Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Tetra-, tetradeca- and octadecametallic clusters of Mn†
Eleftheria
Agapaki
 ,
Angelos B.
Canaj
,
Angelos B.
Canaj
 ,
Gary S.
Nichol
,
Gary S.
Nichol
 and
Euan K.
Brechin
and
Euan K.
Brechin
 *
*
EaStCHEM School of Chemistry, The University of Edinburgh, David Brewster Road, Edinburgh, EH9 3FJ, Scotland, UK. E-mail: ebrechin@ed.ac.uk
First published on 18th April 2023
Abstract
Reaction of equimolar amounts of MnBr2·4H2O and HL1 ((3,5-dimethyl-1H-pyrazol-1-yl)methanol) in a basic MeCN solution leads to the formation of [MnII4(L1)4Br4(H2O)4] (1), whose metallic skeleton is a [MnII4] tetrahedron and cluster core a [MnII4(μ3-O)4] cubane. Replacing MnBr2·4H2O with Mn(O2CMe)2·4H2O affords [MnIII2MnII12O2(L1)4(OAc)22] (2) which is best described as a series of edge-sharing [Mn4] tetrahedra that have self-assembled into a linear array in which each [Mn2] pair is ‘twisted’ with respect to its neighbours in a corkscrew-like manner. Employment of the triangle [MnIII3O(OAc)6(py)3](ClO4) as a reactant instead of a MnII salt results in the formation of [MnIII14MnII4O14(L1)4(HL1)2(OAc)18(H2O)2] (3) whose core is comprised of three vertex-sharing [MnIII4] butterflies flanked on either side by one [MnIII4] cubane and one [MnIII2MnII2] tetrahedron. Dc magnetic susceptibility and magnetisation measurements of polycrystalline samples of 1–3 reveal the predominance of antiferromagnetic exchange interactions. For [Mn4] (1) this leads to a diamagnetic ground state, while for [Mn18] (3) competing exchange interactions result in Single-Molecule Magnet (SMM) behaviour with Ueff = 22 K.
Introduction
The magnetic properties of molecules containing 3d transition metal ions have retained immense scientific interest for decades, originating from initial magneto-structural studies of metal dimers and trimers,1 the construction of models for metalloenzymes2 and the discovery of single-molecule and single-ion magnets,3 to the investigation of geometric spin frustration,4 the exploitation of enhanced magnetocaloric effects for cryogenic refrigeration5 and the emergence of electron-spin based qubits for quantum computation.6 The starting point for all such studies lies in the synthesis of new molecules, and the design of complexes with specific structural characteristics that promote targeted magnetic properties.7 When polymetallic systems are sought-after the nature of the bridging ligands becomes paramount, and for larger nuclearity complexes ‘small and simple’ ligands are often particularly attractive since they offer flexibility in bonding due to (a) the number of coordination modes available, (b) the lack of steric constraints, (c) the ability to accommodate variable metal coordination numbers and geometries, and (d) the ability to stabilise multiple metal oxidation states.8 Alongside the ubiquitous carboxylates,9 N/O-chelate ligands have proved to be highly successful with pertinent examples including amino alcohols,10 polyalkoxides11 and oximes12 (Scheme 1).The pro-ligand (3,5-dimethyl-1H-pyrazol-1-yl)methanol (HL1) belongs to the family of N/O-chelates and has previously been employed to make mono-, di- and tetrametallic compounds of CuII, NiII and CoII.13 In Fe chemistry there is just one example, an [Fe8] cage,14 while there are four examples in Mn chemistry ranging in nuclearity between [Mn7] and [Mn19].15 The relative sparsity of polymetallic cluster compounds reported suggests the ligand has been underemployed, especially given the fascinating topologies already discovered. Here we expand its coordination chemistry to include the synthesis, structure and magnetic characterisation of three new Mn clusters – a [MnII4] cubane, a [MnIII2MnII12] cluster comprising a ‘linear’ array of six shared tetrahedra, and a [MnIII14MnII4] complex containing a [Mn14] plane of vertex- and edge-sharing [Mn4] butterflies, with the four capping ions forming [MnIII4] cubanes and [MnIII2MnII2] tetrahedra with the core.
Experimental
All chemicals were purchased from commercial suppliers and used without further purification. HL1 was prepared as previously described.16 Elemental analyses were performed by the University of Edinburgh microanalytical service in the School of Geosciences.Synthesis of [MnII4(L1)4Br4(H2O)4]·2.25MeCN (1·2.25MeCN)
MnBr2·4H2O (287 mg, 1 mmol), HL1 (126 mg, 1 mmol) and NEt3 (140 μL, 1 mmol) were dissolved in MeCN (15 mL) and stirred for 1 hour. The solution was then filtered and allowed to stand. Pale pink crystals of 1 grew upon slow evaporation of the mother liquor over 2 days. Elemental analysis (%) calculated for Mn4O8N8C24H44Br4: C, 25.92; H, 3.99; N, 10.08. Found: C, 26.00; H, 4.05; N, 10.07. Yield ≤ 40%.Synthesis of [MnIII2MnII12O2(L1)4(OAc)22]·1MeCN·1C3H6O (2·1MeCN·1C3H6O)
Mn(O2CMe)2·4H2O (247 mg, 1 mmol), HL1 (126 mg, 1 mmol) and NEt3 (140 μL, 1 mmol) were dissolved in MeCN (15 mL) and stirred for 1 hour. The solution was then filtered and diffused with acetone. Brown crystals of 2 were obtained over 2 days. Elemental analysis (%) calculated for Mn14O55N8C68H100: C, 30.49; H, 3.76; N, 4.18. Found: C, 30.75; H, 4.09; N, 4.04. Yield ≤ 30%.Synthesis of [MnIII14MnII4O14(L1)4(HL1)2(OAc)18(H2O)2]·2.5MeCN (3·2.5MeCN)
