Open Access Article
Open Access ArticleOne silicon atom of bis(silylene) functions as a selective Lewis base under adduct formation with a Lewis acid†
Yi
Ding
*ab,
Mohd
Nazish
 b,
Paul Niklas
Ruth
b,
Regine
Herbst-Irmer
b,
Paul Niklas
Ruth
b,
Regine
Herbst-Irmer
 b,
Dietmar
Stalke
b,
Dietmar
Stalke
 *b and
Herbert W.
Roesky
*b and
Herbert W.
Roesky
 *b
*b
aCollege of Chemistry and Chemical Engineering, State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering, Ningxia University, Yinchuan 750021, China. E-mail: yiding@nxu.edu.cn
bInstitute of Inorganic Chemistry, Georg-August-Universität Göttingen, Göttingen Tammannstrasse 4, D-37077, Germany. E-mail: dstalke@chemie.uni-goettingen.de; hroesky@gwdg.de
First published on 15th April 2023
Abstract
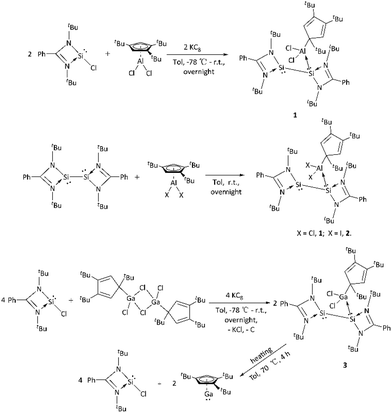
Herein, we describe the facile and selective one-pot synthetic route to silylene-aluminum and silylene-gallium adducts. Reduction of silylene LSiCl (L = PhC(NtBu)2) with KC8 in the presence of bulky and sterically hindered cyclopentadienyl aluminum Cp′′′AlCl2 (Cp′′′ = 1,2,4-tBu3C5H2) and gallium [η1-Cp′′′Ga(μ-Cl)Cl]2 to afford the Lewis acid–base adducts η1-Cp′′′M(Cl2) ← Si(L)-SiL (M = Al, 1; M = Ga, 3). To confirm the formation of the Lewis acid–base adduct, the bis(silylene) LSi(I)-Si(I)L reacts with Cp′′′AlI2 to form η1-Cp′′′Al(I2) ← Si(L)-SiL (2). These are the first examples where one Si atom in the bis(silylene) is a Lewis base and coordinates with aluminum or gallium to form a Lewis acid–base adduct, while the other Si atom in the bis(silylene) still maintains the characteristics of silylene. Compound 3 was heated to 70 °C in toluene for 4 hours and decomposed into the silylene LSiCl and Cp′′′GaI. Compounds 1–3 are well characterized with NMR spectroscopic methods and single-crystal X-ray structural analysis.
Introduction
As a silicon analog of carbene, silylene has a relatively large ΔES–T energy gap and displays fascinating reactivity.1 Bearing vacant orbitals as well as reactive lone pairs of electrons at silicon results in the silylene that can act as a Lewis acid as well as a Lewis base. Silylenes have been widely explored for the formation of transition metal complexes.2 In contrast, the subject of main group metals with silylene or bis(silylene) adducts are not extensively explored. Intrinsically group 13 compounds possess Lewis acidity. Silylene is reported to form Lewis acid–base adducts with organoboron compounds. A number of literature reports on silylene-boron adducts are well documented.3–10In comparison with silylene-boron adducts, reports on silylene-aluminum, silylene-gallium, and silylene-indium are rare (Fig. 1). In 2016, Tacke and co-workers reported the first silylene-aluminum adduct (A) with a Si–Al bond derived from a bis(guanidinato)silylene.4b In 2019, we treated LSi-SiL with AlMe3 to afford the adduct LSi(AlMe3)-Si(AlMe3)L (L = PhC(NtBu)2) (B).11 In addition to that we have also reported adduct C containing an organoaluminum dichloride of a mixed-donor ligand LSiC6H4PPh2.5a In 2021, Radius and co-workers reported the adducts D, which were derived from the reactions with N-heterocyclic silylene (Dipp2NHSi) and AlI3 or Al(C6F5)3.12 Recently, Khan and co-workers reported the reaction of disilene (TMS)2N(η1-Cp*)Si![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Si(η1-Cp*)N(TMS)2 with AlX3 (X = Cl or Br) to form adduct E.13 However, the reactions of the stable N-heterocyclic silylene L′Si: (L′ = (ArN)C(
