Effect of the type of N-substituent in the benzo-18-azacrown-6 compound on copper(ii) chelation: complexation, radiolabeling, stability in vitro, and biodistribution in vivo†
Abstract
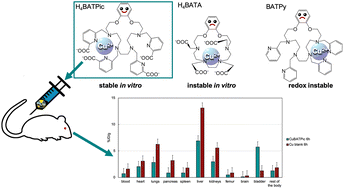
In this work, we synthesized two new benzo-18-azacrown-6 ethers bearing picolinate and pyridine pendant arms and studied the copper complexes of these ligands, as well as those of an acetate analog. All considered ligands were capable of forming mono- and dinuclear complexes due to their large size and large number of donor sites. Among all forms of complexes, the coordination of cations inside the macrocycle has only been shown for the mononuclear form of the acetate complex, while out-cage coordination has been observed for other forms. Electrochemical studies have shown the instability of the mononuclear form of the complex with the pyridine ligand to the reduction in the range of redox potentials of bioreductants. The stabilities of labeled acetate complexes with “in-cage” coordination of the cation and picolinate with “out-cage” coordination were compared in an excess of serum and superoxide dismutase; while the former turned out to be unstable to transchelation, the latter was stable throughout the experiment. Additional studies in biologically relevant media were performed for the picolinate complex and demonstrated its stability in vitro. The biodistribution of this complex in mice after 6 hours post-injection demonstrates a slow excretion from the body; however, the accumulation is noticeably lower than that of free copper cations.


 Please wait while we load your content...
Please wait while we load your content...