[Mn3O(OAc)6(py)3](ClO4) (871 mg, 1 mmol), HL1 (252 mg, 2 mmol) and NEt3 (280 μL, 2 mmol) were dissolved in MeCN (15 mL) and stirred for 1 hour. The solution was then filtered and diffused with Et2O. Dark brown crystals of 3 were obtained over 2 days. Elemental analysis (%) calculated for Mn18O61N12C72H120: C, 27.73; H, 3.88; N, 5.39. Found: C, 27.44; H, 3.76; N, 5.39. Yield ≤ 45%.Single crystal X-ray diffraction data for 1–3
Suitable crystals of 1–3 were selected and mounted on a MITIGEN holder in Paratone oil on either a Rigaku Oxford Diffraction XCalibur (1) or SuperNova (2, 3) diffractometer. Crystals were kept at T = 120 K (1, 2) or 100 K (3) during data collection. The structures were solved with the ShelXT program using dual methods with Olex2 as the graphical interface. Models were refined with ShelXL using full matrix least squares minimisation on F2.17![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 10.1989(2) Å, b = 12.8405(3) Å, c = 18.4168(3) Å, α = 99.820(2)°, β = 90.437(2)°, γ = 105.874(2)°, V = 2282.13(8) Å3, T = 120.00(11) K, Z = 2, Z′ = 1, μ(MoKα) = 4.636, 87
(no. 2), a = 10.1989(2) Å, b = 12.8405(3) Å, c = 18.4168(3) Å, α = 99.820(2)°, β = 90.437(2)°, γ = 105.874(2)°, V = 2282.13(8) Å3, T = 120.00(11) K, Z = 2, Z′ = 1, μ(MoKα) = 4.636, 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 672 reflections measured, 15
672 reflections measured, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 804 unique (Rint = 0.0479) which were used in all calculations. The final wR2 was 0.0626 (all data) and R1 was 0.0332 (I ≥ 2σ(I)). CCDC 2233436.†
804 unique (Rint = 0.0479) which were used in all calculations. The final wR2 was 0.0626 (all data) and R1 was 0.0332 (I ≥ 2σ(I)). CCDC 2233436.†
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 524.6(7) Å3, T = 120.01(10) K, Z = 8, Z′ = 0.25, μ(MoKα) = 1.531, 49
524.6(7) Å3, T = 120.01(10) K, Z = 8, Z′ = 0.25, μ(MoKα) = 1.531, 49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 597 reflections measured, 6437 unique (Rint = 0.0419) which were used in all calculations. The final wR2 was 0.0799 (all data) and R1 was 0.0329 (I ≥ 2σ(I)). CCDC 2233438.†
597 reflections measured, 6437 unique (Rint = 0.0419) which were used in all calculations. The final wR2 was 0.0799 (all data) and R1 was 0.0329 (I ≥ 2σ(I)). CCDC 2233438.†
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2), a = 15.1254(4) Å, b = 15.3885(4) Å, c = 15.9092(3) Å, α = 103.925(2)°, β = 99.153(2)°, γ = 117.635(2)°, V = 3022.24(14) Å3, T = 100.00(10) K, Z = 1, Z′ = 0.5, μ(CuKα) = 15.573, 128
(no. 2), a = 15.1254(4) Å, b = 15.3885(4) Å, c = 15.9092(3) Å, α = 103.925(2)°, β = 99.153(2)°, γ = 117.635(2)°, V = 3022.24(14) Å3, T = 100.00(10) K, Z = 1, Z′ = 0.5, μ(CuKα) = 15.573, 128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 161 reflections measured, 11
161 reflections measured, 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 063 unique (Rint = 0.1085) which were used in all calculations. The final wR2 was 0.1365 (all data) and R1 was 0.0486 (I ≥ 2σ(I)). CCDC 2233437.†
063 unique (Rint = 0.1085) which were used in all calculations. The final wR2 was 0.1365 (all data) and R1 was 0.0486 (I ≥ 2σ(I)). CCDC 2233437.†
Powder X-ray diffraction data for 1–3
Powder XRD data (Fig. S1–S3†) were collected on freshly prepared polycrystalline powder samples of 1–3 using a Bruker D8 ADVANCE with copper radiation at 40 kV, 40 mA and a Johansson monochromator, 2 mm divergence slit and 2.5 degree Soller slits on the incident beam side. LynxEye detector, Bruker DIFFRAC software. Diffraction measured from 2θ = 5°–40°, step size 0.0101°, using borosilicate capillaries of 0.7 mm outside diameter.Magnetometry
Magnetic data for 1–3 were collected on freshly prepared polycrystalline samples restrained in eicosane in gelatine capsules on a Quantum Design PPMS Dynacool in the temperature range T = 300–2 K and field range B = 0.1–9.0 T. Diamagnetic corrections were applied using Pascal's constants.Results and discussion
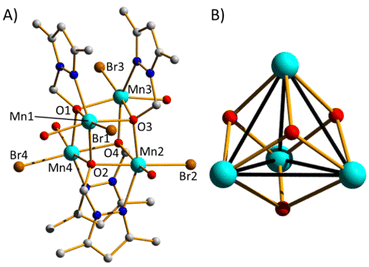
Reaction of equimolar amounts of MnBr2·4H2O and HL1 in a basic MeCN solution leads to the formation of pink crystals of [MnII4(L1)4Br4(H2O)4]·2.25MeCN (1·2.25MeCN). Crystals were in a triclinic cell and structure solution was performed in the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The asymmetric unit contains the whole formula. The metallic skeleton of 1 (Fig. 1) describes a [MnII4] tetrahedron and the cluster core a [MnII4(μ3-O)4] cube (Mn–O–Mn, ∼98–102°; see Table S1† for BVS calculations). The μ3-O-atoms are provided by the L1 ligands, which chelate each metal centre and further bridge to two neighbouring metal ions. The Mn ions are all six-coordinate and in distorted octahedral geometries with their {MnO4NBr} coordination spheres (Mn–O, ∼2.2 Å; Mn–N, ∼2.2 Å, Mn–Br, ∼2.6 Å) completed by the presence of one Br anion and one H2O molecule. The latter form intramolecular H-bonds across four of the six faces of the cube (Br⋯(H)O, ∼3.3 Å). They are also responsible for mediating intermolecular interactions between neighbouring clusters (Br⋯(H)O, ∼3.3 Å) and to the MeCN molecules of crystallisation (O(H)⋯N, ∼2.8 Å). The result is an intricate network of H-bonded clusters in the extended structure (Fig. S4†). A search of the Cambridge Structural Database (CSD) for [Mn4O4] cubanes generates 87 hits, approximately 30% (26) of which are [MnII4O4].