Si(η1-Cp*)N(TMS)2 with AlX3 (X = Cl or Br) to form adduct E.13 However, the reactions of the stable N-heterocyclic silylene L′Si: (L′ = (ArN)C(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2)CH
CH2)CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(Me)-(NAr), Ar = 2,6-iPr2C6H3) with AlMe3 or AlH3·NMe3 leads directly to the 1,1-addition product F.14 In 2013, Aldridge and co-workers reported the silylene-gallium adduct G with metathesis steps from the Si(IV) precursor Si{N(SiMe3)Dipp}Br3.15 In 2017, Driess and co-workers treated LSi-R-BMes2 (L = PhC(NtBu)2; R = 1,12-xanthendiyl spacer; Mes = 2,4,6-Me3C6H2) with equivalent amounts of GaCl3, which resulted in the SiII → GaCl3 adduct (H).16 We have also reported a silylene-gallium adduct I.5a In 2018, Sen and co-worker prepared the first silylene-indium complex J.17
C(Me)-(NAr), Ar = 2,6-iPr2C6H3) with AlMe3 or AlH3·NMe3 leads directly to the 1,1-addition product F.14 In 2013, Aldridge and co-workers reported the silylene-gallium adduct G with metathesis steps from the Si(IV) precursor Si{N(SiMe3)Dipp}Br3.15 In 2017, Driess and co-workers treated LSi-R-BMes2 (L = PhC(NtBu)2; R = 1,12-xanthendiyl spacer; Mes = 2,4,6-Me3C6H2) with equivalent amounts of GaCl3, which resulted in the SiII → GaCl3 adduct (H).16 We have also reported a silylene-gallium adduct I.5a In 2018, Sen and co-worker prepared the first silylene-indium complex J.17
Since we report on the silylene LSiCl,18 many groups have used this precursor for preparing a number of unprecedented compounds.19 Later on, we reported the bis(silylene) LSi-SiL,20 which displayed a silicon(I) dimer, that comprises of a Si–Si single bond with a lone pair of electrons on each silicon center showing very high reactivity.21 Since then this high reactivity of silylene and bis(silylene) were explored by our group and others. However, the reports of the Lewis acid–base adducts based on the silylene or bis(silylene) are limited.
In this report, we reduced the silylene LSiCl with an equivalent amount of KC8 in the presence of half equivalent of bulky and sterically hindered cyclopentadienyl Cp′′′MCl2 (M = Al or Ga) to afford interesting Lewis acid–base adducts η1-Cp′′′M(Cl2) ← Si(L)-SiL. To confirm the formation of this type of novel Lewis acid–base adducts, bis(silylene) LSi-SiL was treated with equivalent amounts of Cp′′′AlI2 to get η1-Cp′′′Al(I2) ← Si(L)-SiL. The new compounds are well characterized by NMR spectroscopic and single-crystal X-ray analyses.
Results and discussion
Reduction of LSiCl proceeds with equivalent amounts of KC8 in the presence of 0.5 equivalents of Cp′′′AlCl2 in the toluene at −78 °C. Then the reaction was slowly warmed up to room temperature and stirring was continued overnight to afford the Lewis acid–base adduct η1-Cp′′′Al(Cl2) ← Si(L)-SiL (1) as colorless crystals in 52% yield (Scheme 1).The 1H NMR spectrum of 1 shows two singlets at δ 1.25 and 1.36 ppm for the tBu of amidinato ligand, two singlets at δ 1.64 and 1.75 ppm for the tBu of cyclopentadienyl ligand. One broad resonance at δ 6.79–6.85 ppm for cyclopentadienyl, and two broad resonances at δ 6.92–7.02 and 7.23–7.25 ppm for phenyl protons. The 29Si NMR shows two resonances at 49.2 and 57.0 ppm caused by the different chemical environments of the two Si atoms in the compound 1, both shift up-field in comparison to the bis(silylene) (76.3 ppm).
