Open Access Article
Open Access ArticleStructural and conformational analysis of a biaryl phosphine integrating a calix[4]arene cavity. Can the phosphorus atom behave as an introverted donor?†
Christophe
Gourlaouen
 a,
Fethi
Elaieb
b,
Eric
Brenner
a,
Fethi
Elaieb
b,
Eric
Brenner
 b,
Dominique
Matt
b,
Dominique
Matt
 *b,
Jack
Harrowfield
*b,
Jack
Harrowfield
 c and
Louis
Ricard
d
c and
Louis
Ricard
d
aLaboratoire de Chimie Quantique, Institut de Chimie, UMR 7177 CNRS-Université de Strasbourg, 4 rue Blaise Pascal, 67070 Strasbourg Cedex, France
bInstitut de Chimie, UMR 7177 CNRS, Université de Strasbourg, 4 Rue Blaise Pascal, 67070 Strasbourg Cedex, France. E-mail: dmatt@unistra.fr
cInstitut de Science et Ingénierie Supramoléculaire (ISIS), UMR 7606 CNRS, Université de Strasbourg, 8 rue Gaspard Monge, 67083 Strasbourg Cedex, France
dLaboratoire de Chimie Moléculaire, CNRS UMR 9168, Ecole Polytechnique, 2 route de Saclay, F-91128 Palaiseau Cedex, France
First published on 10th May 2023
Abstract
The conformational preference of a cavity-based biaryl phosphine, namely 5-(2-diphenylphosphinyl-phenyl)-25,26,27,28-tetrapropyloxycalix[4]arene (L) has been investigated by density functional theory calculations. The analysis showed that the barrier to rotation about the C–C axle of the biaryl unit is only 10.7 kcal mol−1, this rendering possible access to conformers of two types, those in which the P lone pair sits at the cavity entrance and points to the calixarene interior, others with a more open structure where the P atom is located outside the cavity. As revealed by a single crystal X-ray diffraction study, the biaryl phosphine appears virtually as an atropisomer in the solid state in which the phosphorus atom lies totally out of the cavity defined by the four phenoxy rings.
Introduction
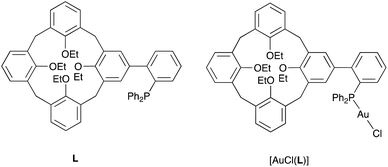
There has been considerable interest in recent years in the development of ligands which covalently link a phosphorus donor atom and a cavity-shaped moiety.1 Most ligands of this kind have been designed with the expectation that the cavity would function as a confining unit towards P-bound metal centres,2 thereby operating as an efficient steric hindrance controller. In other cases, the cavity essentially behaves as a substrate receptor so as to assist the metal in a supramolecular fashion during catalytic transformations.3As an extension to our studies on cavitand-based phosphines, we herein report a structural and conformational analysis of the tertiary phosphine L (Fig. 1), a cone-shaped calix[4]arene4 having a (2-diphenylphosphinyl)-phenyl group grafted at its wider rim.5 With its P(III) atom substituted by a biaryl moiety, L belongs to the family of the so-called biaryl phosphines, a class of ligands of which the relevance to catalysis is well documented. In particular, biaryl phosphines have been successfully used for a variety of palladium-catalyzed carbon–carbon, carbon–nitrogen, and carbon–oxygen forming reactions.6 It is well-known, for example, that in Suzuki–Miyaura cross coupling reactions, their efficiency relies on the ability of the remote aryl ring to bind transiently a P-bound metal centre, thus enabling stabilisation of key, monophosphine intermediates.7 The following computational study was undertaken with the aim of assessing how endo- or exo-orientation of the P-lone pair with respect to the calixarene cavity of L might affect the steric hindrance around the donor atom, and in particular whether such a phosphine could behave as an introverted ligand by binding in its endo conformation.