space group. The asymmetric unit contains the whole formula. The metallic skeleton of 1 (Fig. 1) describes a [MnII4] tetrahedron and the cluster core a [MnII4(μ3-O)4] cube (Mn–O–Mn, ∼98–102°; see Table S1† for BVS calculations). The μ3-O-atoms are provided by the L1 ligands, which chelate each metal centre and further bridge to two neighbouring metal ions. The Mn ions are all six-coordinate and in distorted octahedral geometries with their {MnO4NBr} coordination spheres (Mn–O, ∼2.2 Å; Mn–N, ∼2.2 Å, Mn–Br, ∼2.6 Å) completed by the presence of one Br anion and one H2O molecule. The latter form intramolecular H-bonds across four of the six faces of the cube (Br⋯(H)O, ∼3.3 Å). They are also responsible for mediating intermolecular interactions between neighbouring clusters (Br⋯(H)O, ∼3.3 Å) and to the MeCN molecules of crystallisation (O(H)⋯N, ∼2.8 Å). The result is an intricate network of H-bonded clusters in the extended structure (Fig. S4†). A search of the Cambridge Structural Database (CSD) for [Mn4O4] cubanes generates 87 hits, approximately 30% (26) of which are [MnII4O4].
Repeating the reaction that produces 1 but replacing MnBr2·4H2O with Mn(O2CMe)2·4H2O affords brown crystals of [MnIII2MnII12O2(L1)4(OAc)22]·1MeCN·1C3H6O (2·1MeCN·1C3H6O) when acetone is diffused into the MeCN mother liquor (Fig. 2A–C). Crystals were in an orthorhombic cell and structure solution was performed in the Fddd space group. The asymmetric unit contains one quarter of the formula. The metallic skeleton of 2 (Fig. 3A–C) describes a series of edge-sharing [Mn4] tetrahedra that have self-assembled into a linear array in which each [Mn2] pair is ‘twisted’ with respect to its neighbours in a corkscrew-like manner. In the centre of the cluster lies a [MnIII2MnII4(μ4-O)2] unit (Fig. 2C) that consists of two edge-sharing [MnIII2MnII2] tetrahedra – the {MnIII2} unit being the shared edge.
The μ4-O2− ion (O13 and symmetry equivalent, s.e.) links the two central MnIII ions (Mn4 and s.e.) to each other and to two MnII ions on each side (Mn3 and s.e.). This [Mn6] unit is well documented in Mn carboxylate chemistry.18 The [Mn6] unit is then connected on each side to a [MnII4] tetrahedron (Mn1, Mn2 and s.e.). The metals in the [Mn14] moiety are connected by a series of (twenty two) μ-, μ3- and μ4-carboxylates, while the μ3-L1 ligands are found at the periphery of the molecule, chelating to Mn1 (and s.e.) and further bridging to Mn2 (and s.e., Fig. S5†). All the metal ions are six-coordinate and in distorted octahedral geometries, with the two MnIII ions displaying the expected Jahn–Teller (JT) elongation along the O12–Mn4–O12′ (and s.e.) vector. By symmetry, these are co-planar (Fig. S6†). Closest intermolecular interactions are observed between L1 ligands (C(H)⋯(H)C, ∼3.2–3.8 Å) and carboxylates (C(H)⋯O(C), ∼3.2–3.6 Å), which results in the formation of a brickwork-like motif in the extended structure (Fig. S6†). There are sixteen [Mn14] clusters deposited in the CSD, none with the topology or oxidation state distribution displayed by compound 2.19
The reaction of [Mn3O(OAc)6(py)3](ClO4) with HL1 in a basic MeCN solution affords black crystals of [MnIII14MnII4O14(L1)4(HL1)2(OAc)18(H2O)2]·2.5MeCN (3·2.5MeCN) when Et2O is diffused into the mother liquor (Fig. 4). The crystals were in a triclinic cell and structure solution was performed in the space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) . The asymmetric unit contains half the formula. The metallic skeleton of 3 has a [MnIII14MnII4] oxidation state distribution, the central part of which is a near-planar [MnIII10MnII2] disc (Mn1, Mn3–6, Mn8–9 and s.e.; where Mn5 is the +2 ion) which is constructed from seven vertex- and edge-sharing [Mn4] butterflies (Fig. 5). Two MnII ions (Mn7 and s.e.) and two MnIII ions (Mn2 and s.e.) lie ‘above’ and ‘below’ this plane forming distorted [MnIII2MnII2] and [MnIII4] tetrahedra with three Mn ions in the planar disc, respectively. The metal–oxygen core describes three central vertex-sharing [MnIII4O2] butterflies (Mn8, Mn9, O11, O8, O9 and s.e.), flanked on either side by one [MnIII4O4] cubane (Mn1–4, O4, O5, O7, O14 and s.e.) and one [MnIII2MnII2O] tetrahedron (Mn1, Mn5–7, O6 and s.e.) (Fig. 5).
. The asymmetric unit contains half the formula. The metallic skeleton of 3 has a [MnIII14MnII4] oxidation state distribution, the central part of which is a near-planar [MnIII10MnII2] disc (Mn1, Mn3–6, Mn8–9 and s.e.; where Mn5 is the +2 ion) which is constructed from seven vertex- and edge-sharing [Mn4] butterflies (Fig. 5). Two MnII ions (Mn7 and s.e.) and two MnIII ions (Mn2 and s.e.) lie ‘above’ and ‘below’ this plane forming distorted [MnIII2MnII2] and [MnIII4] tetrahedra with three Mn ions in the planar disc, respectively. The metal–oxygen core describes three central vertex-sharing [MnIII4O2] butterflies (Mn8, Mn9, O11, O8, O9 and s.e.), flanked on either side by one [MnIII4O4] cubane (Mn1–4, O4, O5, O7, O14 and s.e.) and one [MnIII2MnII2O] tetrahedron (Mn1, Mn5–7, O6 and s.e.) (Fig. 5).
 | ||
| Fig. 4 Molecular structure of compound 3. Colour code: MnIII = pink, MnII = light blue, C = grey, O = red, N = dark blue. H atoms and solvent molecules of crystallisation omitted for clarity. | ||
 | ||
| Fig. 5 (A) Metal–oxygen core of 3 minus the carboxylate O-atoms. (B) Metallic skeleton of 3. Colour code: MnIII = pink, MnII = light blue, O = red. | ||
The acetate ligands are of three types (Fig. S7†). Fourteen are μ-bridging, two are μ3-bridging (between Mn1, Mn2, Mn6 and s.e.) and two are μ4-bridging (Mn1, Mn3, Mn4, Mn7 and s.e.) with the μ3-O atom forming a corner of the [MnIII4O4] cubane. The four μ-L−1 ligands are found at the periphery of the cluster, chelating to Mn4 and Mn5 (and s.e.) and bridging to Mn5 and Mn6 (and s.e.), respectively. The two HL1 ligands are found in the centre of the cluster, chelating to Mn7 (and s.e.) and forming a longer contact to Mn8 (and s.e., O–Mn, ∼2.55 Å). The protonated O-atom is H-bonding to both a μ3-O2− ion (O⋯O, ∼2.81 Å) and the O-atom of a bridging OAc (O⋯O, ∼2.61 Å). The two H2O molecules are bonded to Mn9 (and s.e.), H-bonding to the O-atoms of neighbouring μ-OAc ligands (∼2.74–2.88 Å).
