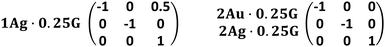Open Access Article
Open Access ArticleGuest-triggered “Soma–Iwamoto-type” penetration complex {Fe(4-methoxypyrimidine)2[M(CN)2]2}·Guest (M = Ag, Au)†
Kosuke
Kitase
 a,
Daisuke
Akahoshi
b and
Takafumi
Kitazawa
*ac
a,
Daisuke
Akahoshi
b and
Takafumi
Kitazawa
*ac
aDepartment of Chemistry, Toho University, Chiba 274-8510, Japan. E-mail: kitazawa@chem.sci.toho-u.ac.jp
bDepartment of Physics, Toho University, Chiba 274-8510, Japan
cResearch Centre for Materials with Integrated Properties, Toho University, Chiba 274-8510, Japan
First published on 8th February 2023
Abstract
Many laboratories have performed extensive studies on the spin-crossover (SCO) phenomenon. Herein, we synthesized novel “Soma–Iwamoto type” complexes containing a 4-methoxypyrimidine (4-MeOPmd) ligand, i.e., {Fe(4-MeOPmd)2[Ag(CN)2]2} (1Ag), {Fe(4-MeOPmd)2[Au(CN)2]2} (1Au), and {Fe(4-MeOPmd)2[Ag(CN)2]2·0.25(4-MeOPmd)} (1Ag·0.25G). Moreover, we synthesized a 4,6-dimethoxypyrimidine (4,6-diMeOPmd) clathrate complex, i.e., {Fe(4-MeOPmd)2[Ag(CN)2]2·0.25(4,6-diMeOPmd)} (2Ag·0.25G) and {Fe(4-MeOPmd)2[Au(CN)2]2·0.25(4,6-diMeOPmd)} (2Au·0.25G). Some of these complexes, i.e., 1Ag·0.25G, 2Ag·0.25G, and 2Au·0.25G, demonstrated penetrated structures, which are very interesting given that they have rarely been reported to date. 1Au and 1Ag did not show the SCO phenomenon, 1Ag·0.25G showed a gradual multi-step SCO phenomenon, and 2Au·0.25G and 2Ag·0.25G showed an abrupt half SCO phenomenon.
Introduction
The spin-crossover (SCO) phenomenon is a reversible spin transition between high-spin (HS) and low-spin (LS) states in response to heat, pressure, or light. Recently, many groups have investigated SCO because of the potential of functional materials for applications such as molecular machines, data storage devices, switching devices, and sensors. Moreover, a Hofmann-type structure is a coordination polymer that contains a center metal ion, bridging cyanometalate ligand, and pillar ligands such as pyridine and pyrazine. The first Hofmann-type complex, Ni(NH3)2[Ni(CN)4]·2(C6H6), was reported by K. A. Hofmann.1 Alternatively, the first Hofmann-type SCO complex, Fe(Py)2[Ni(CN)4], was reported by Kitazawa et al.2 Subsequently, many laboratories have investigated Hofmann-type complexes because of their tunable properties. To date, the reported “Hofmann-type” complexes can be divided into two types. The first type is bridged by square planar tetra-coordinated divalent cyanometalate [M(CN)4]2− (M = Ni, Pd, Pt)3–10 such as Fe(pz)[M(CN)4]·H2O (M = Ni, Pd, Pt),10 while the other is bridged by linear coordinated monovalent cyanometalate [M(CN)2]− (M = Ag, Au).11–16 The first complex possessing the latter type of structure is [Cd(4,4′-bpy)2{Ag(CN)2}2],17 while the second is [Cd(py)2{Ag(CN)2}2],18,19 which were both reported by Soma and Iwamoto, and therefore, the latter type of structures are denoted as “Soma–Iwamoto types”. Coordination polymer frameworks of Hofmann-type ML2M′(CN)4 (M′ = Ni, Pd, Pt) structures are different from that of the Soma–Iwamoto type ML2[M′′(CN)2]2 (M′′ = Ag, Au). The mesh size of the Soma–Iwamoto-type structure is about four-times larger than the Hofmann-type structure because the side length of the former is approximately twice as long as that of the latter. Soma–Iwamoto type Fe complexes were also previously reported, where first reported one was Fe(py)2{Au(CN)2}2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 and Fe(py)2{Ag(CN)2}2.21
20 and Fe(py)2{Ag(CN)2}2.21
However, although many Hofmann-type and Soma–Iwamoto-type complexes have been reported, the pillar ligands in most of these complexes belong to pyridine, pyrazine, or bipyridine-type ligands, and only a few of Soma–Iwamoto-type complexes using pyrimidine-type ligands as a pillar ligand have been reported.