Compound 1 crystallizes in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one molecule and two half toluene solvent molecules in the asymmetric unit. The X-ray crystallography of 1 clearly shows the coordination of the Lewis acid Cp′′′AlCl2 with bis(silylene) LSi-SiL to form the Lewis acid–base adduct (Fig. 2). It is noteworthy that in the bis(silylene) LSi-SiL, one Si atom adopts a four-coordinate arrangement as a Lewis base, while another Si atom also holds a lone pair of electrons with a three-coordinate character. However, the two Si atoms are in an oxidation state I, this is a very rare situation. In the Lewis acid part, the Al atom adopts a four-coordinate oxidation state III. The Al2–Si2 (2.5413(9) Å) bond length is significantly longer than the reported Al–Si covalent bond in compounds [LAlHSiH2Mes]2 (2.4602(8) Å)11 and LAl(SiH2SiH2)2AlL (2.4473(9)–2.4545(9) Å).11 Furthermore, other selected examples with Si–Al bonds are mentioned in brackets (F (2.487(1) Å),14 NacnacAlHSiH2Ph (2.4522(8) Å), and NacnacAlHSiHMePh (2.4548(7) Å)).22 However, the Al–Si coordination bond length which is reported in the following examples is longer in LSi(AlMe3)-Si(AlMe3)L (2.5921(7) and 2.5799(8) Å),11 [PhC(NiPr)2]2Si → AlPh3 (2.5293(14) Å) and [C(NiPr)3]2Si → AlPh3 (2.5544(17) Å).4b This indicates that the Al–Si bond in compound 1 is a coordination bond. The Si1–Si2 bond length is 2.4244(10) Å, which is longer than the corresponding compound in LSi-SiL (2.413(2) Å),20 LSi(AlMe3)-Si(AlMe3)L (2.3937(7) Å),11 and LSi(M)-Si(M)L (M = Ir, 2.376(5) Å; M = Rh, 2.388(2) Å).23 Since only Si2 in LSi-SiL is coordinated with Al from the Cp′′′Al(Cl2), the Si–N bond lengths and the N–Si–N bond angles in the two amidinato silicon four-membered rings are different. The Si2–N bond length (Si2–N3 1.8295(11) Å, Si2–N4 1.8522(11) Å) are shorter than Si1–N (Si1–N1 1.8667(12) Å, Si1–N2 1.8691(11) Å), which makes the bond angle N3–Si2–N4 (71.08(5)°) to be slightly bigger than N1–Si1–N2 (69.50(5)°). It is also noteworthy that the Cp′′′AlCl2 changes from η5 to η1.
with one molecule and two half toluene solvent molecules in the asymmetric unit. The X-ray crystallography of 1 clearly shows the coordination of the Lewis acid Cp′′′AlCl2 with bis(silylene) LSi-SiL to form the Lewis acid–base adduct (Fig. 2). It is noteworthy that in the bis(silylene) LSi-SiL, one Si atom adopts a four-coordinate arrangement as a Lewis base, while another Si atom also holds a lone pair of electrons with a three-coordinate character. However, the two Si atoms are in an oxidation state I, this is a very rare situation. In the Lewis acid part, the Al atom adopts a four-coordinate oxidation state III. The Al2–Si2 (2.5413(9) Å) bond length is significantly longer than the reported Al–Si covalent bond in compounds [LAlHSiH2Mes]2 (2.4602(8) Å)11 and LAl(SiH2SiH2)2AlL (2.4473(9)–2.4545(9) Å).11 Furthermore, other selected examples with Si–Al bonds are mentioned in brackets (F (2.487(1) Å),14 NacnacAlHSiH2Ph (2.4522(8) Å), and NacnacAlHSiHMePh (2.4548(7) Å)).22 However, the Al–Si coordination bond length which is reported in the following examples is longer in LSi(AlMe3)-Si(AlMe3)L (2.5921(7) and 2.5799(8) Å),11 [PhC(NiPr)2]2Si → AlPh3 (2.5293(14) Å) and [C(NiPr)3]2Si → AlPh3 (2.5544(17) Å).4b This indicates that the Al–Si bond in compound 1 is a coordination bond. The Si1–Si2 bond length is 2.4244(10) Å, which is longer than the corresponding compound in LSi-SiL (2.413(2) Å),20 LSi(AlMe3)-Si(AlMe3)L (2.3937(7) Å),11 and LSi(M)-Si(M)L (M = Ir, 2.376(5) Å; M = Rh, 2.388(2) Å).23 Since only Si2 in LSi-SiL is coordinated with Al from the Cp′′′Al(Cl2), the Si–N bond lengths and the N–Si–N bond angles in the two amidinato silicon four-membered rings are different. The Si2–N bond length (Si2–N3 1.8295(11) Å, Si2–N4 1.8522(11) Å) are shorter than Si1–N (Si1–N1 1.8667(12) Å, Si1–N2 1.8691(11) Å), which makes the bond angle N3–Si2–N4 (71.08(5)°) to be slightly bigger than N1–Si1–N2 (69.50(5)°). It is also noteworthy that the Cp′′′AlCl2 changes from η5 to η1.
