Open Access Article
Open Access ArticleSynthesis of π-extended and bowl-shaped sumanene–ferrocene conjugates and their application in highly selective and sensitive cesium cations electrochemical sensors†
Jakub S.
Cyniak
a,
Łucja
Kocobolska
a,
Natalia
Bojdecka
a,
Aleksandra
Gajda-Walczak
b,
Agata
Kowalczyk
 b,
Barbara
Wagner
b,
Barbara
Wagner
 c,
Anna M.
Nowicka
c,
Anna M.
Nowicka
 b,
Hidehiro
Sakurai
b,
Hidehiro
Sakurai
 de and
Artur
Kasprzak
de and
Artur
Kasprzak
 *a
*a
aFaculty of Chemistry, Warsaw University of Technology, Noakowskiego Str. 3, 00-664 Warsaw, Poland. E-mail: artur.kasprzak@pw.edu.pl
bFaculty of Chemistry, University of Warsaw, Pasteura Str. 1, 02-093 Warsaw, Poland
cBiological and Chemical Research Centre, Faculty of Chemistry, University of Warsaw, Zwirki i Wigury Str. 101, 02-093 Warsaw, Poland
dDivision of Applied Chemistry, Graduate School of Engineering, Osaka University, 2-1 Yamadaoka, Suita, 565-0871 Osaka, Japan
eInnovative Catalysis Science Division, Institute for Open and Transdisciplinary Research Initiatives (ICS-OTRI), Osaka University, Suita, Osaka 565-0871, Japan
First published on 13th February 2023
Abstract
Carbon–carbon bond formation, condensation or click chemistry reactions were used to synthesize novel bowl-shaped sumanene–ferrocene conjugates, along with the extended π-electron framework in good yields. For the first time, the present study uses sumanene derivatives tris-substituted at the benzylic positions as the materials to begin the study on the click chemistry or the metal-catalyzed coupling reactions, Suzuki–Miyaura or Sonogashira couplings. The synthesized conjugates exhibited the property of selective recognizing cesium cations. As a result, this led to the development of highly sensitive and selective fluorescent or electrochemical sensors dedicated to the recognition of cesium cations (Cs+) in water. We successfully designed the Cs+ electrochemical sensors, which exhibited an acceptable limit of detection (LOD) values at 0.05–0.38 μM. Spectrofluorimetry, voltammetry, and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS) were used to perform the selectivity studies. The results revealed that the designed sensors are highly Cs+-selective. This work significantly contributes to the design of new methods of sumanene modification. It also provides further information on the electrochemical properties and innovative applications of metallocene-tethered sumanene derivatives.
Introduction
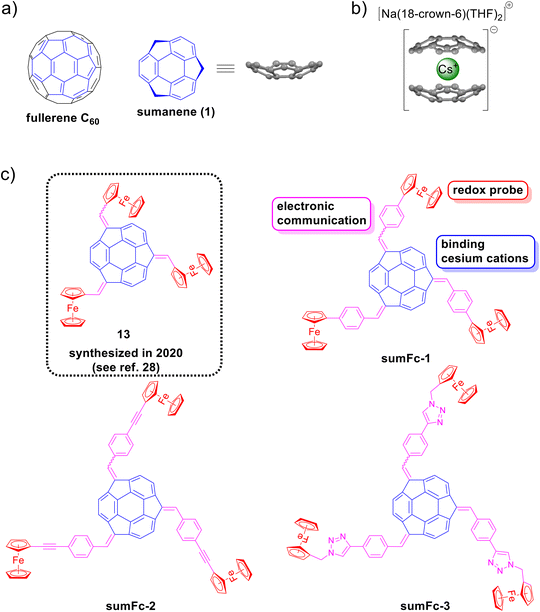
Sumanene (1) is a bowl-shaped compound, first synthesized in 2003,1,2 and is a fragment of fullerene C60 (Fig. 1a). The compound belongs to the class of the so-called buckybowls and features several interesting physicochemical properties related to its specific bowl shape.3–8 Due to the presence of both aromatic and benzylic moieties in the structure of sumanene, the synthetic view of the compound offers interesting opportunities for organic chemists. There have been several demonstrations over the years of the functionalization of sumanene at those positions, as represented by the successful attempts on the electrophilic aromatic substitution (SEAr) reaction,9–11 or reactions including sumanenyl benzylic anions.12,13 As a result, there have been studies reporting several functional organic materials bearing the sumanene skeleton and featuring interesting properties and functions.10,14–18Over the years, there have been theoretical19–21 and experimental22–26 studies on the interactions of sumanene with metal cations. The interaction of sumanene and its derivatives with cesium cations, which results in the formation of the sandwich-type complex is particularly fascinating (Fig. 1b). This interaction occurs in the anionic25 or neutral27–30 state of sumanene and occurs due to the perfect size match between the concave site of the sumanene bowl and the cesium cation's van der Waals radius. Recently, it was demonstrated that sumanene derivatives were used as fluorescent27,29,30 or electrochemical27,28 receptors of cesium cations. The interaction is remarkably specific and has no interfering effects from other cations, including rubidium cations.31,32 The results of the studies have great environmental importance due to significant concentrations of radioactive cesium isotopes, including Cs137, were found in post-disaster areas of nuclear plant accidents in Fukushima and Chernobyl.33–36
In this study, the synthesis of novel sumanene–ferrocene conjugates sumFc-1–sumFc-3 (Fig. 1c) and their application as receptor layers in electrochemical sensors to detect cesium cations in water is reported. The study focused on introducing copper-catalyzed azide–alkyne cycloaddition (CuAAC; click chemistry) or Sonogashira cross-coupling as new tools for sumanene derivatization. While the click chemistry reaction is eagerly used in the synthesis of functional organic compounds,37–39 there are no reports on its use in the modification of sumanene. The design of click chemistry protocol for sumanene chemistry might offer new ways of modification of this bowl-shaped compound, due to the commercial availability or the easiness of preparation of various azide- or alkyne-containing molecules, as well as applicability of click chemistry reactions under aqueous conditions. Additionally, Sonogashira cross-coupling can likely be considered as a method for the expansion of π-electron system of sumanene. However, currently, 2-ethynylsumanene3 is the only very preliminary40 example of sumanene derivatized with Sonogashira cross-coupling.