All the Mn ions in 3 are six-coordinate and in distorted octahedral geometries, with the MnIII ions displaying their expected JT elongation (most of which are approximately co-parallel; Fig. S8†). The only exception is Mn8 (and s.e.) which is five coordinate and square pyramidal, the sixth coordination site being ‘occupied’ by the distal O2 atom of the protonated HL1 ligand.
There are numerous inter-molecular interactions in the structure of 3. The MeCN molecules of crystallisation are in close contact with bridging OAc ligands, C(H)⋯O, ∼3.2 Å, as are peripheral L1 and OAc ligands on neighbouring complexes (C/N/O⋯C/N/O, >3.3 Å). The result is that clusters of 3 pack in a brickwork-like fashion in the extended structure (Fig. S8†). A search of the CSD reveals ten [Mn18] clusters. These include, two [MnIII15MnII3] super-tetrahedra built with triethanolamine,20 a [MnIII18] wheel of triangles using salicylaldoxime,21 a [MnII8MnIII6MnIV4] wheel of defective cubanes made from tripodal alcohols,22 a [MnIV2MnIII16] cage stabilised with phosphonates,23 dimers of [MnIII9] molecules linked with 4,4′-bpy or diamagnetic metal ions,24 a [MnIII14MnII4] carboxylate cage with the ‘rock-salt’ structure,25 a [MnIII18] super-octahedron built from a polydentate amino alcohol26 and a near-planar [MnIII18] cage constructed from [Mn4] butterflies and dicarboxylates.27 While the latter has some structural similarity to 3, the closest structural analogue is the complex [MnIII16MnII2O14(O2CMe)18(hep)4(hepH)2(H2O)2](ClO4)2, built with a different, but related N/O-chelate ligand (hepH = 2-(hydroxyethyl)pyridine).28 This species has a similar Mn–O core and metallic skeleton, but a different oxidation state distribution.
Magnetic properties
Dc magnetic susceptibility (χ) and magnetisation (M) measurements of polycrystalline samples of 1–3 were collected in the T = 300–2 K, B = 0.1 T and T = 2–10 K, B = 0.5–9.0 T temperature and field ranges, respectively. These data are plotted in Fig. 6, 7 and S9.†For 1 the χT value of 17.5 cm3 K mol−1 at T = 300 K is as expected for four uncorrelated S = 5/2 spins, assuming g = 2.00 (Fig. 6). As T is decreased the value of χT remains essentially invariant until approximately T = 25 K wherefrom the value decreases to a minimum value of 4.4 cm3 K mol−1 at T = 2 K. Magnetisation data (Fig. 7A) rise rapidly with increasing field saturating at a maximum value of 19.55 μB at T = 2 K and B = 9.0 T. Both are indicative of very weak, predominantly antiferromagnetic exchange interactions between the MnII ions (the large magnetisation value at B = 9 T is field induced).
The experimental susceptibility and magnetisation data for 1 can be simultaneously fitted with an isotropic spin-Hamiltonian  with a coupling scheme that assumes two unique exchange interactions between neighbouring MnII ions based on differing Mn–O–Mn angles (Fig. 8), J1 = −0.19 cm−1 (between Mn1–Mn2, Mn1–Mn4, Mn2–Mn3 and Mn3–Mn4 with Mn–O–Mn ≥ 100.3°) and J2 = +0.038 cm−1 (between Mn1–Mn3, Mn2–Mn4 with Mn–O–Mn ≤ 98.4°) with g = 2.0. The fit can be improved slightly by including a ferromagnetic intermolecular interaction, zJ = +6.72 × 10−3 cm−1, consistent with the packing of the molecules and H-bonding in the extended structure. The intramolecular exchange coupling constants are consistent with values previously reported for alkoxide-bridged MnII cubes with similar Mn–O–Mn angles where the switch from ferro- to antiferromagnetic occurs at ∼100°.29 This leads to a ground state of S = 0 with multiple low-lying excited states (Fig. 7B).
with a coupling scheme that assumes two unique exchange interactions between neighbouring MnII ions based on differing Mn–O–Mn angles (Fig. 8), J1 = −0.19 cm−1 (between Mn1–Mn2, Mn1–Mn4, Mn2–Mn3 and Mn3–Mn4 with Mn–O–Mn ≥ 100.3°) and J2 = +0.038 cm−1 (between Mn1–Mn3, Mn2–Mn4 with Mn–O–Mn ≤ 98.4°) with g = 2.0. The fit can be improved slightly by including a ferromagnetic intermolecular interaction, zJ = +6.72 × 10−3 cm−1, consistent with the packing of the molecules and H-bonding in the extended structure. The intramolecular exchange coupling constants are consistent with values previously reported for alkoxide-bridged MnII cubes with similar Mn–O–Mn angles where the switch from ferro- to antiferromagnetic occurs at ∼100°.29 This leads to a ground state of S = 0 with multiple low-lying excited states (Fig. 7B).
Given that the magnitude of the exchange calculated is potentially of the same order of magnitude as that of the zero-field splitting expected for MnII ions in a distorted octahedral {MnO4NBr} coordination sphere30 we have also fitted the susceptibility and magnetisation data by including single ion anisotropy  . The best fit afforded J1 = −0.18 cm−1, J2 = +0.053 cm−1 and |D| = 0.05 cm−1, with g = 2.01 and zJ = +7.22 × 10−3 cm−1 (Fig. S9†). The fit is of slightly lower quality, but clearly reveals that J and D are correlated and thus while we can conclude a magnetic interaction of <|0.2| cm−1 is present, the exact contributions to that interaction are somewhat open to debate.