To confirm the formation of Lewis acid–base adduct, we also tried the reaction of bis(silylene) LSi-SiL with Cp′′′AlI2 at room temperature overnight, and then isolated the light yellow crystals η1-Cp′′′Al(I2) ← Si(L)-SiL (2) in the yield of 45% (Scheme 1). The 1H NMR spectrum of 2 shows four singlets at δ 1.21, 1.42, 1.66, and 1.76 ppm for the tBu of amidinato and cyclopentadienyl ligand. One broad resonance at δ 6.88–6.95 ppm for cyclopentadienyl, two broad resonances at δ 6.97–7.06 and 7.20–7.25 ppm for phenyl protons. The 29Si NMR shows two resonances at 47.8 and 54.4 ppm for the two Si atoms in compound 2, which shift to up-field in comparison to compound 1 (49.2 and 57.0 ppm) and the starting material bis(silylene) (76.3 ppm).
2 crystallizes isostructurally to 1 (Fig. 3) in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) with one molecule and two toluene solvent molecules. Due to the larger radius of the two iodine atoms connected to Al in the Lewis acid part, the bond lengths of Al–Si (2.5505(9) Å) and Si–Si (2.4399(8) Å) are both slightly longer than those in compound 1, while all other features mentioned above for 1 are identical.
with one molecule and two toluene solvent molecules. Due to the larger radius of the two iodine atoms connected to Al in the Lewis acid part, the bond lengths of Al–Si (2.5505(9) Å) and Si–Si (2.4399(8) Å) are both slightly longer than those in compound 1, while all other features mentioned above for 1 are identical.
To further elaborate the formation of Lewis acid–base adduct, we repeated the reaction of LSiCl with equivalent amounts of KC8 and 0.25 equivalents of [η1-Cp′′′Ga(μ-Cl)Cl]2 in toluene at −78 °C. From this reaction we obtained as a Lewis acid–base adduct η1-Cp′′′Ga(Cl2) ← Si(L)-SiL (3) as colorless crystals in 65% yield. The 1H NMR spectrum of 3 shows two singlets at δ 1.25 ppm and 1.37 ppm for the tBu of amidinato ligand, two singlets at δ 1.63 ppm and 1.72 ppm for the tBu of cyclopentadienyl ligand. One broad resonance at δ 6.81–6.87 ppm for cyclopentadienyl, two broad resonances at δ 6.92–7.01, 7.10–7.13, and 7.22–7.24 ppm for phenyl protons. The 29Si NMR shows two resonances at 41.1 and 54.7 ppm for the two Si atoms with the different chemical environments in compound 3, which also shift to up-field in comparison to the aluminum compound 1 and starting material bis(silylene) (76.3 ppm).
Compound 3 crystallizes again isostructurally to 1 (Fig. 4) in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , however with two molecules and four toluene solvent molecule in the asymmetric unit. Interestingly, the dimer gallium [η1-Cp′′′Ga(μ-Cl)Cl]2 was split into the monomers Cp′′′GaCl2 by the bis(silylene) to form compound 3. In the Lewis acid part, the Ga atom is four-coordinated in oxidation state III. The Si2–Ga1 bond length (2.4910(7) Å) is longer than those in adducts of G (2.438(1) and 2.452(1) Å),15H (2.3963(17) Å),16 and I (2.3904(6) Å),5a but shorter than the Si–Al bond length in compounds 1 (2.5413(9) Å) and 2 (2.5506 Å). However, the Si1–Si2 bond length (2.4339(7) Å) is longer than in the compound of LSi-SiL (2.413(2) Å),20 the adduct LSi(AlMe3)-Si(AlMe3)L (2.3937(7) Å),11 LSi(M)-Si(M)L (M = Ir, 2.376(5) Å; M = Rh, 2.388(2) Å).23 The above mentioned differences in the two amidinato silicon rings are again valid.
, however with two molecules and four toluene solvent molecule in the asymmetric unit. Interestingly, the dimer gallium [η1-Cp′′′Ga(μ-Cl)Cl]2 was split into the monomers Cp′′′GaCl2 by the bis(silylene) to form compound 3. In the Lewis acid part, the Ga atom is four-coordinated in oxidation state III. The Si2–Ga1 bond length (2.4910(7) Å) is longer than those in adducts of G (2.438(1) and 2.452(1) Å),15H (2.3963(17) Å),16 and I (2.3904(6) Å),5a but shorter than the Si–Al bond length in compounds 1 (2.5413(9) Å) and 2 (2.5506 Å). However, the Si1–Si2 bond length (2.4339(7) Å) is longer than in the compound of LSi-SiL (2.413(2) Å),20 the adduct LSi(AlMe3)-Si(AlMe3)L (2.3937(7) Å),11 LSi(M)-Si(M)L (M = Ir, 2.376(5) Å; M = Rh, 2.388(2) Å).23 The above mentioned differences in the two amidinato silicon rings are again valid.
