 Open Access Article
Open Access ArticleA “gold standard” computational proof for the existence of gold(III) aurophilicity†
Daniel
Blasco‡
 *ab,
Félix
Reboiro‡
a,
Dage
Sundholm
*ab,
Félix
Reboiro‡
a,
Dage
Sundholm
 b,
M. Elena
Olmos
b,
M. Elena
Olmos
 a,
Miguel
Monge
a,
Miguel
Monge
 *a and
José M.
López-de-Luzuriaga
*a and
José M.
López-de-Luzuriaga
 *a
*a
aDepartamento de Química, Centro de Investigación en Síntesis Química (CISQ), Universidad de La Rioja, Madre de Dios 53, 26006, Logroño, Spain. E-mail: miguel.monge@unirioja.es; josemaria.lopez@unirioja.es
bDepartment of Chemistry, Faculty of Science, University of Helsinki, P.O. Box 55 (A.I. Virtasen aukio 1), FIN-00014, Helsinki, Finland. E-mail: daniel.blasco@helsinki.fi
First published on 8th February 2023
Abstract
The existence of aurophilic gold(III)⋯gold(III) interactions has for a long time been neglected due to structural arguments and comparison with the aurophilicity of gold(I) compounds. We show with calculations at the CCSD(T) level of theory that the [AuIII(CH3)3(NH3)]2 dimer has a metallophilic dispersion interaction between the gold(III) atoms of 10.5 kJ mol−1. The aurophilic interaction is illustrated by topological QTAIM calculations and IRI analysis.
The concept of metallophilicity encompasses attractive van der Waals interactions between pairs, strings, or clusters of closed- (d10, s2d10) or seemingly closed-shell (d8) late transition metals that originate from the relativistic mass increase of the s electrons. Among metallophilic interactions, aurophilicity has a privileged status due to its noticeable strength of 30–50 kJ mol−1 that affects the crystalline structure and bulk properties of gold(I)-containing materials.1–3 The character of the interaction is supported by ever-growing irrefutable structural evidence and by computational simulation at post-Hartree–Fock (HF) and density functional theory (DFT) levels that consider van der Waals interactions.4 A gold⋯gold distance of less than twice its van der Waals radius is considered aurophilic. The radius proposed by Bondi for gold, 1.62 Å, is usually chosen,5 although longer values have been reported, such as Allinger's 2.43 Å.6,7
Whereas the existence of the aurophility between gold(I) atoms (AuI⋯AuI; [AuI]: [Xe] 4f145d10) is now out of debate, there is still room for doubts regarding the presence of analogous aurophilicity between gold(III) atoms (AuIII⋯AuIII; [AuIII]: [Xe] 4f145d8). There is still no consensus in the scientific community on its existence.8 Two factors are thought to prevent the formation of AuIII⋯AuIII interactions: (i) the depletion of electron density, since gold(III) is a hard Pearson acid, whereas gold(I) is a soft one, and (ii) the relativistic effects of gold(III) are less pronounced as compared to gold(I).9
However, this reasoning is only enough for explaining the weaker interaction between AuIII⋯AuIII as compared to the AuI⋯AuI one but not to rule out its existence. In our opinion, the different orbital structure (d8vs. d10) and coordination environment (square planar vs. linear) of gold(III) may not prevent the existence of dispersive forces like aurophilicity. Moreover, the contribution of relativistic effects to the aurophilic attraction is not fundamental, accounting for only 22–27% of the total interaction energy.10,11 There are some experimental and computational results that support our claim that AuIII⋯AuIII interactions contribute to the stabilization of gold(III) dimers and polymers, even though they are weaker and overruled by other secondary interactions. The following studies suggest that there is a weak van der Waals-type interaction between the gold(III) atoms:
(i) Mendizabal and Pyykkö calculated at the second order Møller–Plesset (MP2) level of theory a stabilizing interaction of −34.94 or −56.75 kJ mol−1 between the two molecules of the [AuIIICl3(PH3)]2 dimer when the dipole moments of the monomers were perpendicular or antiparallel, respectively. There was an attraction of −20.71 kJ mol−1 even at the HF level, when the molecules were oriented in a perpendicular fashion.12
(ii) In 2005, Klapötke et al. prepared a series of ammonium tetraazidoaurate(III) (Q[AuIII(N3)4]; Q = NMe4, NMe2H2, NH4) complexes. The crystal structure of (NMe4)[AuIII(N3)4] has one-dimensional chains of anions linked by AuIII⋯AuIII contacts, whose bond distances are 3.507(3), 3.584(3) Å.13 However, an attractive nature of such contacts was not obtained in molecular structure optimizations of a [AuIII(N3)4]22− dimer at the DFT level using the B3LYP functional. The dimer dissociated.