Results and discussion
Preliminary structural study
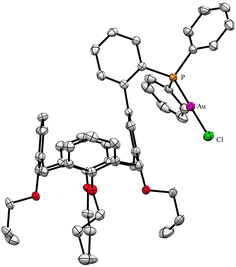
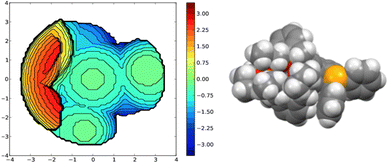
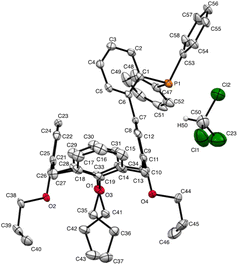
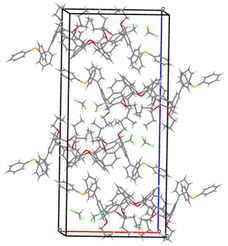
A useful descriptor of the spatial hindrance induced by a phosphine ligand in a transition metal complex is its so-called percent buried volume (% Vbur), a parameter which can be determined by the SambVca web tool recently developed by Cavallo and co-workers.8 The advantage of this particular steric parameter over earlier ones9 lies in its greater reliability in measuring the steric impact proximal to the metal (previous methods, notably Tolman's cone angle,9a being more sensitive to ligand size at a distance), not to mention its facile estimation. The percent buried volume corresponds to the percent of the volume of a sphere occupied by the ligand in a complex and centered at the coordinated metal. Its selected radius is 3.5 Å. It formally represents the space of a potential first coordination sphere. To determine the % Vbur of L, we used the crystallographic data obtained for the previously reported gold complex [AuCl(L)]5 (Fig. 2), from which we “extracted” the structure of the phosphine, exactly as it appears in the complex. Note, in this complex, the P–Au–Cl fragment lies totally outside the calixarene cavity, which has a pinched-cone form leaving little room for any guest inclusion. The calculation, in which H atoms were considered, resulted in a percent buried volume (% Vbur) of 44.5. For comparison, the percent buried volumes reported in the literature for P(Mes)3, PCy3 and PPh3 are respectively 47.6, 31.8, and 29.6.10Fig. 3 shows the steric occupation of the ligand.We deemed it appropriate to compare the structure of L in its gold complex with that of the isolated free ligand, the synthesis of which has been reported elsewhere.5 This structure was expected to provide the preferred positioning of the PPh2 unit of L with respect to the cavity, “in” or “out”. In the following, conformers having the P atom located outside the cavity defined by the calixarene core are termed out-conformers, those with the P located inside the cavity in-conformers. Crystals of free L were readily obtained from a CHCl3 solution of the phosphine. As revealed by a single crystal X-ray diffraction study (Fig. 4), the phosphine crystallised as a chloroform solvate in the orthorhombic space group Pbca with 8 molecules in the unit cell (Fig. 5). The conformer seen in the solid crystal is an out-conformer, with the P-lone pair pointing roughly to the lower rim of the calixarene and lying nearly parallel to the plane of the nearest calixarene phenyl ring, as in [AuCl(L)]. The calixarene core of the phosphine again adopts a pinched-cone conformation, with facing phenoxy ring pairs having interplanar angles of 68.6° and 21.6°. The marked flattening of the calixarene core is best seen by comparing the separations between the centroids of the opposite phenoxy walls, 4.73(1) Å for C21–C26/C7–C12 and 7.80(1) Å for C14–C19/C28–C33 (cf. 4.86(1) Å and 7.70(1) Å in [AuCl(L)]). The angles between the mean plane of the four bridging methylenic carbon atoms (C13, C20, C27, and C34) and the four phenolic rings (C7–C12, C14–C19, C21–C26, C28–C33) are respectively 79.0°, 34.1°, 79.4°, and 34.4°. The twist angle of the biaryl unit is smaller than that seen in [AuCl(L)] (51° vs. 69°), so that the P atom lies here a little closer to the calixarene external wall than in the complex. Finally, we note that, as in [AuCl(L)], the phosphorus atom is linked to one of those facing phenoxy rings having their para-carbon atoms slightly inclined towards the cavity interior. The CHCl3 solvent molecule lies outside the macrocyclic core. Its hydrogen atom (H50) approaches the C7 and C12 atoms of the biaryl fragment. The particular orientation of the C50–H50 bond and the relatively short H50⋯C7 (2.86(1) Å) and H50⋯C12 (2.80(1) Å) distances are obviously indicative of CH–aryl π interactions. While the solvated molecule L·CHCl3 can be described as the “free” ligand, there are, as in any molecular crystal, interactions in the solid state that may have an influence on its conformation. Consideration of the Hirshfeld surface for L in this crystal as obtained by the use of CrystalExplorer11 shows (Fig. S1†), however, that there are very few interactions which exceed dispersion and that more than 70% of the surface contacts are (dispersive) H⋯H. Most of the interactions exceeding dispersion are Cl⋯HC, as indeed is true (Fig. S2†) for the crystal of the complex [AuCl(L)] (where 72% of the surface contacts are H⋯H), but there is one aromatic C⋯C interaction in the ligand crystal that serves to link phenyl groups bound directly to P into chains parallel to the b axis (Fig. S3†). This is not a case of parallel aromatic stacking,12 since the phenyl rings involved are inclined at ∼30° to one another with a rather long centroid⋯centroid separation of 3.8418(3) Å, while the one contact exceeding dispersion is also long (3.251(8) Å) and involves only the phenyl groups on phosphorus, so that it is not an obvious influence upon the orientation of the P lone pair with respect to the cavity.