We heated compound 3 in toluene to 70 °C and kept it for 4 hours, hoping to get some novel structures through rearrangement, but it was found to be decomposed into the silylene LSiCl and Cp′′′GaI according to the NMR spectroscopic methods (Scheme 1).
Conclusions
In summary, we reported the reduction of silylene LSiCl with KC8 in the presence of large sterically hindered cyclopentadienyl aluminum or gallium dichloride to form three novel silylene-aluminum or silylene-gallium Lewis acid–base adducts. In the adducts, one silicon atom in the bis(silylene) is a Lewis base coordinated with large sterically hindered cyclopentadienyl aluminum dihalide or gallium dichloride. One silicon atom in bis(silylene) acts as a Lewis base, while another silicon atom also maintains the characteristics of silylene, making the two amidino silicon four-membered rings in bis(silylene) showing regular changes.Experimental section
All manipulations were carried out using standard Schlenk and glove-box techniques under an atmosphere of high-purity dinitrogen. THF, hexane, and toluene, respectively, were distilled over Na/K alloy (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 75), while diethyl ether was distilled over potassium mirror. Deuterated NMR solvent C6D6 was dried by stirring for 2 days over Na/K alloy followed by distillation in a vacuum and degassing. 1H, 13C{1H}, and 29Si NMR spectra were recorded on Bruker Avance 400 MHz NMR spectrometer and were referenced to the resonances of the solvent used. Microanalyses were performed by the Analytisches Labor für Anorganische Chemie der Universität Göttingen. Melting points were determined in sealed glass capillaries under dinitrogen, and are uncorrected. The starting material LSiCl,18 LSi-SiL,20 η5-Cp′′′AlX2 and [η1-Cp′′′Ga(μ-Cl)Cl]2 were synthesized by following the literature procedure.24 All other reagents were used as received.
75), while diethyl ether was distilled over potassium mirror. Deuterated NMR solvent C6D6 was dried by stirring for 2 days over Na/K alloy followed by distillation in a vacuum and degassing. 1H, 13C{1H}, and 29Si NMR spectra were recorded on Bruker Avance 400 MHz NMR spectrometer and were referenced to the resonances of the solvent used. Microanalyses were performed by the Analytisches Labor für Anorganische Chemie der Universität Göttingen. Melting points were determined in sealed glass capillaries under dinitrogen, and are uncorrected. The starting material LSiCl,18 LSi-SiL,20 η5-Cp′′′AlX2 and [η1-Cp′′′Ga(μ-Cl)Cl]2 were synthesized by following the literature procedure.24 All other reagents were used as received.
Synthesis of η1-Cp′′′Al(Cl2) ← Si(L)-SiL (1)