. The best fit afforded J1 = −0.18 cm−1, J2 = +0.053 cm−1 and |D| = 0.05 cm−1, with g = 2.01 and zJ = +7.22 × 10−3 cm−1 (Fig. S9†). The fit is of slightly lower quality, but clearly reveals that J and D are correlated and thus while we can conclude a magnetic interaction of <|0.2| cm−1 is present, the exact contributions to that interaction are somewhat open to debate.
The χT value for 2 at T = 300 K (47.2 cm3 K mol−1) is below that expected for a [MnIII2MnII12] unit (58.5 cm3 K mol−1), assuming g = 2.00. The value of χT continuously decreases with decreasing temperature to a minimum value of 2.1 cm3 K mol−1 at T = 2 K. Magnetisation data rise in a near linear fashion with increasing field and without saturating, reaching a maximum value of 13.92 μB at T = 2.0 and B = 9.0 T (Fig. S10†). Both are indicative of relatively weak antiferromagnetic exchange interactions between the Mn ions, likely resulting in a diamagnetic ground state but with multiple low-lying, paramagnetic excited states. Weak exchange is commonly observed in clusters with multiple MnII ions.31 The nuclearity and complex topology of compound 2 preclude a quantitative analysis of the susceptibility and magnetisation data. A fit of the inverse susceptibility data (1/χ vs. T, Fig. S11A†) affords C = 54.9 cm3 K mol−1 and θ = −48.6 K.
The χT value for 3 at T = 300 K (41.1 cm3 K mol−1) is below that expected for a [MnIII14MnII4] (59.5 cm3 K mol−1) unit, assuming g = 2.00. As T is decreased χT decreases steadily to a value of ∼32 cm3 K mol−1 at T = 100 K, before decreasing more slowly to a value of ∼29 cm3 K mol−1 at T = 50 K. Below this temperature χT decreases much more rapidly, to a minimum value of 4.4 cm3 K mol−1 at T = 2 K. Magnetisation data for 3 initially rise in a near linear fashion with increasing field (B = 0.5–4.0 T), but thereafter rise more slowly and appear to begin to saturate at ∼16.2 μB at T = 2.0 and B = 9.0 T (Fig. S10†). The data are therefore indicative of weak, but competing antiferromagnetic exchange interactions. A fit of the Curie–Weiss law (Fig. S11B†) affords C = 47.1 cm3 K mol−1 and θ = −51.5 K.
Variable-temperature alternating current (ac) susceptibility measurements were performed to investigate the magnetic relaxation dynamics of 1–3, with only 3 exhibiting frequency dependent out-of-phase χ′′ ac susceptibility peaks at temperatures up to ∼3.5 K in the absence of an external magnetic field (Fig. 9, 10 and S12–S15†). Relaxation times, τ, were extracted by fitting the Cole–Cole χ′′ vs. χ′ plots using the generalised Debye model. The data were analysed with CCFIT.32 The distribution of the α parameter was found to be in the range 0.22 to 0.36 (2.1–3.1 K). An Arrhenius plot of the ln(τ) data versus the inverse temperature (T−1) was fit to an Orbach process yielding Ueff = 22 K (15.3 cm−1) and τ0 = 3.5 × 10−8 s. These values are consistent with other Mn-based single-molecule magnets assuming an Orbach-only relaxation process.28
Conclusions
The reaction of HL1 with the Mn salts MnBr2·4H2O and Mn(O2CMe)2·4H2O in basic MeCN solutions leads to the formation of [MnII4] and [MnIII2MnII12] clusters, respectively. The structure of the former is a cube and that of the latter best described as a series of tetrahedra that have self-assembled into a “twisted” linear array. Switching to the basic carboxylate [MnIII3O(OAc)6(py)3](ClO4) as a reactant results in the formation of a [MnIII14MnII4] cluster whose structure contains vertex-sharing [MnIII4] butterflies flanked on either side by [MnIII4] cubanes and [MnIII2MnII2] tetrahedra. In 1 and 2 the L1 ligand acts solely as a μ3-bridge helping to form an [MnII4O4] cubane in each case, while in 3 it acts as a μ-bridge or remains protonated (HL1) and acts as a simple chelate. Interestingly, the oxidation state distributions in 1 (MnII4) and 2 (MnIII2MnII12) are dominated by MnII, suggesting HL1 to be less acidic than similar ligands such as hmpH, hepH and pdmH2 (Scheme 1) which normally result in MnIII-dominated clusters.28,33 This is also reflected in the L1 ligands always coordinating to at least one MnII ion in 1–3. A larger ratio of MnIII![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MnII (MnIII14MnII4, 3) in the product can be obtained by employing an [MnIII3] triangle as the reactant, although we note that there has been some metal reduction, hinting at some complex redox behaviour in the formation of 3. For both 2 and 3 the ratio of OAc
MnII (MnIII14MnII4, 3) in the product can be obtained by employing an [MnIII3] triangle as the reactant, although we note that there has been some metal reduction, hinting at some complex redox behaviour in the formation of 3. For both 2 and 3 the ratio of OAc![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) L1 in the complex is heavily in favour of the former, despite being used in equimolar amounts in 2. All of the above suggests that examining increased ratios of base, the addition of oxidising agents (e.g. MnO4−), and the role of carboxylate sterics will be key in making new family members. The apparent preference of L1 for MII ions over MII ions also suggests the ligand may be a good choice for targeting polymetallic FeII clusters.
L1 in the complex is heavily in favour of the former, despite being used in equimolar amounts in 2. All of the above suggests that examining increased ratios of base, the addition of oxidising agents (e.g. MnO4−), and the role of carboxylate sterics will be key in making new family members. The apparent preference of L1 for MII ions over MII ions also suggests the ligand may be a good choice for targeting polymetallic FeII clusters.
Dc magnetic susceptibility and magnetisation measurements of polycrystalline samples of 1–3 reveal the predominance of antiferromagnetic exchange interactions. For 1 two very weak exchange interactions (J1 = −0.19 cm−1, J2 = +0.038 cm−1) lead to a diamagnetic S = 0 ground state with multiple low-lying excited states, assuming an isotropic model. A similar scenario, i.e. multiple weak magnetic interactions, is inferred for complex 2, given the presence of numerous MnII ions. For 3 competing exchange interactions arising from the presence of multiple edge-sharing [Mn3] triangles, and the presence of multiple co-parallel JT axes, results in SMM behaviour with Ueff = 22 K (15.3 cm−1).
