Synthesis and catalytic performance of nickel phosphinite pincer complexes in deoxygenative hydroboration of amides†‡
Abstract
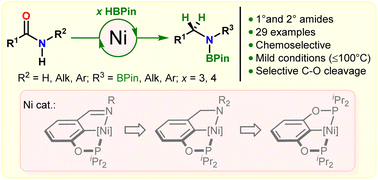
A series of imino-POCNR, amino-POCNR2, and bis(phosphinite) POCOP pincer complexes of Ni(II) were prepared and tested in catalytic deoxygenative hydroboration of amides with HBPin to the corresponding amines. In contrast to the deoxygenative hydrosilylation approach, primarily developed for tertiary amides, superior reactivity in Ni-catalyzed deoxygenative hydroboration was demonstrated for secondary carboxamides. The bis(phosphinite) hydride complex (POCOP)NiH proved the most active in these reactions, tolerating potentially reducible functionalities such as internal alkenes, esters, nitriles, heteroaromatic compounds, and tertiary amides. Preferable hydroboration of secondary amides was also demonstrated in the presence of primary amide functionalities. The reactions were conducted at 60–80 °C, representing a rare example of a base-metal catalytic system for selective deoxygenation of secondary amides to the corresponding amines under mild conditions. In contrast to secondary amides, deoxygenative hydroboration of primary amides was demonstrated using an iminophosphinite pre-catalyst (POCNDmp)Ni(CH2TMS) (Dmp = 2,6-Me2C6H3). Deoxygenation reactions were suggested to proceed via a direct C–O bond cleavage mechanism, which is triggered by dehydrogenative N-borylation to access more electrophilic N-borylamides amenable to the addition of HBPin to the carbonyl group.


 Please wait while we load your content...
Please wait while we load your content...