To a mixture of LSiCl (294 mg, 1.0 mmol), KC8 (135 mg, 1.0 mmol) and η5-Cp′′′AlCl2 (166 mg, 0.5 mmol) or Cp′′′AlBr2 (210 mg, 0.5 mmol) was added toluene (30 mL) at −78 °C, and the resulting mixture was allowed to warm to room temperature slowly and stirred at room temperature overnight. After filtration, the solvent was concentrated to 10 mL under vacuum. The orange solution was stored in a freezer at −30 °C for 7 days to isolate X-ray quality colorless block-shaped crystals of 1 (yield: 247 mg, 52%). 1H NMR (400 MHz, C6D6, 298 K, ppm): δ 7.25–7.23, 7.02–6.92 (m, 10H, Ar–H), 6.85–6.79 (m, 2 H, C5H2(tBu)3), 1.75 (s, 18 H, C5H2(tBu)3), 1.64 (s, 9 H, C5H2(tBu)3), 1.36 (s, 18 H, NtBu), 1.25 (s, 18 H, NtBu); 13C NMR (126 MHz, C6D6, 298 K, ppm): δ 163.6 (NCN), 154.1, 152.3 (PhCN), 133.7, 131.8, 131.1, 130.5, 130.1, 129.7, 129.0, 128.5, 128.4, 127.5, 126.8 (Ar–C, Cp–C), 53.9, 53.2 (C(CH3)), 35.4, 33.9, 33.5, 32.6, 31.9, 31.7, 31.6, 31.0, 28.8 C(CH3); 29Si NMR (99 MHz, 298 K, C6D6, ppm): δ 57.0, 49.2. Mp: 208 °C (dec). Anal. (%) calcd for C47H75AlCl2N4Si2·C7H8 (940.3): C, 68.83; H, 8.88; N, 5.95 Found: C, 69.11; H, 9.01; N, 5.54.Synthesis of η1-Cp′′′Al(I2) ← Si(L)-SiL (2)
To a mixture of LSi-SiL (259 mg, 0.5 mmol) and η5-Cp′′′AlI2 (258 mg, 0.5 mmol) were added toluene (30 mL) at room temperature and stirred overnight. After filtration, the solvent was concentrated to 10 mL under vacuum. The red solution was stored in a freezer at −30 °C for 3 days to get X-ray quality colorless block-shaped crystals of 2 (yield: 247 mg, 43%). 1H NMR (400 MHz, C6D6, 298 K, ppm): δ 7.25–7.20, 7.06–6.93 (m, 10H, Ar–H), 6.92–6.88 (m, 2 H, C5H2(tBu)3), 1.76 (s, 18 H, C5H2(tBu)3), 1.66 (s, 9 H, C5H2(tBu)3), 1.42–1.21 (m, 36 H, NtBu); 13C NMR (100 MHz, C6D6, 298 K, ppm): δ 170.1 (NCN), 164.9, 164.8 (PhCN), 133.4, 131.6, 131.4, 130.3, 130.1, 129.8, 129.1, 129.0, 128.5, 128.4, 128.2, 126.9, 125.3 (Ar–C, Cp–C), 54.4, 53.1 (C(CH3)), 35.9, 34.0, 33.7, 33.5, 32.2, 32.2, 32.0, 31.5 C(CH3); 29Si NMR (99 MHz, 298 K, C6D6, ppm): δ 54.4, 47.8. Mp: 219 °C (dec). Anal. (%) calcd for C47H75AlI2N4Si2·2 C7H8 (1217.33): C, 60.18; H, 7.53; N, 4.60 Found: C, 60.31; H, 7.73; N, 4.39.Synthesis of η1-Cp′′′Ga(Cl2) ← Si(L)-SiL (3)
To a mixture of LSiCl (294 mg, 1.0 mmol), KC8 (135 mg, 1.0 mmol) and [η1-Cp′′′Ga(μ-Cl)Cl]2 (188 mg, 0.25 mmol) was added toluene (30 mL) at −78 °C, and the resulting mixture was allowed to warm to room temperature slowly and stirred at room temperature overnight. After filtration, the solvent was concentrated to 10 mL under vacuum. The yellow solution was stored in a freezer at −30 °C for 2 weeks to get X-ray quality yellow block shaped crystals of 3 (yield: 315 mg, 65%). 1H NMR (400 MHz, C6D6, 298 K, ppm): δ 7.24–6.89 (m, 10 H, Ar–H), 6.89–6.84 (m, 2 H, C5H2(tBu)3), 1.72 (s, 18 H, C5H2(tBu)3), 1.63 (s, 9 H, C5H2(tBu)3), 1.37 (s, 18 H, NtBu), 1.25 (s, 18 H, NtBu). 13C{1H} NMR (100 MHz, C6D6, 298 K, ppm δ 166.2 (NCN), 155.5, 152.3 (PhCN), 133.3, 131.4, 130.9, 130.3, 129.9, 128.8, 128.5, 128.2, 127.8, 127.5, 126.8 (Ar–C, Cp–C), 54.1, 53.4 (C(CH3)3), 35.8, 34.6, 33.9, 33.4, 31.7, 31.5, 31.0, 30.5 (C(CH3)3). 29Si NMR (99 MHz, C6D6, 298 K, ppm): 54.7, 41.1. Mp: 187 °C (dec). Anal. (%) calcd for C47H75GaCl2N4Si2·2 C7H8 (1077.17): C, 68.01; H, 8.52; N, 5.20 Found: C, 68.25; H, 8.81; N, 5.14.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. Ding thanks the National First-Rate Discipline Construction Project of Ningxia (Chemical Engineering and Technology NXY-LXK2017A04) and NYG2022024. D. S. and P. N. R. are grateful for financial support by the RTG “BENCh”, which was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)-389479699/GRK2455.References