(iii) A year later, Doerrer et al. synthesized up to eleven double salts of [PtII(tpy)X]+ and [AuIII(bpy)X2]+ (tpy = 2,2′:6′,2′′-terpyridine; bpy = 2,2′-bipyridine; X = Cl, Br, CN) cations that were prepared by anion metathesis in aqueous solution.14 Among them, the crystal structures of [AuIII(bpy)Cl2][AuIIIBr4] and [AuIII(bpy)Br2][AuIIIBr4] with the gold(III)-gold(III) ion pairs are noteworthy, since they have AuIII (cation)⋯AuIII (anion) distances of 3.518(1) Å and ca. 3.54 Å, respectively. The quality of [AuIII(bpy)Br2][AuIIIBr4] was unpublishable. A similar study was reported in 2015 by Haukka et al. including quantum theory of atoms in molecules (QTAIM) calculations of the topology of the charge density.7 The authors proposed a combination of structural measurements and bond critical point (BCP) descriptors to identify AuIII⋯AuIII aurophilicity. They also report AuIII⋯AuIII interaction energies at the DFT/PBE0 level of theory in the range 3.6–9.3 kJ mol−1.
(iv) Extremely short intramolecular AuIII⋯AuIII distances ranging between 2.984–3.080 Å were obtained by Bessonov et al. for the molecular structure of doubly supported dimethylgold(III) carboxylates ([{AuIII(CH3)2}2(μ-OC(R)O)2]; R = H, CF3, C(CH3)3, Ph).15
(v) Che et al. contributed to the search for these interactions with the article from 2012 where they prepared [AuIII(C^N^N)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NMe2)](PF6) (C^N^N = 6-phenyl-2,2′-bipyridine) with the shortest unsupported AuIII⋯AuIII distance to date of 3.495 Å.16
CC6H4-4-NMe2)](PF6) (C^N^N = 6-phenyl-2,2′-bipyridine) with the shortest unsupported AuIII⋯AuIII distance to date of 3.495 Å.16
The gold(III) ions of examples (ii) and (v) would not be expected to aggregate by AuIII⋯AuIII contacts based on Coulomb repulsion, and therefore gold(III) aurophilicity is reasonably invoked in these cases. Also note that in examples (iii) and (iv) the presumed gold(III) aurophilic interaction is assisted by Coulomb attraction and ligand support, respectively.
Here, we demonstrate that at the “gold standard” level of theory i.e., at the coupled cluster singles and doubles level with a perturbative treatment of the triples (CCSD(T)) in combination with the def2-TZVP basis sets,17 the AuIII⋯AuIII interactions explain a part of the total interaction energy between neutral gold(III) complexes.
For achieving a clearer-as-possible description of the AuIII⋯AuIII interaction without losing chemical representativeness, we have built a very simple dimer model by substituting the C3N donor atoms of the well-known bis-orthometallated [AuIII(C^N^C)(alkynyl)] complexes18 with methyl and ammonia ligands, respectively. The small size of [AuIII(CH3)3(NH3)]2 (Fig. 1, inset; molecule 1) allows us to employ correlated ab initio levels of theory such as RI-MP2/def2-TZVP and CCSD(T)/def2-TZVP. The computational details are given in the ESI.† The molecular structure of 1 optimized at the RI-MP2/def2-TZVP level has been employed in the calculations of the interaction energies. The potential energy curves (PECs) have been obtained by stretching the AuIII⋯AuIII distance to the selected values, without reoptimization of the rest of the dimer. The counterpoise-corrected RHF, RI-MP2 and CCSD(T) interaction energies (ΔEint, eqn (S1)†) as functions of the AuIII⋯AuIII distance (R) are plotted in Fig. 1. The equilibrium distances (Re) and interaction energies (ΔEint(Re)) derived from fitting the points to the Herschbach–Laurie four-parameter function (eqn (S2)†) are given in Table 1.