Previously, we reported the preparation of two Soma–Iwamoto-type SCO complexes, {Fe(4-methylpyrimidine)2[Au(CN)2]2}15 and {Fe(4-methylpyrimidine)2[Ag(CN)2]2},16 using 4-methylpyrimidine. These complexes show “normal” stacking of Soma–Iwamoto-type single or double-layer and Soma–Iwamoto type-structure, where the unique stacking of the Soma–Iwamoto-type layer has not been obtained in pyrimidine-type complexes to date. The interaction between the Ag atom in the bridging ligand and lone pair of the N atom in the 4-methylpyrimidine ligand was observed in {Fe(4-methylpyrimidine)2[Ag(CN)2]2}. In this study, the methyl group in the pyrimidine-type ligand is replaced with a methoxy group, causing additional M–ligand interaction due to the lone pair in the O atom. Thus, we improved the subtle interactions between the cyano-coordinate framework and pyrimidine-type molecule as a second bond, which is associated with spin transition behavior.
Herein, we synthesized novel Soma–Iwamoto type SCO complexes using the 4-methoxypyrimidine ligand, including {Fe(4-methoxypyrimidine)2[Ag(CN)2]2} (1Ag), {Fe(4-methoxypyrimidine)2[Au(CN)2]2} (1Au), and {Fe(4-methoxypyrimidine)2[Ag(CN)2]2·0.25(4-methoxypyrimidine)} (1Ag·0.25G). We also used 4,6-dimethoxypyrimidine as a guest. The reason why this molecule has a similar structure to 4-methoxypyrimidine but cannot coordinate to the Fe center is due to the steric hindrance of both nitrogen atoms in the pyrimidine ring, and consequently this molecule can only act as a guest molecule. Furthermore, we synthesized two complexes, i.e., {Fe(4-methoxypyrimidine)2[Ag(CN)2]2·0.25(4,6-dimethoxypyrimidine)} (2Ag·0.25G) and {Fe(4-methoxypyrimidine)2[Au(CN)2]2·0.25(4,6-dimethoxypyrimidine)} (2Au·0.25G), using this molecule.
Experimental
The complexes were synthesized using Mohr's salt and cyanometalate.Mohr's salt was purchased from Kanto Chemical Co., L-ascorbic acid was purchased from Nacalai Tesque Co., K[Au(CN)2] was purchased from Toyo Chemical Industrial Co., and 4-methoxypyrimidine and 4,6-dimethoxypyrimidine were purchased from TCI.
The Fe–Ag Soma–Iwamoto-type complexes (1Ag, 1Ag·0.25G, 2Ag·0.25G) and Fe–Au Soma–Iwamoto-type complexes (1Au, 2Au·0.25G) were synthesized via a direct method and vapor diffusion method, respectively.
Complex 1Au was synthesized using Mohr's salt Fe(NH4)2(SO4)2·6H2O, L-ascorbic acid and K[Au(CN)2] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio, and 4-methoxypyrimidine. In the synthesis of the powder sample, 0.3 mmol of Mohr's salt (0.1194 g), 0.28 mmol of L-ascorbic acid (0.0493 g) and 0.6 mmol of K[Au(CN)2] (0.1740 g) were dissolved in 10 mL of water. Then excess 4-methoxypyrimidine (0.3302 g, 3.0 mmol) was dropped into this solution. Subsequently, 0.1522 g (0.196 mmol, 64% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.0392 g), 0.1 mmol of L-ascorbic acid (0.0176 g) and 0.2 mmol of K[Au(CN)2] (0.0576 g) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then, this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). Colorless crystals were obtained using this method.‡
2 molar ratio, and 4-methoxypyrimidine. In the synthesis of the powder sample, 0.3 mmol of Mohr's salt (0.1194 g), 0.28 mmol of L-ascorbic acid (0.0493 g) and 0.6 mmol of K[Au(CN)2] (0.1740 g) were dissolved in 10 mL of water. Then excess 4-methoxypyrimidine (0.3302 g, 3.0 mmol) was dropped into this solution. Subsequently, 0.1522 g (0.196 mmol, 64% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.0392 g), 0.1 mmol of L-ascorbic acid (0.0176 g) and 0.2 mmol of K[Au(CN)2] (0.0576 g) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then, this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). Colorless crystals were obtained using this method.‡
Complexes 1Ag and 1Ag·0.25G were obtained as a mixture. These complexes were synthesized using Mohr's salt Fe(NH4)2(SO4)2·6H2O and K[Ag(CN)2] in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and 4-methoxypyrimidine. In the synthesis of the powder sample, 0.4 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.1569 g) and 0.96 mmol of 4-methoxypyrimidine (0.1060 g) were dissolved in 3 mL of water (solution 1) and 0.8 mmol of K[Ag(CN)2] (0.1664 g) was dissolved in 7 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2079 g of white powder of 1Ag and 1Ag·0.25G was obtained as a mixture by this method. In the synthesis of a single crystal, 0.1 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.0392 g) and 0.2 mmol of K[Ag(CN)2] (0.0398 g) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). Clear (1Ag) and orange (1Ag·0.25G) crystals were obtained and separated.