Additionally, the study used sumanene derivatives tris-substituted at the benzylic positions for the first time as the starting materials in CuAAC or metal-catalyzed coupling reactions, namely Suzuki–Miyaura or Sonogashira couplings. The study further anticipated the enhanced electronic communication between the ferrocene units (redox probe) and sumanene (receptor site) in those compounds, considering the expansion of π-electron networks in sumFc-1–sumFc-3 (see Fig. 1c). We suspected that this would result in good optical and beneficial properties of the electrochemical sensors. Thus, inspired by the our recent preliminary work28 on tris(ferrocenylmethidene)sumanene (13) in electrochemical sensors, herein the successful design of highly selective and sensitive cesium cations electrochemical sensors comprising sumFc-1–sumFc-3 in the role of receptor layers is reported.
Results and discussion
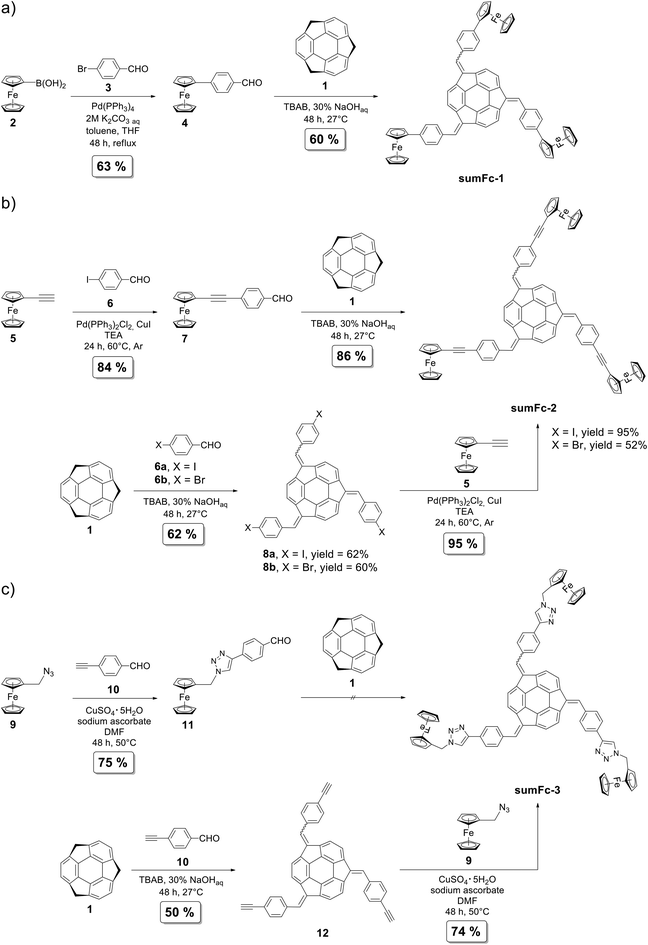
The synthesis of sumanene–ferrocene conjugates sumFc-1–sumFc-3 is presented in Scheme 1. For the full experimental details, see section 1 in ESI.†The synthesis of sumanene–ferrocene conjugate sumFc-1 (Scheme 1a) was based on a two-step process. In the first stage, the Suzuki–Miyaura cross-coupling reaction was used to synthesize 4-(formylphenyl)ferrocene (4) in good yield (63%) from ferroceneboronic acid (2). In the second stage, the condensation-type reaction (isolated yield: 60%) was employed to synthesize the compound sumFc-1 from sumanene (1) and compound 4. The condensation occurred under phase transfer catalyst (PTC) conditions13,28,29 with sodium hydroxide as a base and n-tetrabutylammonium bromide (TBAB) as a PTC catalyst.
Two synthetic pathways toward sumanene–ferrocene conjugate sumFc-2 were studied (Scheme 1b). Both synthetic pathways consisted of two steps and were based on the Sonogashira cross-coupling and condensation-type reactions. There were differences in the reaction sequence in the synthetic pathways. In the first synthetic path, the ferrocene derivative bearing an aldehyde group was synthesized using the Sonogashira cross-coupling and was further subjected to the condensation-type reaction with sumanene (1). In the second path, the sumanene derivative bearing the halogen group was synthesized via the condensation-type reaction. Following this, it was subjected to the Sonogashira cross-coupling reaction with ethynylferrocene (5). In the first stage of the first synthetic path toward sumFc-2, a Sonogashira cross-coupling reaction was used to synthesize compound 7 from ethynylferrocene (5). In the second stage, compound 7 was subjected to the condensation-type reaction with sumanene (1). There were high yields for both steps (84–86%). In the second synthetic path toward sumFc-2, sumanene (1) was subjected to the condensation-type reaction with 4-iodobenzaldehyde (6), which yielded tris[(4-iodophenyl)methidene]sumanene (8a; 62%). Sonogashira cross-coupling reaction was used to synthesize the target sumFc-2 from 8a and 5. The reaction yield was very high and equaled 95%. The reactivity of the respective tris[(4-bromophenyl)methidene]sumanene (8b) in the Sonogashira cross-coupling reaction with 5 was also investigated. The yield of sumFc-2 synthesis starting from 8b was significantly lower (52%) compared to the reaction yield starting from tris-iodo sumanene derivative 8a. This denotes that the reactivity of halogen-containing bowl-shaped π-conjugated sumanene derivatives shall follow the general trend of the reactivity of aromatic halides in the Sonogashira cross-coupling reaction.