Conformational study
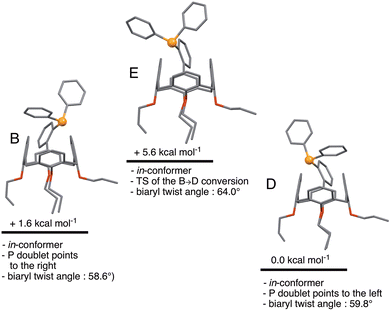
We began the conformational study by minimising the energy of the out-conformer revealed by the above X-ray study. This investigation resulted in conformer A (Fig. 6) displaying structural characteristics close, if not identical, to those of L in the solid (P lone pair pointing to the lower part of the cavity; calixarene moiety adopting a pinched-cone conformation; interplane angles between the facing phenoxy rings of 12.3° and 73.6°; biaryl twist angle: 68.6°). We then gradually changed the dihedral biaryl angle in A in order to explore the potential energy surface leading to an in-conformer. Optimisation of the energy maximum resulted in transition state (TS) C (Fig. 6), the imaginary frequency of which led to in-conformer B (Fig. 6). For this transformation an energy barrier of 10.7 kcal mol−1 was established, thus reflecting the ease of displacing the phosphorus atom from “out” to “in” positions, and vice versa. It should be recalled that that conformational locking of atropisomers‡ only occurs for rotational barriers above 30 kcal mol−1.13 It should also be mentioned here that B was not the most stable in-conformer found in this study (vide infra).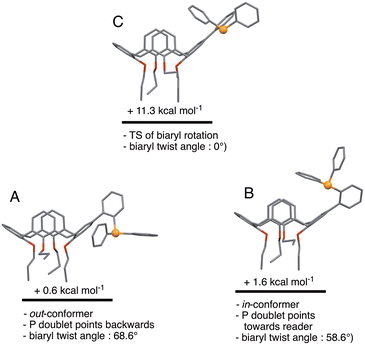 | ||
| Fig. 6 Relative energies of in- and out-conformers of L. Conformer C represents the transition state for the A → B conversion (i.e. rotation about the biaryl single bond). | ||
A further consideration was how much energy it would cost, starting from B, to orientate the phosphorus doublet to the interior of the cavity. In fact, no conformer with this feature could be found. Instead, structure E was found, which is a transition state connecting two in-conformers, namely B and D (Fig. 7). The TS character of E arises from attractive dispersion forces between the P atom and an upper-rim H atom of the calixarene unit, as evidenced by NCI analysis on D (Fig. S4†). Such stabilising interactions were not seen in E. Overall, the B → E → D conversion can be seen as a combined rotation about the C–C(biaryl) and the P–C(C6H4) bonds. The corresponding energy barrier is here only 3.0 kcal mol−1. Thus, there is virtually no impediment for the P atom of L to behave as an introverted donor in solution, although such a coordinative behaviour has not yet been observed experimentally.
