2017, 28, 773 CrossRef CAS.
- D. Visinescu, F. Papa, A. C. Ianculescu, I. Balint and O. Carp, J. Nanopart. Res., 2013, 15, 1456 CrossRef.
- D. Li, Y. Li, X. Liu, Y. Guo, C.-W. Pao, J.-L. Chen, Y. Hu and Y. Wang, ACS Catal., 2019, 9, 9671 CrossRef CAS.
- L. Li, D. L. King, Z. Nie, X. S. Li and C. Howard, Energy Fuels, 2010, 24, 3698 CrossRef CAS.
- Y. ElJabbar, H. Lakhlifi, R. ElOuatib, L. Er-Rakho, S. Guillemet-Fritsch and B. Durand, J. Non-Cryst. Solids, 2020, 542, 120115 CrossRef.
- S. D. Kapse, F. C. Raghuwanshi, V. D. Kapse and D. R. Patil, Curr. Appl. Phys., 2012, 12, 307 CrossRef.
- J. P. Morán-Lázaroa, O. Blanco, V. M. Rodríguez-Betancourtt, J. Reyes-Gómez and C. R. Michel, Sens. Actuators, B, 2016, 226, 518 CrossRef.
- P. Panda, R. Mishra, S. Panigrahy and S. Barman, ACS Appl. Nano Mater., 2022, 5, 5176 CrossRef CAS.
- N. Srisawad, W. Chaitree, O. Mekasuwandumrong, P. Praserthdam and J. Panpranot, J. Nanomater., 2012, 108369 Search PubMed.
- D. F. L. Horsth, J. O. Primo, N. Balaba, F. J. Anaissi and C. Bittencourt, RSC Sustainability, 2023, 1, 159 RSC.
- J. R. Hackman, in Pigment Handbook, Volume 1: Properties and Economics, ed. P. A. Lewis, Wiley-Interscience Publication, 2nd edn, 1988, p. 389 Search PubMed.
- A. Zhang, B. Mu, Z. Luo and A. Wang, Dyes Pigm., 2017, 139, 473 CrossRef CAS.
- Sh. Salem, J. Ind. Eng. Chem., 2014, 20, 818 CrossRef CAS.
- F. Tielens, M. Calatayud, R. Franco, J. M. Recio, J. Pérez-Ramírez and C. Minot, J. Phys. Chem. B, 2006, 110, 988 CrossRef CAS PubMed.
- M. Taguchi, T. Nakane, K. Hashi, S. Ohki, T. Shimizu, Y. Sakka, A. Matsushita, H. Abe, T. Funazukuria and T. Naka, Dalton Trans., 2013, 42, 7167 RSC.
- Z. Chen, E. Shi, W. Li, Y. Zheng and W. Zhong, Mater. Lett., 2002, 55, 281 CrossRef CAS.
- W. Li, J. Li and J. Guo, J. Eur. Ceram. Soc., 2003, 23, 2289 CrossRef CAS.
- T. Suzuki, H. Nagai, M. Nohara and H. Takagi, J. Phys.: Condens. Matter, 2007, 19, 145265 CrossRef.
- A. Maljuk, V. Tsurkan, O. Zeharko, A. Cervellino, A. Loidl and D. N. Argyriou, J. Cryst. Growth, 2009, 311, 3997 CrossRef CAS.
- A. Stan, C. Munteanu, A. M. Musuc, R. Birjega, R. Ene, A. Ianculescu, I. Raut, L. Jecu, M. Badea-Doni, E. M. Angel and O. Carp, Dalton Trans., 2015, 44, 7844 RSC.
- B. Serment, C. Brochon, G. Hadziioannou, S. Buffière, A. Demourguesa and M. Gaudon, RSC Adv., 2019, 9, 34125 RSC.
- K. Mokhtari and Sh. Salem, RSC Adv., 2017, 7, 29899 RSC.
- C. M. Alvarez-Docio, J. J. Reinosa, A. del Campo and J. F. Fernández, Dyes Pigm., 2017, 137, 1 CrossRef CAS.