The preliminary trials toward the synthesis of sumFc-3 were based on a similar pathway as those mentioned above. The pathways included the modification of the ferrocene derivative in the first stage and the subsequent condensation-type reaction with sumanene (Scheme 1c) in the second stage. The CuAAC reaction was employed to synthesize 1,2,3-triazole containing ferrocene derivative 11 efficiently (75%) from ferrocenemethylazide (9). Newly synthesized compound 11 was subjected to the condensation-type reaction with sumanene. However, despite several attempts, the target product sumFc-3 was not obtained. We suspected that this resulted due to the presence of an active methylene group in 11, which under basic conditions would likely interfere with the expected reaction pathway. In order to omit this severe limitation, the second synthetic pathway toward sumFc-3 was studied. In the first stage, tris[(4-ethynylphenyl)methidene]sumanene (12) was synthesized from sumanene using the condensation-type reaction. This alkyne containing sumanene derivative was then used as the starting material in the synthesis of sumFc-3. The CuAAC reaction between 9 and 12 enabled the efficient (74%) synthesis of target sumFc-3. It is to be noted that sumFc-3 is the first example of a sumanene derivative synthesized employing the click chemistry reaction.
Sumanene–ferrocene conjugates sumFc-1–sumFc-3 were analyzed using an NMR spectroscopy and a high-resolution mass spectrometry (HRMS). The NMR spectra of sumFc-1–sumFc-3 consisted of signals originating due to the presence of C3 symmetrical and asymmetrical diastereomer.13,28,29 The NMR spectra and a detailed discussion on the NMR spectra of sumFc-1–sumFc-3 are presented in section 2 of the ESI.† Briefly, 7–9 signals or 24–30 signals were observed in the 1H NMR analyses for symmetrical or unsymmetrical diastereomers, respectively. There were 16–19 signals or 48–57 signals in the 13C{1H} NMR analyses for symmetrical or unsymmetrical diastereomers, respectively. Due to the overlapped signals observed in the NMR spectra, the presence of many multiplets in the spectra was observed. In each case, the observed number of signals was highly consisted with the anticipated values, also within the given groups of multiplets originating from the presence of structural motifs introduced to the sumanene framework. The analysis of the cross-coupling signals in the 1H–1H COSY NMR spectra supported the proper assignments of the signals, as presented graphically in Fig. 2 for the representative sumanene derivative sumFc-1. Additionally, the signals originating from the presence of ferrocene in the synthesized compounds overlapped in the 1H NMR and 13C{1H} NMR spectra. Therefore, the 1H–13C HSQC NMR analyses were especially helpful in supporting the ascription of the signals, which originated due to the presence of ferrocene in the synthesized conjugates sumFc-1–sumFc-3 (see Fig. S45 in section 2 in ESI†).
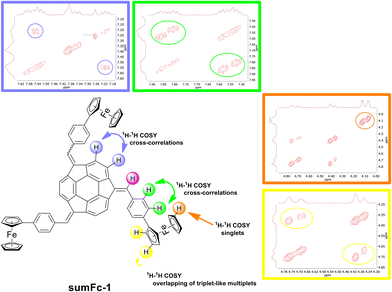 | ||
| Fig. 2 The graphical representation of the cross-correlations in the 1H–1H COSY NMR spectra of sumanene–ferrocene conjugate sumFc-1 together with the crucial insets of this spectra. The same color does not correspond to the same chemical shift in the NMR spectrum. For the detailed discussion on the NMR spectra of sumFc-1–sumFc-3, see section 2 in ESI.† | ||
To our delight, despite relatively sophisticated structures, compounds sumFc-1–sumFc-3 exhibited good solubility in many commonly used organic solvents, including chloroalkanes (chloroform, dichloromethane, 1,2-dichloroethane), aromatics (benzene, toluene, chlorobenzene, o-dichlorobenzene), dimethylsulfoxide or tetrahydrofuran. The absorption spectra of sumFc-1–sumFc-3 featured three absorption maxima (λmax) located at 260–278 nm, 365–370 nm and 480–510 nm (for the spectra, see section 4 in ESI†). In comparison to the absorption spectrum of sumanene (1; λmax = 278 cm−1 in CH2Cl2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11,41), the presence of two batochromically shifted absorption maxima for sumFc-1–sumFc-3 was observed. It was ascribed13,28 to the expansion of π-electron system and the presence of ferrocene units in sumFc-1–sumFc-3 structures. The highest emission intensity was found for compound sumFc-3 (for the spectra, see section 4, ESI†).
11,41), the presence of two batochromically shifted absorption maxima for sumFc-1–sumFc-3 was observed. It was ascribed13,28 to the expansion of π-electron system and the presence of ferrocene units in sumFc-1–sumFc-3 structures. The highest emission intensity was found for compound sumFc-3 (for the spectra, see section 4, ESI†).