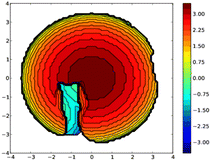
A topographic steric map, based on such an hypothetical conformation is represented in Fig. 8. As expected, the buried volume of this conformer, 79.4%, is considerably higher than that of the “out” version displayed above. Overall, the bulkiness of L should be regarded as variable, its magnitude being related to the place a coordinated metal centre would occupy, inside or outside the cavity. Although not observed yet with L, we anticipate that “intra-cavity” complexes should form with L, provided the coordinated metal fragment possesses a size compatible with that of the calixarene inner space. In this regard, we compared the energies of two hypothetical isomers of formula [Au(CO)L]+, one containing an inwardly oriented P atom, the other with a P atom directed away from the calixarene (Fig. 9) (examples of carbonyl complexes of the type [Au(CO)PR3]+ have been reported in the literature14). Calculations revealed that the introverted complex is by ca. 6.5 kcal mol−1 more stable than its exo-counterpart. This may be largely attributed to weak inner-cavity interactions involving the carbonyl moiety and two facing phenoxy rings (distances between the CO rod and these rings: ca. 3.1 Å). NCI analysis confirmed these findings (Fig. S5†).
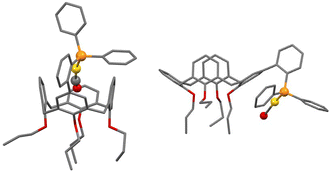 | ||
| Fig. 9 Calculated structures of cationic [Au(CO)L]+ complexes having their “Au(CO)” moiety positioned either inside (left) or outside (right) the macrocyclic cavity. | ||
Experimental
Computational details for the theoretical study
All calculations were performed with GAUSSIAN 09 (D.01)15 at DFT level of theory (B3LYP functional16) and 6-31+G** basis set for all atoms.17 Dispersion forces were introduced using Grimme's corrections.18 Solvent was included through a PCM model of chloroform.19 All structures were fully optimized and the nature of the encountered stationary point checked by the computation of the frequencies. All minima were characterized by a complete set of real frequencies and the transition states (TS) by one and only imaginary frequency.Synthesis
The free biaryl phosphine 5-(2-diphenylphosphanyl-phenyl)-25,26,27,28-tetrapropyloxy calix[4]arene (L) was prepared according to a reported procedure.5Crystallography
Single crystals of L were grown as colorless prisms by cooling to −15 °C a CHCl3 solution of the compound. Data were collected at 150 K on a Nonius Kappa APEX II CCD diffractometer using an MoKα (λ = 0.71069 Å) X-ray source and a graphite monochromator. Formula: C58H61O4P·CHCl3; Mr = 972.40 g mol−1; orthorhombic; space group Pbca; a = 16.8473(19), b = 16.4750(19), c = 37.556(4) Å, V = 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 424(2) Å3; Z = 8; Dcalcd = 1.239 g cm−3; μ = 0.253 mm−1; F(000) = 4112. Total reflections collected 40
424(2) Å3; Z = 8; Dcalcd = 1.239 g cm−3; μ = 0.253 mm−1; F(000) = 4112. Total reflections collected 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 548, 7373 with I > 2σ(I). Goodness of fit on F2 1.076; R(I > 2σ(I)) = 0.0967, wR2 = 0.2991 (all data), 628 parameters; maximum/minimum residual density 1.106/−1.391 e Å−3. The crystal structure was solved in SHELXT20 and refined in SHELXL97
548, 7373 with I > 2σ(I). Goodness of fit on F2 1.076; R(I > 2σ(I)) = 0.0967, wR2 = 0.2991 (all data), 628 parameters; maximum/minimum residual density 1.106/−1.391 e Å−3. The crystal structure was solved in SHELXT20 and refined in SHELXL97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 21 by full-matrix least-squares using anisotropic thermal displacement parameters for all non-hydrogen atoms. Each phosphine unit is associated to a molecule of CHCl3. Crystal data and further information on the structure determination are summarized in Table S1.† Selected bond lengths and angles are given in Table S2.†