- N. El Habra, L. Crociani, C. Sada, P. Zanella, M. Casarin, G. Rossetto, G. Carta and G. Paolucci, Chem. Mater., 2007, 19, 3381 CrossRef CAS.
- C. Feldmann and H. O. Jungk, Angew. Chem., Int. Ed., 2001, 40, 359 CrossRef CAS PubMed.
- A. Forés, M. Llusar, J. A. Badenes, J. Calbo, M. A. Tena and G. Monrós, Green Chem., 2000, 2, 93 RSC.
- M. De Boeck, M. Kirsch-Volders and D. Lison, Mutat. Res.-Fund. Mol. M., 2003, 533, 135 CrossRef CAS PubMed.
- A. Yurdakul and H. Gocmez, Ceram. Int., 2019, 45, 5398 CrossRef CAS.
- J. Lu, K. Minami, S. Takami and T. Adschiri, Chem. Eng. Sci., 2013, 85, 50 CrossRef CAS.
- R. Dumitru, F. Manea, L. Lupa, C. Pacurariu, A. Ianculescu, A. Baciu and S. Negrea, J. Therm. Anal. Calorim., 2017, 128, 1305 CrossRef CAS.
- A. A. Ali, E. El Fadaly and I. S. Ahmed, Dyes Pigm., 2018, 158, 451 CrossRef CAS.
- A. Tirsoaga, D. Visinescu, B. Jurca, A. Ianculescu and O. Carp, J. Nanopart. Res., 2011, 13, 6397 CrossRef CAS.
- D. Visinescu, B. Jurca, A. Ianculescu and O. Carp, Polyhedron, 2011, 30, 2824 CrossRef CAS.
- S. Zhao, J. Guob, W. Lia, H. Guo and B. You, Dyes Pigm., 2018, 151, 130 CrossRef CAS.
- D. Visinescu, C. Paraschiv, A. Ianculescu, B. Jurca, B. Vasile and O. Carp, Dyes Pigm., 2010, 87, 125 CrossRef CAS.
- D. Visinescu, G. Patrinoiu, A. Tirsoaga and O. Carp, in Environmental chemistry for a sustainable world, ed. E. Lichtfouse, J. Schwarbauer and D. Roberts, Springer, Dordrecht, Heidelberg, London, New York, 2013, ch. 5, p. 119 Search PubMed.
- T. C. de Sousa Santos, D. do Rosario Pinheiro, C. M. L. Costa, D. C. Estumano and N. F. da Paixão Ribeiro, Ceram. Int., 2020, 46, 2332 CrossRef.
- R. Ianos, E. Muntean, C. Păcurariu, R. Lazău, C. Bandas and G. Delinescu, Dyes Pigm., 2017, 142, 24 CrossRef CAS.
- T. Nakane, T. Naka, K. Sato, M. Taguchi, M. Nakayama, T. Mitsui, A. Matsushita and T. Chikyowa, Dalton Trans., 2015, 44, 997 RSC.
- N. Zhou, Y. Li, Y. Zhang, Y. Shu, S. Nian, W. Cao and Z. Wu, Dyes Pigm., 2018, 148, 25 CrossRef CAS.
- F. Fiévet, S. Ammar-Merah, R. Brayner, F. Chau, M. Giraud, F. Mammeri, J. Peron, J.-Y. Piquemal, L. Sicard and G. Viau, Chem. Soc. Rev., 2018, 47, 5187 RSC.
- H. Dong, Y.-C. Chen and C. Feldmann, Green Chem., 2015, 17, 4107 RSC.
- N. Pinna and M. Niederberger, Angew. Chem., Int. Ed., 2008, 47, 5292 CrossRef CAS PubMed.
- A. Kumar, A. Daverey, N. K. Sahu and D. Bahadur, J. Mater. Chem. B, 2013, 1, 3652 RSC.
- K. Vamvakidis, M. Katsikini, G. Vourlias, M. Angelakeris, E. C. Paloura and C. Dendrinou-Samara, Dalton Trans., 2015, 44, 5396 RSC.