Author contributions
EA performed the synthesis, GSN/ABC the single crystal XRD/PXRD measurements, respectively. EA/ABC collected and fitted the magnetic data. EKB conceived the idea. All authors contributed to the preparation of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
EKB thanks The Leverhulme Trust (RPG-2021-176).Notes and references
- (a) B. Bleaney and K. D. Bowers, Proc. R. Soc. London, Ser. A, 1952, A214, 451 Search PubMed; (b) W. H. Crawford, H. W. Richardson, J. R. Wasson, D. J. Hodgson and W. E. Hatfield, Inorg. Chem., 1976, 15, 2107 CrossRef; (c) W. E. Hatfield, Comments Inorg. Chem., 1981, 1, 105 CrossRef CAS; (d) J. Glerup, D. J. Hodgson and E. Petersen, Acta Chem. Scand., 1983, A37, 161 CrossRef CAS.
- G. Aromí, S. M. J. Aubin, M. A. Bolcar, G. Christou, H. J. Eppley, K. Folting, D. N. Hendrickson, J. C. Huffman, R. C. Squire, H.-L. Tsai, S. Wang and M. W. Wemple, Polyhedron, 1998, 17, 3005 CrossRef.
- (a) A. Caneschi, D. Gatteschi, R. Sessoli, A.-L. Barra, L.-C. Brunel and M. J. Guillot, J. Am. Chem. Soc., 1991, 113, 5873 CrossRef CAS; (b) R. Sessoli, H.-L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS; (c) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS; (d) G. Christou, D. Gatteschi, D. N. Hendrickson and R. Sessoli, MRS Bull., 2000, 25, 66 CrossRef CAS; (e) N. Ishikawa, M. Sugita, T. Ishikawa, S. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS PubMed; (f) J. M. Zadrozny and J. R. Long, J. Am. Chem. Soc., 2011, 133, 20732 CrossRef CAS PubMed.
- J. Schnack, Dalton Trans., 2010, 39, 4677 RSC.
- M. Evangelisti and E. K. Brechin, Dalton Trans., 2010, 39, 4672 RSC.
- (a) K. S. Pedersen, A.-M. Ariciu, S. McAdams, H. Weihe, J. Bendix, F. Tuna and S. Piligkos, J. Am. Chem. Soc., 2016, 138, 5801 CrossRef CAS PubMed; (b) G. A. Timco, T. B. Faust, F. Tuna and R. E. P. Winpenny, Chem. Soc. Rev., 2011, 40, 3067 RSC.
- (a) G. Aromí and E. K. Brechin, Synthesis of 3d Metallic Single-Molecule Magnets, in Single-Molecule Magnets and Related Phenomena. Structure and Bonding, ed. R. Winpenny, Springer, Berlin, Heidelberg, 2006, vol. 122 Search PubMed; (b) C. J. Milios and R. E. P. Winpenny, Cluster-Based Single-Molecule Magnets, in Molecular Nanomagnets and Related Phenomena. Structure and Bonding, ed. S. Gao, Springer, Berlin, Heidelberg, 2014, vol. 164 Search PubMed.
- (a) N. Hoshino, A. M. Ako, A. K. Powell and H. Oshio, Inorg. Chem., 2009, 48, 3396 CrossRef CAS PubMed; (b) C. Papatriantafyllopoulou, E. E. Moushi, G. Christou and A. J. Tasiopoulos, Chem. Soc. Rev., 2016, 45, 1597 RSC.
- (a) R. Bagai and G. Christou, Chem. Soc. Rev., 2009, 38, 1011 RSC; (b) M. Affronte, F. Troiani, A. Ghirri, S. Carretta, P. Santini, V. Corradini, R. Schuecker, C. Muryn, G. Timco and R. E. Winpenny, Dalton Trans., 2006, 2810–2817 RSC.
- (a) A. J. Tasiopoulos and S. P. Perlepes, Dalton Trans., 2008, 5537 RSC; (b) L. Ungur, S. K. Langley, T. N. Hooper, B. Moubaraki, E. K. Brechin, K. S. Murray and L. F. Chibotaru, J. Am. Chem. Soc., 2012, 134, 18554 CrossRef CAS; (c) A. Baniodeh, N. Magnani, Y. Lan, G. Buth, C. E. Anson, J. Richter, M. Affronte, J. Schnack and A. K. Powell, npj Quantum Mater., 2018, 3, 10 CrossRef; (d) D. J. Cutler, M. K. Singh, G. S. Nichol, M. Evangelisti, J. Schnack, L. Cronin and E. K. Brechin, Chem. Commun., 2021, 57, 8925 RSC.
- (a) E. K. Brechin, Chem. Commun., 2005, 5141 RSC; (b) L. R. B. Wilson, M. Coletta, M. Evangelisiti, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Dalton Trans., 2022, 51, 4213 RSC.
- (a) C. J. Milios, T. C. Stamatatos and S. P. Perlepes, Polyhedron, 2006, 25, 134 CrossRef CAS; (b) R. Inglis, C. J. Milios, L. F. Jones, S. Piligkos and E. K. Brechin, Chem. Commun., 2012, 48, 181 RSC; (c) R. Inglis, S. M. Taylor, L. F. Jones, G. S. Papaefstathiou, S. P. Perlepes, S. Datta, S. Hill, W. Wernsdorfer and E. K. Brechin, Dalton Trans., 2009, 9157 RSC.