| Level of theory | R e | −ΔEint(Re) | |||
|---|---|---|---|---|---|
| Total | AuIII⋯[ligand] | [ligand]⋯[ligand] | AuIII⋯AuIII | ||
| a −ΔEint(AuIII⋯AuIII) = ΔEint(total) − 2 × ΔEint(AuIII⋯[ligand]) + ΔEint([ligand]⋯[ligand]). | |||||
| RHF | 4.11 | 15.9 | — | — | — |
| RI-MP2 | 3.48 | 56.6 | 25.3 | 10.7 | 16.7 |
| CCSD(T) | 3.59 | 45.1 | 23.1 | 11.6 | 10.5 |
The PECs are markedly different depending on the chosen level of theory. In other words, the PEC depends on how electron correlation is considered in the computational framework. At the RHF level that does not consider electron correlation, the interaction curve is non-bonding and flat at long distances, although an overall binding of the dimer (ΔEint < 0) is found at distances longer than ca. 3.48 Å. This finding agrees with the results obtained by Mendizabal and Pyykkö for antiparallel [AuIIICl3(PH3)]2, and the same explanation based on long-range dipole–dipole attraction may be invoked here. When electron correlation is considered an interaction minimum is predicted, suggesting a dispersive origin for the intermolecular attraction. Whereas both RI-MP2 and CCSD(T) predict a minimum, RI-MP2 calculations find it at a shorter distance of 3.48 Å as compared to 3.59 Å at the CCSD(T) level. The binding energy of 56.64 kJ mol−1 obtained at the RI-MP2 level is also somewhat larger than the one of 45.05 kJ mol−1 calculated at CCSD(T) level, which is in line with the notion that MP2 overestimates van der Waals interaction energies. The total interaction energy between the monomers of 1 can be approximated to the sum of AuIII⋯AuIII, twice AuIII⋯[ligand], and [ligand]⋯[ligand] contributions {[ligand] = [(CH3)3(NH3)]}. The contribution from the AuIII⋯[ligand] interactions to the total interaction energy has been partially removed by subtracting twice that calculated for a monomer of 1 and the saturated ligands of the other monomer at the RI-MP2 and CCSD(T) AuIII⋯AuIII equilibrium distances, respectively. The extra [ligand]⋯[ligand] interaction energy removed in this way has been restored by adding that of the saturated ligands of [(CH4)3(NH3)]2 (see Fig. S1 and the ESI for further details†). Correcting for AuIII⋯[ligand] and [ligand]⋯[ligand] interaction leads to an approximate attractive interaction energy of 16.7 (RI-MP2) and 10.5 kJ mol−1 (CCSD(T)) when considering only the AuIII⋯AuIII interactions.
We have repeated this procedure with Klapötke's anionic [AuIII(N3)4]22− dimer (molecule 2)13 and with a theoretical cationic {cis-[AuIII(CH3)2(NH3)2]}22+ dimer (molecule 3) as a model of the interaction found in [AuIII(C^N^N)(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CC6H4-4-NMe2)](PF6),16 as proofs of concept. Due to the larger size of 2 and the repulsive character of the interaction found within 3 (vide infra), we only report results obtained at the RHF/def2-TZVP and RI-MP2/def2-TZVP levels of theory. We also found that optimizing the bound dimer of 2 at the RI-DFT/B3LYP-D3(BJ)/def2-TZVP level of theory results in its dissociation. However, if the same calculation is done at the RI-MP2/def2-TZVP level, a short AuIII⋯AuIII distance of 3.09 Å is obtained (Fig. S2,† inset). The PECs of 2 are repulsive at all distances due to the coulombic force between the anions, but an MP2 minimum at ca. 3.21 Å is found (Fig. S2†). Thus, AuIII⋯AuIII interactions may play a role in directing the crystal packing of [NMe4][AuIII(N3)4]. However, the free optimization of 3 at the same level of theory as 2 led to the complete dissociation of the dimer. A starting structure was therefore obtained by fixing the AuIII⋯AuIII distance during the optimization. The absence of a minimum in the PECs of 3 (Fig. S3†) shows that cation-cation repulsion overcomes gold(III) aurophilicity if no other interligand interactions are present.
CC6H4-4-NMe2)](PF6),16 as proofs of concept. Due to the larger size of 2 and the repulsive character of the interaction found within 3 (vide infra), we only report results obtained at the RHF/def2-TZVP and RI-MP2/def2-TZVP levels of theory. We also found that optimizing the bound dimer of 2 at the RI-DFT/B3LYP-D3(BJ)/def2-TZVP level of theory results in its dissociation. However, if the same calculation is done at the RI-MP2/def2-TZVP level, a short AuIII⋯AuIII distance of 3.09 Å is obtained (Fig. S2,† inset). The PECs of 2 are repulsive at all distances due to the coulombic force between the anions, but an MP2 minimum at ca. 3.21 Å is found (Fig. S2†). Thus, AuIII⋯AuIII interactions may play a role in directing the crystal packing of [NMe4][AuIII(N3)4]. However, the free optimization of 3 at the same level of theory as 2 led to the complete dissociation of the dimer. A starting structure was therefore obtained by fixing the AuIII⋯AuIII distance during the optimization. The absence of a minimum in the PECs of 3 (Fig. S3†) shows that cation-cation repulsion overcomes gold(III) aurophilicity if no other interligand interactions are present.