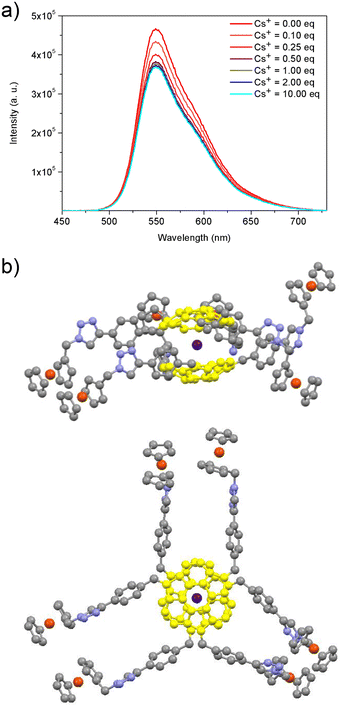
As discussed above, the presence of sumanene skeleton in sumFc-1–sumFc-3 opens up the possibility of the selective recognition of cesium cations by the compounds. At first, spectrofluorimetry was used to track the supramolecular interactions between the cesium cations and sumFc-1–sumFc-3. The addition of further portions (molar equivalents) of cesium cations caused a significant lowering of the emission intensity for each sumanene derivative. This is presented in Fig. 3a, for the representative compound sumFc-3 (for the respective spectra for sumFc-1 and sumFc-2, see section 5, ESI†). The dynamic interactions of the sumanene–ferrocene conjugates and cesium cations caused this turn-off behavior.27,29,30 While there were further decreases in the emission intensity due to an increase in the cesium cations concentration, the changes were not the same at each interval. It was a direct result of the stoichiometry of the non-covalent systems formed. A continuous variation method (Job's plot method)42 was employed to estimate stoichiometry for each system (see data in section 5, ESI†). A 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry (sumanene derivative
1 stoichiometry (sumanene derivative![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cesium cations) was concluded for each system. The stoichiometry corresponds to the anticipated sandwich-type complexes composed of one cesium cation encapsulated by two sumanene bowls.27,29,30Fig. 3b shows the illustrative visualization of the potential structure of those dynamically formed systems (for details of the calculation, refer to section 6 of ESI†).
cesium cations) was concluded for each system. The stoichiometry corresponds to the anticipated sandwich-type complexes composed of one cesium cation encapsulated by two sumanene bowls.27,29,30Fig. 3b shows the illustrative visualization of the potential structure of those dynamically formed systems (for details of the calculation, refer to section 6 of ESI†).
The Benesi–Hildebrand method29,30,43,44 was used to estimate the apparent binding constant (Kapp) for each system. The values of Kapp for newly synthesized sumanene–ferrocene conjugates sumFc-1–sumFc-3 were comparable with the Kapp values for other sumanene-based receptors.27,29,30 The values were equaled to 3.3 × 104 M−2, 8.4 × 104 M−2 and 7.3 × 104 M−2, for sumFc-1, sumFc-2, and sumFc-3, respectively. The selectivity of the interaction between sumFc-1–sumFc-3 and cesium cations was also investigated. There were no significant changes in the emission spectrum of sumFc-1–sumFc-3 measured in the presence of 10 equivalents of rubidium cations. Since rubidium cations were previously reported to be the major interferent to recognizing the cesium cations, this study supported that there was a high specificity in the interaction between cesium cations and sumFc-1–sumFc-3. Additionally, there was no interfering effect of other metal cations, i.e., sodium cations, potassium cations, magnesium cations, calcium cations, or barium cations. The high Cs+ selectivity of the designed receptors was ascribed to the perfect size match between sumanene concave site and van der Waals radius of cesium cations (ca. 3.4 Å). This conclusion is highly consistent with the reported results of theoretical19,21,25 and experimental25,27,30 studies. This perfect size match favors the cation–π interactions toward the dynamic formation of the complex, as visualized in Fig. 3b.
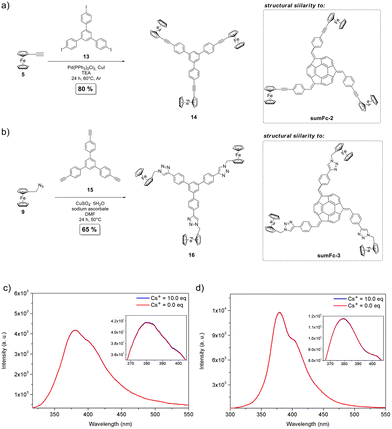
The ferrocenylated derivatives of the C3-symmetric non-bowl-shaped 1,3,5-triphenylbenzene were synthesized to elucidate the importance of the presence of sumanene skeleton in the receptors sumFc-1–sumFc-3 toward enabling Cs+ recognition. The derivatives were compounds 14 and 16, featuring structural similarity to sumFc-2 and sumFc-3, respectively (see Fig. 4). The experimental details on their synthesis are presented in section 1 in ESI.† For compounds 14 and 16, no changes in emission intensity were found when the spectra were measured even in the presence of the excess (10 eq.) of cesium cations (see Fig. 4 and data in section 6 in ESI†). Therefore, there was no interaction of 14 and 16 in a solution with cesium cations. This supports the crucial role of the presence of sumanene skeleton in compounds sumFc-1–sumFc-3 to provide the Cs+ recognition ability of those receptors.