- (a) Y.-W. Li, L.-Y. Guo, L. Feng, Z. Jagličić, S.-Y. Zeng and D. Sun, CrystEngComm, 2017, 19, 5897 RSC; (b) W.-F. Xie, L.-Y. Guo, J.-H. Xu, M. Jagodič, Z. Jagličić, W.-G. Wang, G.-L. Zhuang, Z. Wang, C.-H. Tung and D. Sun, Eur. J. Inorg. Chem., 2016, 3253 CrossRef CAS; (c) B. Barszcz, T. Głowiak, J. Jezierska and A. Tomkiewicz, Polyhedron, 2004, 23, 1309 CrossRef CAS; (d) I. Banerjee, M. Dolai, A. D. Jana, K. K. Das and M. Ali, CrystEngComm, 2012, 14, 4972 RSC; (e) B. Barszcz, T. Głowiak, J. Jezierska and K. Kurdziel, Inorg. Chem. Commun., 2002, 1056–1058 CrossRef CAS; (f) V. M. Leovac, R. Petković, A. Kovács, G. Pokol and K. M. Szécsény, J. Therm. Anal. Calorim., 2007, 89, 267 CrossRef CAS; (g) R. W. M. ten Hoedt, F. B. Hulsbergen, G. C. Verschoor and J. Reedijk, Inorg. Chem., 1982, 21, 2369 CrossRef CAS; (h) A. Beheshti, E. S. M. Fard, M. Kubicki, P. Mayer, C. T. Abrahams and S. E. Razatofighie, CrystEngComm, 2019, 21, 251 RSC; (i) J. Lim, G. Kim, K. Do, S. Lee, S. Ryu, D. Yoshioka and M. Mikuriya, X-Ray Struct. Anal. Online, 2015, 31, 49 CrossRef CAS; (j) A. Mar, S. J. Retting, A. Storr and J. Trott, Can. J. Chem., 1988, 66, 101 CrossRef CAS; (k) F. Paap, E. Bouwman, W. L. Driessen, R. A. G. de Graaff and J. Reedijk, J. Chem. Soc., Dalton Trans., 1985, 737 RSC.
- R. Touzani, M. Haibach, A. J. Nawara-Hultzsch, S. El Kadiri, T. J. Emge and A. S. Goldman, Polyhedron, 2011, 30, 2530 CrossRef CAS.
- (a) L.-Y. Guo, H.-F. Su, M. Kurmoo, C.-H. Tung, D. Sun and L.-S. Zheng, J. Am. Chem. Soc., 2017, 139, 14033–14036 CrossRef CAS PubMed; (b) F. Yang, Y.-K. Deng, L.-Y. Guo, H.-F. Su, Z. Jagličić, Z.-Y. Feng, G.-L. Zhuang, S.-Y. Zenge and D. Sun, CrystEngComm, 2016, 18, 1329 RSC; (c) B.-Q. Ji, H.-F. Su, M. Jagodič, Z. Jaglicič, M. Kurmoo, X.-P. Wang, C.-H. Tung, Z.-Z. Cao and D. Sun, Inorg. Chem., 2019, 58, 3800 CrossRef CAS PubMed; (d) Y.-K. Deng, H.-F. Su, J.-H. Xu, W.-G. Wang, M. Kurmoo, S.-C. Lin, Y.-Z. Tan, J. Jia, D. Sun and L.-S. Zheng, J. Am. Chem. Soc., 2016, 138, 1328 CrossRef CAS.
- W. L. Driessen, Recl. Trav. Chim. Pays-Bas, 1982, 101, 441 CrossRef CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, A71, 3 CrossRef PubMed; (b) O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, J. Appl. Crystallogr., 2009, 42, 339 CrossRef CAS; (c) G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3 Search PubMed.
- (a) A. R. E. Baikie, A. J. Howes, M. B. Hursthouse, A. B. Quick and P. Thornton, J. Chem. Soc., Chem. Commun., 1986, 1587 RSC; (b) A. G. Blackman, J. C. Huffman, E. B. Lobkovsky and G. Christou, Polyhedron, 1992, 11, 251 CrossRef CAS.
- (a) S.-H. Huang, C.-I. Yang, S.-Y. Jhan and H.-L. Tsai, Polyhedron, 2013, 66, 245 CrossRef CAS; (b) M. Charalambous, E. E. Moushi, T. N. Nguyen, A. M. Mowson, G. Christou and A. J. Tasiopoulos, Eur. J. Inorg. Chem., 2018, 3905 CrossRef CAS; (c) M. Manoli, A. Collins, S. Parsons, A. Candini, M. Evangelisti and E. K. Brechin, J. Am. Chem. Soc., 2088, 130, 11129 CrossRef PubMed; (d) Y. Okui, F. A. Catusanu, R. Kubota, B. Kure, T. Nakajima, T. Tanase, T. Kajiwara, M. Mikuriya, H. Miyasaka and M. Yamashita, Eur. J. Inorg. Chem., 2011, 4325 CrossRef CAS; (e) L. Zhang, R. Clérac, P. Heijboer and W. Schmitt, Angew. Chem., Int. Ed., 2012, 51, 3007 CrossRef CAS PubMed; (f) A. Mishra, J. Yano, Y. Pushkar, V. K. Yachandra, K. A. Abboud and G. Christou, Chem. Commun., 2007, 1538 RSC; (g) A. Mishra, Y. Pushkar, J. Yano, V. K. Yachandra, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2008, 47, 1940 CrossRef CAS PubMed; (h) K. Su, F. Jiang, J. Qian, J. Pan, J. Pang, X. Wan, F. Hu and M. Hong, RSC Adv., 2015, 5, 33579 RSC; (i) C. C. Stoumpos, I. A. Gass, C. J. Milios, N. Lalioti, A. Terzis, G. Aromi, S. J. Teat, E. K. Brechin and S. P. Perlepes, Dalton Trans., 2009, 307 RSC; (j) C. J. Milios, E. Kefalloniti, C. P. Raptopoulou, A. Terzis, R. Vicente, N. Lalioti, A. Escuer and S. P. Perlepes, Chem. Commun., 2003, 819 RSC; (k) D. I. Alexandropoulos, C. Papatriantafyllopoulou, C. Li, L. Cunha-Silva, M. J. Manos, A. J. Tasiopoulos, W. Wernsdorfer, G. Christou and T. C. Stamatatos, Eur. J. Inorg. Chem., 2013, 2286 CrossRef CAS; (l) M. U. Anwar, Y. Lan, L. M. C. Beltran, R. Clérac, S. Pfirrmann, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 5177 CrossRef CAS PubMed; (m) F.-P. Xiao and L.-F. Jin, Inorg. Chem. Commun., 2008, 11, 717 CrossRef CAS; (n) X. Jiang, G.-Y. An, C.-M. Liu and H.-Z. Kou, Eur. J. Inorg. Chem., 2015, 5314 CrossRef CAS; (o) J. S. Costa, L. A. Barrios, G. A. Craig, S. J. Teat, F. Luis, O. Roubeau, M. Evangelisti, A. Camon and G. Aromí, Chem. Commun., 2012, 48, 1413 RSC; (p) G. Aromí, A. Bell, S. J. Teat, A. G. Whittaker and R. E. P. Winpenny, Chem. Commun., 2002, 1896 RSC; (q) X.-Y. Wang, A. V. Prosvirin and K. R. Dunbar, Angew. Chem., Int. Ed., 2010, 49, 5081 CrossRef CAS PubMed.