- J. Bai, X. Li, G. Liu, Y. Qian and S. Xiong, Adv. Funct. Mater., 2014, 24, 3012 CrossRef CAS.
- J. Merikhi, H.-O. Jungk and C. Feldmann, J. Mater. Chem., 2000, 10, 1311 RSC.
- C. Feldmann, Adv. Mater., 2001, 13, 1301 CrossRef CAS.
- D. Visinescu, M. Scurtu, R. Negrea, R. Birjega, D. C. Culita, M. C. Chifiriuc, C. Draghici, J. Calderon Moreno, A. M. Musuc, I. Balint and O. Carp, RSC Adv., 2015, 5, 99976 RSC.
- D. Visinescu, M. D. Hussien, J. Calderon Moreno, R. Negrea, R. Birjega, S. Somacescu, C. D. Ene, M. C. Chifiriuc, M. Popa, M. S. Stan and O. Carp, Langmuir, 2018, 34, 13638 CrossRef CAS PubMed.
- A. A. Khassin, V. F. Anufrienko, V. N. Ikorskii, L. M. Plyasova, G. N. Kustova, T. V. Larina, I. Yu. Molina and V. N. Parmon, Phys. Chem. Chem. Phys., 2002, 4, 4236 RSC.
- J. Zhang, S. Wei, J. Lin, J. Luo, S. Liu, H. Song, E. Elawad, X. Ding, J. Gao, S. Qi and C. Tang, J. Phys. Chem. B, 2006, 110, 21680 CrossRef CAS PubMed.
- X. Bai, G. Caputo, Z. Hao, V. T. Freitas, J. Zhang, R. L. Longo, O. L. Malta, R. A. S. Ferreira and N. Pinna, Nat. Commun., 2014, 5, 5702 CrossRef CAS PubMed.
- U. Lavrenčič Štangar, B. Orel and M. Krajnc, J. Sol-Gel Sci. Technol., 2003, 26, 771 CrossRef.
- M. Zayat and D. Levy, Chem. Mater., 2000, 12, 2763 CrossRef CAS.
- F. Tielens, M. Calatayud, R. Franco, J. M. Recio, J. Pérez Ramírez and C. Minot, J. Phys. Chem. B, 2006, 110, 988 CrossRef CAS PubMed.
- A. M. Wahba, N. G. Imam and M. B. Mohamed, J. Mol. Struct., 2016, 1105, 61 CrossRef CAS.
- V. G. Hadjiev, M. N. Iliev and I. V. Vergilov, J. Phys. C: Solid State Phys., 1988, L199 CrossRef.
- C. M. Álvarez-Docio, J. J. Reinosa, A. Del Campo and J. F. Fernández, J. Alloys Compd., 2019, 779, 244 CrossRef.
- B. Jongsomjit, J. Panpranot and J. J. Goodwin, J. Catal., 2001, 204, 98 CrossRef CAS.
- M. Bouchard and A. Gambardella, J. Raman Spectrosc., 2010, 41, 1477 CrossRef.
- A. Rahman, M. S. Charoo and R. Jayaganthan, Mater. Technol., 2015, 30, 168 CrossRef CAS.
- J. Gangwar, B. K. Gupta, S. K. Tripathi and A. K. Srivastava, Nanoscale, 2015, 7, 13313 RSC.
- S. Petrescu, S. Avramescu, A. M. Musuc, F. Neatu, M. Florea and P. Ionita, Mater. Res. Bull., 2020, 122, 110643 CrossRef CAS.
- E. Pargoletti, U. H. Hossain, I. Di Bernardo, H. Chen, T. Tran-Phu, G. L. Chiarello, J. Lipton-Duffin, V. Pifferi, A. Tricoli and G. Cappelletti, ACS Appl. Mater. Interfaces, 2020, 12, 39549 CrossRef CAS PubMed.
- M. C. Biesinger, B. P. Payne, A. P. Grosvenor, L. W. M. Lau, A. R. Gerson and R. St. C. Smart, Appl. Surf. Sci., 2011, 257, 2717 CrossRef CAS.