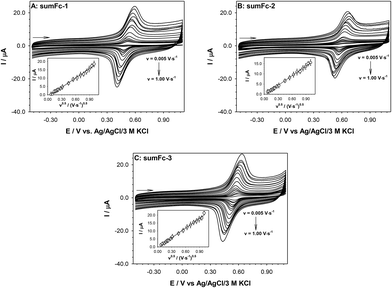
The presence of ferrocene in the sumanene–ferrocene conjugates sumFc-1–sumFc-3 makes them electroactive. The electrochemical characterization of sumFc-1–sumFc-3 was performed in DCM with the addition of 100 mM TBAPF6 as the supporting electrolyte. The representative cyclic voltammograms are presented in Fig. 5. One pair of the well-defined current signals, related to the Fc/Fc+ electrode process was visible on the voltammetric curves. It shows that all Fc molecules are oxidized or reduced at the same potential. The intensity of the currents signals in the studied scan rate range (0.005 to 1.0 V s−1) changed linearly with the square root of the scan rate. The insets in Fig. 5 present only the dependencies of Iox,Fcversus v0.5. The linear character of the plotted dependencies Iox, Fc = f(v0.5) indicates the diffusive nature of each electrode process in each studied compound. The intensity of the current signal in the case of diffusion controlled reversible or quasi-reversible electrochemical process is described by the Randles–Sevcik equation45 (eqn (1)):
| IFc = 2.69 × 105n3/2D1/2AC0v0.5 | (1) |
| Compound | Regression equation | r | D × 105 [cm2 s−1] |
|---|---|---|---|
| sumFc-1 | I Fc = 18.77·v0.5 − 1.21 | 0.9973 | 9.04 |
| sumFc-2 | I Fc = 15.72·v0.5 − 0.35 | 0.9988 | 6.34 |
| sumFc-3 | I Fc = 20.20·v0.5 − 0.36 | 0.9977 | 10.50 |
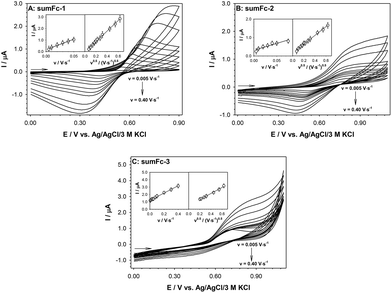
The receptor or analyte must have appropriate properties depending on the detection technique of the recognition process, enabling the use of a specific measurement technique. The required condition for the electrochemical detection methods is electroactivity, i.e., the ability of a molecule to replace an electron with the electrode surface. The electrochemical molecular recognition process should result in a change in the intensity of the current signal or a large shift in the redox potential of the analyte if it is electroactive, or an electroactive signaling unit. The signaling unit is usually performed by metallocenes, which include ferrocene and its derivatives. Thus, the first step was the voltammetric characterization of the containing receptor layers that contained the ferrocene-sumanene derivatives sum-Fc-1–sum-Fc-3. The cyclic voltammetry experiment was used to perform the electrochemical characteristics of the obtained receptor layers (GC/sumFc-TBAPF6-nafion™). The measurements were carried out in 50 mM TBABF4 aqueous solution. The recorded cyclic voltammograms for the various scan rates are presented in Fig. 6. The cyclic voltammograms of the recognition layers, obtained at the scan rates between 0.005 and 0.400 V s−1, exhibited well-developed anodic peak. However, the cathodic peak was only visible in the case of sumFc-1 and sumFc-2.
The electrode process should be a surface process since the studied sumanene–ferrocene receptors were immobilized on the electrode surface. In order to confirm this thesis, the dependencies of the anodic current signal intensity as a function of the electrode scan rate were plotted. The obtained dependencies are presented in the insets of Fig. 6. The linear dependencies of Iox, Fc = f(v) in the whole range of the studied scan rates were observed only for sumFc-3. The plotted dependencies of the sumFc-1 and sumFc-2 were linear in the range 0.005–0.075 V s−1. It should be noted that the intensities of the anodic current signal concerning the square root of the scan rate for all studied ferrocene–sumanene derivatives also showed linearity. The receptors differ between each other in the structure and the length of the linker between sumanene molecule and the ferrocene units. The linear nature of the Iox, Fc = f(v0.5) relationship confirms the lability of the redox probe in the layer.
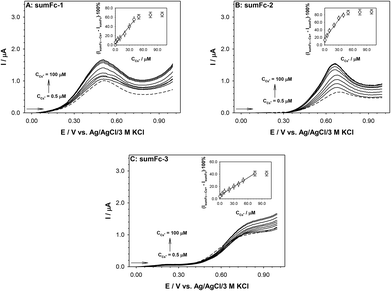
The ability to use sumFc-1–sumFc-3 containing receptor layer in the detection of cesium cations (Cs+) was instigated in the final stage of the research program. We decided to analyze the sensitivity of such a sensor, and whether it is influenced by the linker through which the ferrocene units are conjugated to the sumanene molecule. The sensitivity of the receptors to the presence of the Cs+ in the solution was determined based on the changes in the ferrocene electrooxidation current signal intensity. Differential pulse (DP) voltammetry was used to carry out the measurements in water in the Cs+ concentration ranging from 0.5 to 100 μM. The recorded DP voltammograms are presented in Fig. 7. The obtained DP voltammograms showed that with the increase in the Cs+ concentration in the analyzed solution, there was also an increase in the intensity of the ferrocene electrooxidation current signal. The difference in the ferrocene current signal intensities, recorded in the presence and absence of the cesium cation, was used in the construction of the calibration curves (IsumFc⋯Cs+ − IsumFc)·100% = f(CCs+) (see insets in Fig. 7). The analytical parameters of the receptor layer were determined based on these curves, such as the linear regression equation, analytical range of work, limits of detection (LOD) and limits of quantification (LOQ). The values of these parameters are given in Table 2. The LOD and (LOQ) were determined for each receptor according to the equations:45
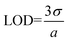 | (2) |
| LOQ = 3.3·LOD | (3) |
| Compound | Regression equation | r | Analytical work of range [μM] | LOD/LOQ [μM] |
|---|---|---|---|---|
| sumFc-1 | (IsumFc⋯Cs+ − IsumFc)·100% = 1.25·CCs+ + 2.79 | 0.9907 | 0.5–40 | 0.05/0.16 |
| sumFc-2 | (IsumFc⋯Cs+ − IsumFc)·100% = 1.67·CCs+ + 17.0 | 0.9876 | 0.5–40 | 0.09/0.30 |
| sumFc-3 | (IsumFc⋯Cs+ − IsumFc)·100% = 0.48·CCs+ + 5.45 | 0.9967 | 0.5–75 | 0.38/1.24 |
The lowest value of the limit of detection (LOD) had the sensor containing the sumFc-1 receptor, what might be a result of the lowest Kapp value (3.3 × 104 M−2 by spectrofluorimetry) for sumFc-1 receptor. Notably, the sensors containing sumFc-1 and sumFc-2 that were synthesized by means of the carbon–carbon bond formation reaction (Suzuki–Miyaura and Sonogashira coupling, respectively) featured relatively similar LOD values (0.05–0.09 μM). The LOD value for the sensor containing sumFc-3 was higher (0.38 μM). This difference might be a result of better electronic communication between sumanene and ferrocene units in sumFc-1 and sumFc-2 in comparison to the click chemistry derived sumFc-3.