- (a) S. K. Langley, K. J. Berry, B. Moubaraki and K. S. Murray, Dalton Trans., 2009, 973 RSC; (b) T. Stamatatos, D. Foguet-Albiol, W. Wernsdorfer, K. A. Abboud and G. Christou, Chem. Commun., 2011, 47, 274 RSC.
- M. Manoli, R. Inglis, S. Piligkos, L. Yanhua, W. Wernsdorfer, E. K. Brechin and A. J. Tasiopoulos, Chem. Commun., 2016, 52, 12829 RSC.
- C.-M. Liu, D.-Q. Zhang and D.-B. Zhu, Chem. – Asian J., 2011, 6, 74 CrossRef CAS PubMed.
- M. Shanmugam, G. Chastanet, T. Mallah, R. Sessoli, S. J. Teat, G. A. Timco and R. E. P. Winpenny, Chem. – Eur. J., 2006, 12, 8777 CrossRef CAS PubMed.
- (a) H. J. Eppley, N. de Vries, S. Wang, S. M. Aubin, H.-L. Tsai, K. Folting, D. N. Hendrickson and G. Christou, Inorg. Chim. Acta, 1997, 263, 323 CrossRef CAS; (b) A. M. Ako, B. Burger, Y. Lan, V. Mereacre, R. Clérac, G. Buth, S. Gomez-Coca, E. Ruiz, C. E. Anson and A. K. Powell, Inorg. Chem., 2013, 52, 5764 CrossRef CAS PubMed.
- E. K. Brechin, W. Clegg, M. Murrie, S. Parsons, S. J. Teat and R. E. P. Winpenny, J. Am. Chem. Soc., 1998, 120, 7365 CrossRef CAS.
- A.-J. Zhou, J.-D. Leng, J.-S. Hu and M.-L. Tong, Dalton Trans., 2013, 42, 9428 RSC.
- R. C. Squire, S. M. J. Aubin, K. Folting, W. E. Streib, D. N. Hendrickson and G. Christou, Angew. Chem., Int. Ed. Engl., 1995, 34, 887 CrossRef CAS.
- (a) E. K. Brechin, C. Boskovic, W. Wernsdorfer, J. Yoo, A. Yamaguchi, E. C. Sañudo, T. R. Concolino, A. L. Rheingold, H. Ishimoto, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 2002, 124, 9710 CrossRef CAS PubMed; (b) E. K. Brechin, E. C. Sañudo, W. Wernsdorfer, C. Boskovic, J. Yoo, D. N. Hendrickson, A. Yamaguchi, H. Ishimoto, T. E. Concolino, A. L. Rheingold and G. Christou, Inorg. Chem., 2005, 44, 502 CrossRef CAS PubMed; (c) E. C. Sanudo, E. K. Brechin, C. Boskovic, W. Wernsdorfer, J. Yoo, A. Yamaguchi, T. R. Concolino, K. A. Abboud, A. L. Rheingold, H. Ishimoto, D. N. Hendrickson and G. Christou, Polyhedron, 2003, 22, 2267 CrossRef CAS.
- (a) M. Nihei, N. Hoshino, T. Ito and H. Oshio, Polyhedron, 2003, 22, 2359 CrossRef CAS; (b) G. S. Papaefstathiou, A. Escuer, F. A. Mautner, C. Raptopoulou, A. Terzis, S. P. Perlepes and R. Vicente, Eur. J. Inorg. Chem., 2005, 879 CrossRef CAS; (c) D. Sivanesan, K. Son, H. J. Lee, K. T. Park, Z. Jang, B. J. Suh and S. Yoon, Polyhedron, 2013, 50, 339 CrossRef CAS; (d) C. C. Stoumpos, I. A. Gass, C. J. Milios, N. Lalioti, A. Terzis, G. Aromí, S. J. Teat, E. K. Brechin and S. P. Perlepes, Dalton Trans., 2009, 2, 307 RSC; (e) T. Taguchi, M. R. Daniels, K. A. Abboud and G. Christou, Inorg. Chem., 2009, 48, 9325 CrossRef CAS PubMed.
- (a) A. Abragam and B. Bleaney, Electron Paramagnetic Resonance of Transition Ions, Clarendon Press, Oxford, 1970 Search PubMed; (b) F. E. Mabbs and D. Collison, Electron Paramagnetic Resonance of d Transition Metal Compounds, Elsevier, Amsterdam, 1992 Search PubMed; (c) C. Duboc, M.-N. Collomb and F. Neese, Appl. Magn. Reson., 2010, 37, 229 CrossRef; (d) R. Boča, Coord. Chem. Rev., 2004, 248, 757 CrossRef.
- (a) M. Soler, E. Rumberger, K. Folting, D. N. Hendrickson and G. Christou, Polyhedron, 2001, 20, 1365 CrossRef CAS; (b) C. Boskovic, E. K. Brechin, W. E. Streib, K. Folting, J. C. Bollinger, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 2002, 124, 3725 CrossRef CAS PubMed.
- D. Reta and N. F. Chilton, Phys. Chem. Chem. Phys., 2019, 21, 23567–23575 RSC.
- See for example: (a) S. M. Taylor, R. D. McIntosh, S. Piligkos, S. J. Dalgarno and E. K. Brechin, Chem. Commun., 2012, 48, 11190 RSC; (b) C. Boskovic, E. K. Brechin, W. E. Streib, K. Folting, D. N. Hendrickson and G. Christou, Chem. Commun., 2001, 467–468 RSC; (c) E. K. Brechin, J. C. Huffman, G. Christou, J. Yoo, M. Nakano and D. N. Hendrickson, Chem. Commun., 1999, 783 RSC; (d) C. Boskovic, E. K. Brechin, W. E. Streib, K. Folting, J. C. Bollinger, D. N. Hendrickson and G. Christou, J. Am. Chem. Soc., 2002, 124, 3725 CrossRef CAS PubMed; (e) J. Yoo, E. K. Brechin, A. Yamaguchi, M. Nakano, J. C. Huffman, A. L. Maniero, L.-C. Brunel, K. Awaga, H. Ishimoto, G. Christou and D. N. Hendrickson, Inorg. Chem., 2000, 39, 3615–3623 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2233436 (1), 2233437 (3) and 2233438 (2). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt00732d |
| This journal is © The Royal Society of Chemistry 2023 |