- A. Celebioglu, S. Vempati, C. Ozgit-Akgun, N. Biyikli and T. Uyar, RSC Adv., 2014, 4, 61698 RSC.
- H. Jian-kang, J. Li-tao, H. Bo, L. De-bao, L. Yan and L. Ya-chun, J. Fuel Chem. Technol., 2015, 43, 846 CrossRef.
- B. R. Strohmeier, Surf. Sci. Spectra, 1994, 3, 128 CrossRef CAS.
- E. Agostinelli, C. Batistooni, D. Fiorani, G. Mattogno and M. Nogue, J. Phys. Chem. Solids, 1989, 50, 269 CrossRef CAS.
- J. Ji, W. Zhu, J. Li, P. Wang, Y. Liang, W. Zhang, X. Yin, B. Wu and G. Li, ACS Appl. Mater. Interfaces, 2017, 9, 19124 CrossRef CAS PubMed.
- F.-H. Du, Y. Ni, Y. Wang, D. Wang, Q. Ge, S. Chen and H. Y. Yang, ACS Nano, 2017, 11, 8628 CrossRef CAS PubMed.
- J. Zhu, K. Y. Simon Ng and D. Deng, ACS Appl. Mater. Interfaces, 2014, 6, 2996 CrossRef CAS PubMed.
- Q. Feng, M. Hirasawa, K. Kajiyoshi and K. Yanagisawa, J. Mater. Sci., 2007, 42, 640 CrossRef CAS.
- X. Yu, J. He, D. Wang, Y. Hu, H. Tian and Z. He, J. Phys. Chem. C, 2012, 116, 851 CrossRef CAS.
- D. Larcher, G. Sudant, R. Patrice and J. M. Tarascon, Chem. Mater., 2003, 15, 3543 CrossRef CAS.
- L. Poul, N. Jouini and F. Fiévet, Chem. Mater., 2000, 12, 3123 CrossRef CAS.
- R. J. Joseyphus, T. Matsumoto, H. Takahashi, D. Kodama, K. Tohji and B. Jeyadevan, J. Solid State Chem., 2007, 180, 3008 CrossRef CAS.
- K. J. Carroll, J. U. Reveles, M. D. Shultz, S. N. Khanna and E. E. Carpenter, J. Phys. Chem. C, 2011, 115, 2656 CrossRef CAS.
- T. Matsumoto, K. Takahashi, K. Kitagishi, K. Shinoda, J. L. Cuya Huaman, J.-Y. Piquemal and B. Jeyadevan, New J. Chem., 2015, 39, 5008 RSC.
- K. Takahashi, S. Yokoyama, T. Matsumoto, J. L. Cuya Huaman, H. Kaneko, J.-Y. Piquemal, H. Miyamura and J. Balachandran, New J. Chem., 2016, 40, 8632 RSC.
- H. Kaneko, T. Matsumoto, J. L. Cuya Huaman, M. Ishijima, K. Suzuki, H. Miyamura and J. Balachandran, Inorg. Chem., 2021, 60, 3025 CrossRef CAS PubMed.
- R. Deshmukh and M. Niederberger, Chem. – Eur. J., 2017, 23, 8542 CrossRef CAS PubMed.
- B. Ludi and M. Niederberger, Dalton Trans., 2013, 42, 12554 RSC.
- J. Livage and C. Sanchez, J. Non-Cryst. Solids, 1992, 145, 11 CrossRef CAS.
- S. Zhou, M. Antonietti and M. Niederberger, Small, 2007, 3, 763 CrossRef CAS PubMed.
- L. T. Anh, A. K. Rai, T. V. Thi, J. Gim, S. Kim, V. Mathew and J. Kim, J. Mater. Chem. A, 2014, 2, 6966 RSC.
- A.-M. Cao, J.-S. Hu, H.-P. Liang, W.-G. Song, L.-J. Wan, X.-L. He, X.-G. Gao and S.-H. Xia, J. Phys. Chem. B, 2006, 110, 15858 CrossRef CAS PubMed.
- W. Q. Jiao, X. M. Liang, Y. M. Wang and M. Y. He, CrystEngComm, 2014, 16, 3348 RSC.