21 by full-matrix least-squares using anisotropic thermal displacement parameters for all non-hydrogen atoms. Each phosphine unit is associated to a molecule of CHCl3. Crystal data and further information on the structure determination are summarized in Table S1.† Selected bond lengths and angles are given in Table S2.†
Conclusions
In conclusion, the present study illustrates the potential of calix[4]arenes having an o-(PPh2)–C6H4 substituent appended at their upper rim to behave either as classical triaryl phosphines displaying an open structure or as bulky, introverted ligands in which the P lone pair is directed towards the cavity interior. The study constitutes a reminder that the values which usually express the size of a phosphine (cone angle, % buried volume) are inferred from static structures and thus do not take into account possible solution dynamics that may lead to particular conformers able to trap a metal atom inside a cavity-shaped subunit. It would therefore probably make sense to introduce a dual size parameter for all phosphines in which the phosphorus atom is appended to a cavity-shaped subunit.Conflicts of interest
There are no conflicts to declare.Notes and references
- (a) D. Matt and J. Harrowfield, ChemCatChem, 2021, 13, 153–168 CrossRef CAS; (b) A. Molnár, ChemCatChem, 2021, 13, 1424–1474 CrossRef; (c) G. Cera, G. Giovanardi, A. Secchi and A. Arduini, Chem. – Eur. J., 2021, 27, 10261–10266 CrossRef CAS PubMed; (d) Z. Kaya, E. Bentouhami, K. Pelzer and D. Armspach, Coord. Chem. Rev., 2021, 445, 214066 CrossRef CAS; (e) G. S. Ananthnag, D. Mondal, J. T. Mague and M. S. Balakrishna, Dalton Trans., 2019, 48, 14632–14641 RSC; (f) S. Tilloy, H. Bricout, S. Menuel, F. Hapiot and E. Monflier, Curr. Organocatal., 2016, 3, 24–31 CrossRef CAS; (g) N. Endo, M. Inoue and T. Iwasawa, Eur. J. Org. Chem., 2018, 1136–1140 CrossRef CAS; (h) I. R. Knyazeva, A. R. Burilov, M. A. Pudovik and W. D. Habicher, Russ. Chem. Rev., 2013, 82, 150–186 CrossRef; (i) R. Gramage-Doria, D. Armspach and D. Matt, Coord. Chem. Rev., 2013, 257, 776–816 CrossRef CAS; (j) Phosphorus(III) Ligands in Homogeneous Catalysis: Design and Synthesis, ed. P. C. J. Kamer and P. W. N. M. van Leeuwen, John Wiley & Sons, Ltd, 2012 Search PubMed; (k) J. Vachon, S. Harthong, E. Jeanneau, C. Aronica, N. Vanthuyne, C. Roussel and J. P. Dutasta, Org. Biomol. Chem., 2011, 9, 5086–5091 RSC; (l) S. D. Alexandratos and S. Natesan, Ind. Eng. Chem. Res., 2000, 39, 3998–4010 CrossRef CAS; (m) D. Over, A. de la Lande, X. S. Zeng, O. Parisel and O. Reinaud, Inorg. Chem., 2009, 48, 4317–4330 CrossRef CAS PubMed; (n) F. J. Parlevliet, M. A. Zuideveld, C. Kiener, H. Kooijman, A. L. Spek, P. C. J. Kamer and P. W. N. M. van Leeuwen, Organometallics, 1999, 18, 3394–3405 CrossRef CAS; (o) C. Wieser, C. B. Dieleman and D. Matt, Coord. Chem. Rev., 1997, 165, 93–161 CrossRef CAS.
- J. Emerson-King, S. Pan, M. R. Gyton, R. Tonner-Zech and A. B. Chaplin, Chem. Commun., 2023, 59, 2150–2152 RSC.
- H. K. A. C. Coolen, P. W. N. M. van Leeuwen and R. J. M. Nolte, Angew. Chem., Int. Ed., 1992, 31, 905–907 CrossRef.
- D. Gutsche, Calixarenes, in Monographs in Supramolecular Chemistry, ed. J. F. Stoddart, RSC, Cambridge, UK, 1989 Search PubMed.
