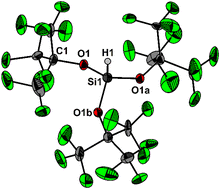
The perfluorinated alkoxy silanes {(F3C)3CO}3SiH (1) and {(F5C6)3CO}2Si(Cl)H (2) were prepared and fully characterized. Despite the high calculated Brønsted acidities, all attempts to deprotonate 1 and 2 to give the conjugate silanide ions failed due to the exceptionally short and strong Si–H bonds. In the solid state, the Si–H units are not involved in any intermolecular interactions, but instead the crystal packing consists of exceptionally short and strong F⋯F interactions. The cohesive energies are entirely comprised of London dispersion interactions, similarly as in the crystal structures of noble gases.