The stability and selectivity of the receptors were also investigated. The electrochemical measurements were performed every 3 days. The sensors between the measurements were kept in the desiccator. There were no significant changes in the intensity and the position of the recorded current signal during the 2-month period. The observed differences were not higher than 4.5%. Based on the results described for the changes in the intensity of the Fc electrooxidation current signal since the construction of the sensor, it is concluded that the constructed sensor was very stable.
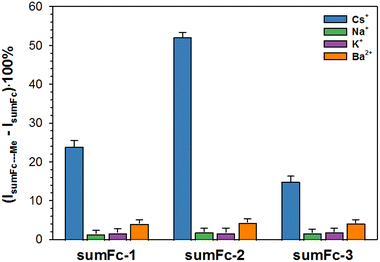
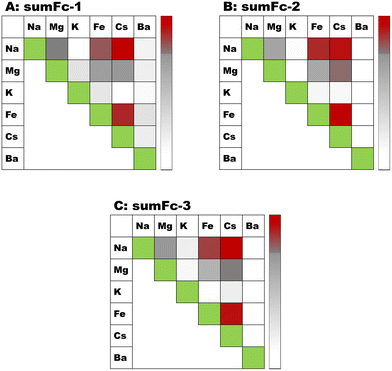
The selectivity studies were carried out against other cations: Na+, K+ and Ba2+. The diagram is presented in Fig. 8. The influence of other metal cations on the Fc current signal was negligible. Thus, the electrochemical studies further indicated that the designed sensors are highly selective versus Cs+. The laser ablation inductively coupled plasma mass spectrometry (LA-IC-MS) analysis was performed to further prove that the designed ferrocene–sumanene derivatives sumFc-1–sumFc-3 are very selective receptors toward Cs+. The substrate (glass), modified with the sumanene–ferrocene receptor, was immersed in an equimolar mixture of (20 μM) with Cs+, Na+, K+, and Ba2+ for 1 hour before the measurements. Raw signals were background corrected for each isotope individually for Ar flow only. Spikes (defined as a single data point exceeding the intensities of the neighboring data more than 2 times) were replaced with a value of the average intensity of two neighboring signals. A custom-written formula in Excel® (Microsoft Corp.) was used for all the recalculations and visualization of the LA-ICP-MS data. Fluctuations of transient signals recorded during line ablation for the respective isotopes of Na, K, Fe, Cs, and Ba allowed for the calculation of correlation coefficients of co-occurrences of all elements against each other. A percentile scale, setting the maximum (red color) for the 95th percentile value and the minimum (white color) for the 5th percentile value, was used as a reference for the relative formatting of cells containing calculated correlation coefficients. The results obtained for three independent samples are presented in Fig. 9. The correlation coefficient indicating the co-occurrence of Fe with other elements for each sample was calculated in descending order: Cs > Na > Mg. However, there was no rule of constant behavior for K and Ba.
The comparison of the LOD values for the designed electrochemical sensors, comprising sumFc-1–sumFc-3 as the receptor layer, with the performance of the other reported Cs+ receptors is presented in Table 3. As it can be seen, the LOD values for sumFc-1–sumFc-3 are lower in comparison to the other, non-sumanene receptors. The better performance of sumanene-based receptors in comparison with the calixarene-based ones, is especially important, also from the viewpoint of the high Cs+ selectivity of sumFc-1–sumFc-3. Notably, the performance of sumFc-1–sumFc-3 and tris(ferrocenylmethidene)sumanene (13) based sensors is better than the performance of the monoferrocenylsumanenes. SumFc-1 and sumFc-2 together with tris(ferrocenylmethidene)sumanene (13), show the most satisfactory LOD values (0.02–0.09 μM). From the application viewpoint, it is also important that the sensors comprising sumFc-1–sumFc-3 as the receptor layer featured good time stability.