The RI-DFT/PBE0-D3(BJ)/def2-TZVP interaction energy (ΔEint)s§ between the gold(III) monomers of molecule 1 has been decomposed into:
| ΔEint = ΔEele + ΔEex-rep + ΔEorb + ΔEcorr + ΔEdisp |
 | ||
| Fig. 2 Relative contribution of ΔEele (black), ΔEorb (green), ΔEcorr (blue) and ΔEdisp (pink) to the stabilization of molecule 1. | ||
The CCSD(T) electron density of molecule 1 has been examined using QTAIM19 and topological calculations with the interaction region indicator (IRI) analysis method.20 The relationship between the QTAIM (3, −1) BCPs, bond paths i.e., the maximal gradient path connecting two BCPs, and sign(λ2) × ρe-weighted IRI isosurfaces in a single image is a powerful tool for gaining visual insight into the covalent and non-covalent interactions. Fig. 3 shows how molecule 1 is bound by attractive, van der Waals, and repulsive interactions and their strength. A BCP is found in the bond path connecting the two gold(III) atoms with an electron density (ρe(BCP)) of 0.0055 e Å3. Its Laplacian (∇2[ρe(BCP)]) is 0.0136. The ρe(BCP) value between gold(III)–gold(III) ion pairs is even one order of magnitude smaller than the one reported by Haukka et al.,7 overruling any covalent character of the modelled interaction. Moreover, the positive ∇2[ρe(BCP)] value indicates reduction and expansion of the electron density as in closed-shell bonds.21 The expected region for van der Waals interactions between the ligands are depicted in the IRI isosurface as a bright green area around the corresponding BCPs. More importantly, an area with similar characteristics (electron-poor, attractive character) coinciding with the gold(III)⋯gold(III) intermetallic axis is also seen in Fig. 3. The physical meaning of bond paths has been controversial because some authors incorrectly assigned them to chemical bonds.22 It should be stressed that bond paths do not reflect chemical bonds of Lewis type but they provide a much broader concept of bonded interactions including the van der Waals ones.23 Thus, the bond path connecting the two gold(III) atoms is the ultimate topological proof of the existence of a van der Waals-type interaction acting between the metals, which can be consequently addressed as gold(III) aurophilicity. A similar IRI isosurface and QTAIM bond path between the gold(III) atoms is seen for molecule 2 in Fig. S5 in the ESI.†
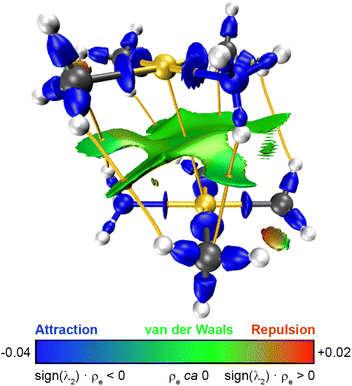 | ||
| Fig. 3 The QTAIM (3, −1) BCPs (orange dots), bond paths (yellow strings) and the IRI isosurface (isovalue = 1.0) are superimposed for molecule 1. The RGB colour scale refers to the IRI isosurface (adapted from ref. 16). Colour code: C, grey; H, white; Au, yellow, N, blue. | ||
To conclude, we believe that the lack of structural evidence of gold(III) aurophilicity is partly a consequence of the current interests in gold(III) chemistry, which focusses on the production of phosphors and the tailoring of their emission energies by pre- and post-synthetic modifications.8,18 Strongly σ-donating ligands consisting of large aromatic polycyclic pincers are needed and routinely employed for achieving luminescence. As expected, π-stacking interactions are the most important supramolecular motif that overrules AuIII⋯AuIII interactions. We hope that this communication will pave the way for accepting gold(III) aurophilicity as a weak metallophilic interaction and that it stimulates further experimental research on this less developed topic.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The research has been supported by MCIN/AEI/10.13039/501100011033 through project PID2019-104379RB-C22, and by The Academy of Finland through project 340583. DB acknowledges Universidad de La Rioja for the concession of a Margarita Salas post-doctoral scholarship financed by the Spanish Ministerio de Universidades and the European Union-NextGenerationEU program. This work used the Beronia cluster (Universidad de La Rioja), which is supported by FEDER-MINECO grant number UNLR-094E-2C-225. The authors also acknowledge CSC-IT Center for Science, Finland and the Finnish Grid and Cloud Infrastructure (persistent identifier urn:nbn:fi:research-infras-2016072533) for computational resources.JML, MM and MEO designed the project. DS and MM provided the computational resources. DB and FR conducted the calculations. All authors have contributed to the text of the final version of the manuscript. DB and FR contributed equally.