- (a) R. S. Grev, H. F. Schaefer and P. P. Gaspar, J. Am. Chem. Soc., 1991, 113, 5638–5643 CrossRef CAS; (b) M. C. Holthausen, W. Koch and Y. Apeloig, J. Am. Chem. Soc., 1999, 121, 2623–2624 CrossRef CAS; (c) M. Haaf, T. A. Schmedake and R. West, Acc. Chem. Res., 2000, 33, 704–714 CrossRef CAS PubMed; (d) B. Gehrhus and M. F. Lappert, J. Organomet. Chem., 2001, 617–618, 209–223 CrossRef CAS; (e) P. P. Gaspar, M. Xiao, D. H. Pae, D. J. Berger, T. Haile, T. Chen, D. Lei, W. R. Winchester and P. Jiang, J. Organomet. Chem., 2002, 646, 68–79 CrossRef CAS; (f) M. Yoshida and N. Tamaoki, Organometallics, 2002, 21, 2587–2589 CrossRef CAS; (g) Y. Apeloig, R. Pauncz, M. Karni, R. West, W. Steiner and D. Chapman, Organometallics, 2003, 22, 3250–3256 CrossRef CAS; (h) Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479–3511 CrossRef CAS PubMed; (i) M. Kira, Chem. Commun., 2010, 46, 2893–2903 RSC; (j) M. Asay, C. Jones and M. Driess, Chem. Rev., 2011, 111, 354–396 CrossRef CAS PubMed; (k) M. Kosa, M. Karni and Y. Apeloig, J. Am. Chem. Soc., 2013, 135, 9032–9040 CrossRef CAS PubMed.
- (a) L. Alvarez-Rodriguez, J. A. Cabeza, P. Garcia-Alvarez and D. Polo, Coord. Chem. Rev., 2015, 300, 1–28 CrossRef CAS; (b) Y.-P. Zhou and M. Driess, Angew. Chem., Int. Ed., 2019, 58, 3715–3728 CrossRef CAS PubMed; (c) C. Shan, S. Yao and M. Driess, Chem. Soc. Rev., 2020, 49, 6733–6754 RSC; (d) W. Yang, Y. Dong, H. Sun and X. Li, Dalton Trans., 2021, 50, 6766–6772 RSC; (e) M. Ghosh and S. Khan, Dalton Trans., 2021, 50, 10674–10688 RSC.
- M. P. Luecke, E. Pens, S. Yao and M. Driess, Chem. – Eur. J., 2020, 26, 4500–4504 CrossRef CAS PubMed.
- (a) R. Tacke and T. Ribbeck, Dalton Trans., 2017, 46, 13628–13659 RSC; (b) F. M. Mück, J. A. Baus, R. Bertermann, C. Burschka and R. Tacke, Organometallics, 2016, 35, 2583–2588 CrossRef.
- (a) M. Nazish, M. M. Siddiqui, S. K. Sarkar, A. Münch, C. M. Legendre, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Chem. – Eur. J., 2021, 27, 1744–1752 CrossRef CAS PubMed; (b) J. Li, Y. Liu, S. Kundu, H. Keil, H. Zhu, R. Herbst-Irmer, D. Stalke and H. W. Roesky, Inorg. Chem., 2020, 59, 7910–7914 CrossRef CAS PubMed; (c) A. Jana, D. Leusser, I. Objartel, H. W. Roesky and D. Stalke, Dalton Trans., 2011, 40, 5458–5463 RSC; (d) R. Azhakar, G. Tavčar, H. W. Roesky, J. Hey and D. Stalke, Eur. J. Inorg. Chem., 2011, 475–477 CrossRef CAS; (e) A. Jana, R. Azhakar, S. P. Sarish, P. P. Samuel, H. W. Roesky, C. Schulzke and D. Koley, Eur. J. Inorg. Chem., 2011, 5006–5013 CrossRef CAS; (f) R. S. Ghadwal, H. W. Roesky, S. Merkel and D. Stalke, Chem. – Eur. J., 2010, 16, 85–88 CrossRef CAS PubMed.
- (a) G. Dübek, D. Franz, C. Eisenhut, P. J. Altmann and S. Inoue, Dalton Trans., 2019, 48, 5756–5765 RSC; (b) S. Inoue and K. Leszczyńska, Angew. Chem., Int. Ed., 2012, 51, 8589–8593 CrossRef CAS PubMed.