- A. Dandapat and G. De Besides, ACS Appl. Mater. Interfaces, 2012, 4, 228 CrossRef CAS PubMed.
- K. S. W. Sing, D. H. Everett, R. A. W. Haul, L. Moscou, R. A. Pierotti, J. Rouquerol and T. Siemieniewska, Pure Appl. Chem., 1985, 57, 603 CrossRef CAS.
- A. Grossman and C. Ortega, Langmuir, 2008, 24, 3977 CrossRef PubMed.
- E. Escalona Platero, C. Otero Areán and J. B. Parra, Res. Chem. Intermed., 1999, 25, 187 CrossRef CAS.
- C. Otero Areán, M. Peñarroya Mentruit, E. Escalona Platero, F. X. Llabrés i Xamena and J. B. Parra, Mater. Lett., 1999, 39, 22 CrossRef.
- W. Xu, X. Liu, J. Ren, H. Liu, Y. Ma, Y. Wang and G. Lu, Microporous Mesoporous Mater., 2011, 142, 251 CrossRef CAS.
- L. Xu, J. Zhang, F. Wang, K. Yuan, L. Wang, K. Wu, G. Xua and W. Chen, RSC Adv., 2015, 5, 48256 RSC.
- A. A. S. Gonçalves, M. J. F. Costa, L. Zhang, F. Ciesielczyk and M. Joroniec, Chem. Mater., 2018, 30, 436 CrossRef.
- J. M. Saniger, Mater. Lett., 1995, 22, 109 CrossRef CAS.
- G. B. Kunde, G. D. Yadava and A. K. Ganguli, J. Environ. Chem. Eng., 2019, 7, 102834 CrossRef.
- U. L. Stangar, B. Orel, M. Krajnc, R. C. Korošec and P. Bukovec, Mater. Tehnol., 2002, 36, 387 Search PubMed.
- A. Nakatsuka, Y. Ikeda, Y. Yamasaki, N. Nakayama and T. Mizota, Solid State Commun., 2003, 128, 85 CrossRef CAS.
- T. Tatarchuka, A. Shyichuk, J. Lamkiewicz and J. Kowalik, Ceram. Int., 2020, 46, 14674 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: FTIR spectra (Fig. S1 and S2) and NIR-UV-Vis spectra (Fig. S3 and S4) of wPZnxCo1−xAl and PZnxCo1−xAl. XRD patterns (Fig. S5), PL spectra (Fig. S6), SEM micrographs (Fig. S7 and S8) and EDX analysis results (Fig. S9) of PZnxCo1−xAl. TG, DTG and DSC curves of wPZnxCo1−xAl (Fig. S10 and S11) and PZnxCo1−xAl (Fig. S12 and S13) precursors (x = 0.2, 0.4). Results of the Rietveld refinement on the XRD data for wZnxCo1−xAl (Fig. S14–S16) and ZnxCo1−xAl (Fig. S17–S19) oxides. Raman spectra for ZnxCo1−xAl oxides (Fig. S20). High-resolution C 1s XPS spectra for wZnxCo1−xAl and ZnxCo1−xAl oxides (Fig. S21). N2 adsorption–desorption isotherms of wZnxCo1−xAl (Fig. S22–S24) and ZnxCo1−xAl (Fig. S25–S27) oxides. Overlaid FTIR spectra (Fig. S28–S30) and NIR-UV-Vis spectra (Fig. S31 and S32) of wZnxCo1−xAl and ZnxCo1−xAl oxides, schematic representation of the two-step polyol-assisted reaction including pictures of the water-added, wPZnxCo1−xAl, and water free, PZnxCo1−xAl, precursors and their thermolysis products, wZnxCo1−xAl and ZnxCo1−xAl oxides (Fig. S33). Results of the XPS survey scan (Table S1) and EDX energies (eV) and atomic ratio (%) of water-added wZnxCo1−xAl and water-free ZnxCo1−xAl oxides (Table S2). See DOI: https://doi.org/10.1039/d3dt00972f |
| This journal is © The Royal Society of Chemistry 2023 |