Conclusions
In conclusion, this work designed new, efficient methods of sumanene modification employing click chemistry of metal-catalyzed carbon–carbon bond formation reactions. As a result, novel, bowl-shaped, and π-extended sumanene derivatives bearing three ferrocene units (sumFc-1–sumFc-3) were synthesized. Sophisticated sumanene derivatives tris-substituted at the benzylic positions were used for the first time as the starting materials in the click chemistry reaction and Suzuki–Miyaura or Sonogashira couplings. Our findings significantly contribute to further development in sumanene chemistry toward the design of novel functional organic compounds bearing a sumanene motif. All the synthesized compounds have been comprehensively characterized with the spectroscopic techniques. Spectrofluorimetric analyzes revealed that compounds sumFc-1–sumFc-3 selectively interact with cesium cations. The apparent binding constants for the dynamically formed sandwich-type complexes equals (3.3–7.3) × 104 M−2. Voltammetry was used to electrochemically analyze the designed sumanene–ferrocene conjugates. Each compound featured one pair of well-defined current signals, which is related to the Fc/Fc+ electrode process. Eventually, herein, the design of the electrochemical sensors toward the fast, sensitive, and selective detection of cesium cations (Cs+) in water was described. The lowest limit of detection (LOD) of Cs+ was found for sumFc-1 containing sensor (0.05 μM). The LOD values for sumFc-2 and sumFc-3 containing sensors equaled 0.09 μM and 0.38 μM, respectively. The effect of the linker between the sumanene and ferrocene units in the studied conjugates on the LOD of Cs+ sensors was observed. The sensors, which contained the carbon–carbon bonded sumFc-1 (synthesized via Suzuki–Miyaura coupling) and sumFc-2 (synthesized via Sonogashira coupling), featured lower LOD values (0.05–0.09 μM) compared to the click chemistry derived sumFc-3 (0.38 μM). It might be ascribed to the better electronic communication between the sumanene and the ferrocene units in sumFc-1 and sumFc-2 compared to sumFc-3. Notably, these LOD values indicate the potential usefulness of the designed sensors in the analyses of real samples, such as the ones from post-disaster areas. For example, the Mano River (Japan, northwest of the Fukushima–Daiichi plant) contaminated with radioactive toxic cesium was estimated to be ca. 0.06 mM.28,33 Eventually, the high selectivity of the designed sumanene–ferrocene receptors toward recognizing Cs+ was also evidenced with fluorescence spectroscopy, electrochemistry, and laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Considering the presented results, this work significantly contributes to further the progress in the organic chemistry of sumanene by providing new protocols for its modification. Additionally, it broadens the knowledge of the electrochemical properties of the sumanene–ferrocene conjugates as well as their applications in environmental chemistry, especially Cs+ detection.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The financial support the National Science Centre, Poland, OPUS grant no. 2021/43/B/ST4/00114 (A. K.), Warsaw University of Technology (WUT; A. K.), and JSPS KAKENHI grant no. JP19H00912 and JP21H05233 (H. S.) are acknowledged. We would like to thank Dr hab. Mariola Koszytkowska-Stawińska, prof. WUT, for fruitful discussions, Mr Sergei Molchanov (WUT) for his assistance in 2-D NMR spectra measurements, and Mr Maurycy Krzyżanowski (WUT) for his assistance in calculation part.References
- H. Sakurai, T. Daiko and T. Hirao, Science, 2003, 301, 1878–1878 CrossRef CAS PubMed.
- H. Sakurai, Bull. Chem. Soc. Jpn., 2021, 94, 1579–1587 CrossRef.
- T. Amaya and T. Hirao, Chem. Rec., 2015, 15, 310–321 CrossRef CAS PubMed.
- T. Amaya and T. Hirao, Chem. Commun., 2011, 47, 10524 RSC.
- S. Higashibayashi and H. Sakurai, Chem. Lett., 2011, 40, 122–128 CrossRef CAS.
- M. Saito, H. Shinokubo and H. Sakurai, Mater. Chem. Front., 2018, 2, 635–661 RSC.
- S. Mebs, M. Weber, P. Luger, B. M. Schmidt, H. Sakurai, S. Higashibayashi, S. Onogi and D. Lentz, Org. Biomol. Chem., 2012, 10, 2218 RSC.
- T.-C. Wu, H.-J. Hsin, M.-Y. Kuo, C.-H. Li and Y.-T. Wu, J. Am. Chem. Soc., 2011, 133, 16319–16321 CrossRef CAS PubMed.
- B. B. Shrestha, S. Karanjit, G. Panda, S. Higashibayashi and H. Sakurai, Chem. Lett., 2013, 42, 386–388 CrossRef CAS.
- I. Hisaki, H. Toda, H. Sato, N. Tohnai and H. Sakurai, Angew. Chem., Int. Ed., 2017, 56, 15294–15298 CrossRef CAS PubMed.
- H. Toda, Y. Uetake, Y. Yakiyama, H. Nakazawa, T. Kajitani, T. Fukushima and H. Sakurai, Synthesis, 2019, 4576–4581 CAS.
- H. Sakurai, T. Daiko, H. Sakane, T. Amaya and T. Hirao, J. Am. Chem. Soc., 2005, 127, 11580–11581 CrossRef CAS PubMed.
- T. Amaya, K. Mori, H.-L. Wu, S. Ishida, J. Nakamura, K. Murata and T. Hirao, Chem. Commun., 2007, 1902–1904 RSC.
- T. Amaya, T. Nakata and T. Hirao, J. Am. Chem. Soc., 2009, 131, 10810–10811 CrossRef CAS PubMed.
- S. Alvi and R. Ali, Beilstein J. Org. Chem., 2020, 16, 2212–2259 CrossRef CAS PubMed.
- Y. Yakiyama, T. Hasegawa and H. Sakurai, J. Am. Chem. Soc., 2019, 141, 18099–18103 CrossRef CAS PubMed.
- Y. Shoji, T. Kajitani, F. Ishiwari, Q. Ding, H. Sato, H. Anetai, T. Akutagawa, H. Sakurai and T. Fukushima, Chem. Sci., 2017, 8, 8405–8410 RSC.
- T. Amaya, K. Kobayashi and T. Hirao, Asian J. Org. Chem., 2013, 2, 642–645 CrossRef CAS.