- (a) H. Braunschweig, T. Bruckner, A. Deißenberger, R. D. Dewhurst, A. Gackstatter, A. Gartner, A. Hofmann, T. Kupfer, D. Prieschl, T. Thiess and S. R. Wang, Chem. – Eur. J., 2017, 23, 9491–9494 CrossRef CAS PubMed; (b) A. Gackstatter, H. Braunschweig, T. Kupfer, C. Voigt and N. Arnold, Chem. – Eur. J., 2016, 22, 16415–16419 CrossRef CAS PubMed.
- Y. Suzuki, S. Ishida, S. Sato, H. Isobe and T. Iwamoto, Angew. Chem., Int. Ed., 2017, 56, 4593–4597 CrossRef CAS PubMed.
- S. Khoo, Y. L. Shan, M. C. Yang, Y. Li, M. D. Su and C. W. So, Inorg. Chem., 2018, 57, 5879–5887 CrossRef CAS PubMed.
- (a) M. K. Bisai, V. S. V. S. N. Swamy, K. V. Raj, K. Vanka and S. S. Sen, Inorg. Chem., 2021, 60, 1654–1663 CrossRef CAS PubMed; (b) S. Pahar, S. Karak, M. Pait, K. V. Raj, K. Vanka and S. S. Sen, Organometallics, 2018, 37, 1206–1213 CrossRef CAS.
- J. Li, M. Zhong, H. Keil, H. Zhu, R. Herbst-Irmer, D. Stalke, S. De, D. Koley and H. W. Roesky, Chem. Commun., 2019, 55, 2360–2363 RSC.
- M. J. Krahfuss and U. Radius, Eur. J. Inorg. Chem., 2021, 548–561 CrossRef CAS.
- N. Sen, N. Parvin, S. Tothadi and S. Khan, Organometallics, 2021, 40, 1874–1883 CrossRef CAS.
- Y. Xiong, S. Yao and M. Driess, Chem. – Eur. J., 2012, 18, 3316–3320 CrossRef CAS PubMed.
- A. V. Protchenko, A. D. Schwarz, P. M. Blake, C. Jones, N. Kaltsoyannis, P. Mountford and S. Aldridge, Angew. Chem., Int. Ed., 2013, 52, 568–571 CrossRef CAS PubMed.
- Z. Mo, T. Szilvási, Y. P. Zhou, S. Yao and M. Driess, Angew. Chem., Int. Ed., 2017, 56, 3699–3702 CrossRef CAS PubMed.
- S. Pahar, S. Karak, M. Pait, K. V. Raj, K. Vanka and S. S. Sen, Organometallics, 2018, 37, 1206–1213 CrossRef CAS.
- (a) C. W. So, H. W. Roesky, J. Magull and R. B. Oswald, Angew. Chem., Int. Ed., 2006, 45, 3948–3950 CrossRef CAS PubMed; (b) S. S. Sen, H. W. Roesky, D. Stern, J. Henn and D. Stalke, J. Am. Chem. Soc., 2010, 132, 1123–1126 CrossRef CAS PubMed.
- (a) S. Nagendran and H. W. Roesky, Organometallics, 2008, 27, 457–492 CrossRef CAS; (b) S. Yao, Y. Xiong and M. Driess, Organometallics, 2011, 30, 1748–1767 CrossRef CAS; (c) S. S. Sen, S. Khan, P. P. Samuel and H. W. Roesky, Chem. Sci., 2012, 3, 659–682 RSC.
- S. S. Sen, A. Jana, H. W. Roesky and C. Schulzke, Angew. Chem., Int. Ed., 2009, 48, 8536–8538 CrossRef CAS PubMed.
- S. S. Sen, S. Khan, S. Nagendran and H. W. Roesky, Acc. Chem. Res., 2012, 45, 578–587 CrossRef CAS PubMed.
- T. Chu, I. Korobkov and G. I. Nikonov, J. Am. Chem. Soc., 2014, 136, 9195–9202 CrossRef CAS PubMed.
- S. Khoo, H. X. Yeong, Y. Li, R. Ganguly and C. W. So, Inorg. Chem., 2015, 54, 9968–9975 CrossRef CAS PubMed.
- Y. Ding, P. N. Ruth, R. Herbst-Irmer, D. Stalke, Z. Yang and H. W. Roesky, Dalton Trans., 2021, 50, 2067–2074 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: NMR spectra of new compounds and complexes prepared during this study. CCDC 2248551–2248553. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt00811h |
| This journal is © The Royal Society of Chemistry 2023 |