References
- N. Mirzadeh, S. H. Privér, A. J. Blake, H. Schmidbaur and S. K. Bhargava, Chem. Rev., 2020, 120, 7551–7591 CrossRef CAS PubMed.
- H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2012, 41, 370–412 RSC.
- H. Schmidbaur and A. Schier, Chem. Soc. Rev., 2008, 37, 1931–1951 RSC.
- P. Pyykkö, Angew. Chem., Int. Ed., 2004, 43, 4412–4456 CrossRef PubMed.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- N. L. Allinger, X. Zhou and J. Bergsma, J. Mol. Struct.: THEOCHEM, 1994, 312, 69–83 CrossRef.
- A. N. Chernyshev, M. V. Chernysheva, P. Hirva, V. Y. Kukushkin and M. Haukka, Dalton Trans., 2015, 44, 14523–14531 RSC.
- L. Rocchigiani and M. Bochmann, Chem. Rev., 2021, 121, 8364–8451 CrossRef CAS PubMed.
- P. Schwerdtfeger, P. D. W. Boyd, S. Brienne and A. K. Burrell, Inorg. Chem., 1992, 31, 3411–3422 CrossRef CAS.
- P. Pyykko, N. Runeberg and F. Mendizabal, Chem. – Eur. J., 1997, 3, 1451–1457 CrossRef CAS.
- N. Runeberg, M. Schütz and H.-J. Werner, J. Chem. Phys., 1999, 110, 7210–7215 CrossRef CAS.
- F. Mendizabal and P. Pyykkö, Phys. Chem. Chem. Phys., 2004, 6, 900–905 RSC.
- T. M. Klapötke, B. Krumm, J.-C. Galvez-Ruiz and H. Nöth, Inorg. Chem., 2005, 44, 9625–9627 CrossRef PubMed.
- R. Hayoun, D. K. Zhong, A. L. Rheingold and L. H. Doerrer, Inorg. Chem., 2006, 45, 6120–6122 CrossRef CAS PubMed.
- A. A. Bessonov, N. B. Morozova, N. V. Kurat'eva, I. A. Baidina, N. V. Gel'fond and I. K. Igumenov, Russ. J. Coord. Chem., 2008, 34, 73–80 Search PubMed.
- W. Lu, K. T. Chan, S.-X. Wu, Y. Chen and C.-M. Che, Chem. Sci., 2012, 3, 752–755 RSC.
- J. Řezáč and P. Hobza, J. Chem. Theory Comput., 2013, 9, 2151–2155 CrossRef PubMed.
- C. Bronner and O. S. Wenger, Dalton Trans., 2011, 40, 12409–12420 RSC.
- R. F. W. Bader, Chem. Rev., 1991, 91, 893–928 CrossRef CAS.
- T. Lu and Q. Chen, Chem.: Methods, 2021, 1, 231–239 CAS.
- R. Bianchi, G. Gervasio and D. Marabello, Inorg. Chem., 2000, 39, 2360–2366 CrossRef CAS PubMed.
- R. F. W. Bader, J. Phys. Chem. A, 2009, 113, 10391–10396 CrossRef CAS PubMed.
- R. F. W. Bader, J. Phys. Chem. A, 1998, 102, 7314–7323 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Computational details, interaction energy calculations on molecules 1–3, EDA of molecule 1, IRI analysis of molecule 2, Cartesian coordinates of molecules 1–3, [AuIII(CH3)3(NH3)]⋯ [(CH4)3(NH3)] and [(CH4)3(NH3)]2. See DOI: https://doi.org/10.1039/d2dt03731a |
| ‡ These authors contributed equally. |
| § The energy decomposition analysis (EDA) is currently implemented in TURBOMOLE v.7.5.1 at the RHF and DFT levels of theory only. |
| This journal is © The Royal Society of Chemistry 2023 |