- U. D. Priyakumar and G. N. Sastry, Tetrahedron Lett., 2003, 44, 6043–6046 CrossRef CAS.
- I. K. Petrushenko, N. V. Shipitsin and K. B. Petrushenko, Phys. E, 2022, 135, 114949 CrossRef CAS.
- D. Vijay, H. Sakurai, V. Subramanian and G. N. Sastry, Phys. Chem. Chem. Phys., 2012, 14, 3057 RSC.
- T. Amaya, W. Wang, H. Sakane, T. Moriuchi and T. Hirao, Angew. Chem., Int. Ed., 2010, 49, 403–406 CrossRef CAS PubMed.
- T. Amaya, H. Sakane and T. Hirao, Angew. Chem., 2007, 119, 8528–8531 CrossRef.
- T. Amaya and T. Hirao, in Advances in Organometallic Chemistry and Catalysis, ed. A. J. L. Pombeiro, John Wiley & Sons, Inc., Hoboken, NJ, USA, 2013, pp. 473–483 Search PubMed.
- S. N. Spisak, Z. Wei, A. Yu. Rogachev, T. Amaya, T. Hirao and M. A. Petrukhina, Angew. Chem., Int. Ed., 2017, 56, 2582–2587 CrossRef CAS PubMed.
- T. Amaya, Y. Takahashi, T. Moriuchi and T. Hirao, J. Am. Chem. Soc., 2014, 136, 12794–12798 CrossRef CAS PubMed.
- A. Kasprzak and H. Sakurai, Dalton Trans., 2019, 48, 17147–17152 RSC.
- A. Kasprzak, A. Kowalczyk, A. Jagielska, B. Wagner, A. M. Nowicka and H. Sakurai, Dalton Trans., 2020, 49, 9965–9971 RSC.
- A. Kasprzak and H. Sakurai, Chem. Commun., 2021, 57, 343–346 RSC.
- A. Kasprzak, A. Tobolska, H. Sakurai and W. Wróblewski, Dalton Trans., 2022, 51, 468–472 RSC.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- N. Kumar, I. Leray and A. Depauw, Coord. Chem. Rev., 2016, 310, 1–15 CrossRef CAS.
- T. Mizuno and H. Kubo, Sci. Rep., 2013, 3, 1742 CrossRef PubMed.
- Y. Morino, T. Ohara and M. Nishizawa, Geophys. Res. Lett., 2011, 38, L00G11 CrossRef.
- A. T. Kotilainen, M. M. Kotilainen, V.-P. Vartti, K.-L. Hutri and J. J. Virtasalo, Mar. Pollut. Bull., 2021, 172, 112924 CrossRef CAS PubMed.
- V. Saenko, V. Ivanov, A. Tsyb, T. Bogdanova, M. Tronko, Yu. Demidchik and S. Yamashita, Clin. Oncol., 2011, 23, 234–243 CrossRef CAS PubMed.
- P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905–4979 CrossRef CAS PubMed.
- K. Sada and K. Kokado, in Advances in Organic Crystal Chemistry, ed. M. Sakamoto and H. Uekusa, Springer Singapore, Singapore, 2020, pp. 523–538 Search PubMed.
- Y. Goto, H. Sato, S. Shinkai and K. Sada, J. Am. Chem. Soc., 2008, 130, 14354–14355 CrossRef CAS PubMed.
- 2-Ethynylsumanene was a part of unpublished results. This compound was introduced in the account (see ref. 3). No spectral data was provided for this compound. A preliminary trial on using this compound for the Glaser coupling reaction was reported (yield 12%; MALDI-MS data was provided for the Glaser coupling product, no NMR data was provided).
- Q. Tan, P. Kaewmati, S. Higashibayashi, M. Kawano, Y. Yakiyama and H. Sakurai, Bull. Chem. Soc. Jpn., 2018, 91, 531–537 CrossRef CAS.
- J. S. Renny, L. L. Tomasevich, E. H. Tallmadge and D. B. Collum, Angew. Chem., Int. Ed., 2013, 52, 11998–12013 CrossRef CAS PubMed.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS.
- S. Goswami, K. Aich, S. Das, A. K. Das, A. Manna and S. Halder, Analyst, 2013, 138, 1903 RSC.
- A. J. Bard and L. R. Faulkner, Electrochemical methods: fundamentals and applications, Wiley, New York, 2nd edn, 2001 Search PubMed.
- N. S. Neghmouche and T. Lanez, Int. Lett. Chem., Phys. Astron., 2013, 9, 37–45 Search PubMed.
- M. Arvand, M. Moghimi and M. A. Bagherinia, Anal. Lett., 2009, 42, 393–408 CrossRef CAS.
- B. Radaram, T. Mako and M. Levine, Dalton Trans., 2013, 42, 16276 RSC.
- X. Q. Pham, L. Jonusauskaite, A. Depauw, N. Kumar, J. P. Lefevre, A. Perrier, M.-H. Ha-Thi and I. Leray, J. Photochem. Photobiol., A, 2018, 364, 355–362 CrossRef CAS.
- N. Kumar, Q. Pham-Xuan, A. Depauw, M. Hemadi, N.-T. Ha-Duong, J.-P. Lefevre, M.-H. Ha-Thi and I. Leray, New J. Chem., 2017, 41, 7162–7170 RSC.
- E. Özcan and B. Çoşut, ChemistrySelect, 2018, 3, 7940–7944 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, compounds characterization data, discussion on the NMR spectra, data on the spectrofluorimetric analyses of the complexes, calculation details. See DOI: https://doi.org/10.1039/d3dt00084b |
| This journal is © The Royal Society of Chemistry 2023 |