- F. Elaieb, D. Sémeril, D. Matt, M. Pfeffer, P. A. Bouit, M. Hissler, C. Gourlaouen and J. Harrowfield, Dalton Trans., 2017, 46, 9833–9845 RSC.
- (a) R. Martin and S. L. Buchwald, Acc. Chem. Res., 2008, 41, 1461–1473 CrossRef CAS PubMed; (b) D. S. Surry and S. L. Buchwald, Chem. Sci., 2011, 2, 27–50 RSC; (c) P. Ruiz-Castillo and S. L. Buchwald, Chem. Rev., 2016, 116, 12564–12649 CrossRef CAS PubMed; (d) R. Dorel, C. P. Grugel and A. M. Haydl, Angew. Chem., Int. Ed., 2019, 58, 17118–17129 CrossRef CAS PubMed.
- J. L. Lamola, P. T. Moshapo, C. W. Holzapfel and M. C. Maumela, RSC Adv., 2021, 11, 26883–26891 RSC.
- L. Falivene, R. Credendino, A. Poater, A. Petta, L. Serra, R. Oliva, V. Scarano and L. Cavallo, Organometallics, 2016, 35, 2286–2293 CrossRef CAS.
- (a) C. A. Tolman, Chem. Rev., 1977, 77, 313–348 CrossRef CAS; (b) A. Immirzi and A. Musco, Inorg. Chim. Acta, 1977, 25, L41–L42 CrossRef CAS.
- H. Clavier and S. P. Nolan, Chem. Commun., 2010, 46, 841–861 RSC.
- (a) C. F. McKenzie, P. R. Spackman, D. Jayatilaka and M. A. Spackman, IUCrJ, 2017, 4, 575–587 CrossRef PubMed; (b) P. R. Spackman, M. J. Turner, J. J. McKinnon, S. K. Wolff, D. J. Grimwood, D. Jayatilaka and M. A. Spackman, Crystal Explorer 21.5, University of Western Australia, Perth, Australia, 2021 Search PubMed.
- K. Carter-Fenk and J. M. Herbert, Phys. Chem. Chem. Phys., 2020, 22, 24870–24886 RSC.
- (a) F. R. Leroux and H. Mettler, Adv. Synth. Catal., 2007, 349, 323–336 CrossRef CAS; (b) V. Zeindlhofer, P. Hudson, A. M. Pálvölgyi, M. Welsch, M. Almarashi, H. L. Woodcock, B. Brooks, K. Bica-Schröder and C. Schröder, Int. J. Mol. Sci., 2020, 21, 6222–6241 CrossRef CAS PubMed.
- (a) G. Ciancaleoni, N. Scafuri, G. Bistoni, A. Macchioni, F. Tarantelli, D. Zuccaccia and L. Belpassi, Inorg. Chem., 2014, 53, 9907–9916 CrossRef CAS PubMed; (b) H. V. R. Dias, C. Dash, M. Yousufuddin, M. A. Celik and G. Frenking, Inorg. Chem., 2011, 50, 4253–4255 CrossRef CAS PubMed; (c) D. A. Roşca, J. A. Wright and M. Bochmann, Dalton Trans., 2015, 44, 20785–20807 RSC.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery, Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 09, Gaussian, Inc., Wallingford CT, 2016 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- R. Ditchfield, W. J. Hehre and J. A. Pople, J. Chem. Phys., 1971, 54, 724 CrossRef CAS.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed.
- S. Miertuš, E. Scrocco and J. Tomasi, Chem. Phys., 1981, 55, 117–129 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Adv., 2015, A71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, C71, 3–8 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Cartesian coordinates of calculated structures. Crystal and refinement data, selected bond lengths and angles, Hirshfeld surfaces, NCI analyses, and CIF for L·CHCl3. CCDC 2142053. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3dt00612c |
| ‡ The term atropisomers does formally not apply for conformers such as A and B. Atropisomers are stereoisomers arising because of hindered rotation about a single bond, where energy differences due to steric strain create a barrier to rotation which is sufficiently high to allow isolation of individual conformers. Thus the stereoisomer found in the solid should merely be regarded as an out-conformer. |
| This journal is © The Royal Society of Chemistry 2023 |