2 molar ratio and 4-methoxypyrimidine. In the synthesis of the powder sample, 0.4 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.1569 g) and 0.96 mmol of 4-methoxypyrimidine (0.1060 g) were dissolved in 3 mL of water (solution 1) and 0.8 mmol of K[Ag(CN)2] (0.1664 g) was dissolved in 7 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2079 g of white powder of 1Ag and 1Ag·0.25G was obtained as a mixture by this method. In the synthesis of a single crystal, 0.1 mmol of Mohr's salt Fe(NH4)2(SO4)2·6H2O (0.0392 g) and 0.2 mmol of K[Ag(CN)2] (0.0398 g) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). Clear (1Ag) and orange (1Ag·0.25G) crystals were obtained and separated.
Complex 2Au·0.25G was synthesized using Mohr's salt Fe(NH4)2(SO4)2·6H2O, L-ascorbic acid, K[Au(CN)2] and 4-methoxypyrimidine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and excess 4,6-dimethoxypyrimidine. In the synthesis of a powder sample, 0.4 mmol (0.1590 g) of Mohr's salt, 0.4 mmol (0.0707 g) of L-ascorbic acid, excess 4,6-dimethoxypyrimidine (0.3535 g, 2.4 mmol) and 0.8 mmol (0.0881 g) of 4-methoxypyrimidine were dissolved in 6 mL of water (solution 1) and 0.8 mmol (0.2314 g) of K[Au(CN)2] was dissolved in 4 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2097 g (0.259 mmol, 32% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol (0.0392 g) of Mohr's salt, 0.1 mmol (0.0176 g) of L-ascorbic acid, 0.2 mmol (0.0576 g) of K[Au(CN)2] and excess of 4,6-dimethoxypyrimidine (0.1401 g, 1.0 mmol) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). A colorless crystal was obtained using this method.
2 molar ratio and excess 4,6-dimethoxypyrimidine. In the synthesis of a powder sample, 0.4 mmol (0.1590 g) of Mohr's salt, 0.4 mmol (0.0707 g) of L-ascorbic acid, excess 4,6-dimethoxypyrimidine (0.3535 g, 2.4 mmol) and 0.8 mmol (0.0881 g) of 4-methoxypyrimidine were dissolved in 6 mL of water (solution 1) and 0.8 mmol (0.2314 g) of K[Au(CN)2] was dissolved in 4 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2097 g (0.259 mmol, 32% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol (0.0392 g) of Mohr's salt, 0.1 mmol (0.0176 g) of L-ascorbic acid, 0.2 mmol (0.0576 g) of K[Au(CN)2] and excess of 4,6-dimethoxypyrimidine (0.1401 g, 1.0 mmol) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). A colorless crystal was obtained using this method.
Complex 2Ag·0.25G was synthesized using Mohr's salt Fe(NH4)2(SO4)2·6H2O, K[Ag(CN)2] and 4-methoxypyrimidine in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 molar ratio and excess of 4,6-dimethoxypyrimidine. In the synthesis of the powder sample, 0.5 mmol (0.1956 g) of Mohr's salt and excess 4,6-dimethoxypyrimidine (0.2802 g, 2.0 mmol) were dissolved in 10 mL of water (solution 1) and 1.0 mmol (0.2041 g) of K[Ag(CN)2] and 1.1 mmol (0.1171 g) of 4-methoxypyrimidine were dissolved in 5 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2197 g (0.348 mmol, 35% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol (0.0392 g) of Mohr's salt, 0.05 mmol (0.0088 g) of L-ascorbic acid, 0.2 mmol (0.0398 g) of K[Ag(CN)2] and excess 4,6-dimethoxypyrimidine (0.1401 g, 1.0 mmol) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). A colorless crystal was obtained using this method.
2 molar ratio and excess of 4,6-dimethoxypyrimidine. In the synthesis of the powder sample, 0.5 mmol (0.1956 g) of Mohr's salt and excess 4,6-dimethoxypyrimidine (0.2802 g, 2.0 mmol) were dissolved in 10 mL of water (solution 1) and 1.0 mmol (0.2041 g) of K[Ag(CN)2] and 1.1 mmol (0.1171 g) of 4-methoxypyrimidine were dissolved in 5 mL of water (solution 2). Then solution 2 was dropped into solution 1. Subsequently, 0.2197 g (0.348 mmol, 35% yield) of white powder was obtained by this method. In the synthesis of a single crystal, 0.1 mmol (0.0392 g) of Mohr's salt, 0.05 mmol (0.0088 g) of L-ascorbic acid, 0.2 mmol (0.0398 g) of K[Ag(CN)2] and excess 4,6-dimethoxypyrimidine (0.1401 g, 1.0 mmol) were dissolved in a 10 mL sample bottle filled with 10 mL water. Then this solution was exposed to 4-methoxypyrimidine vapour in a larger airtight bottle (50 mL sample bottle). A colorless crystal was obtained using this method.
The expected compositions of the synthesized complexes were confirmed by elemental analysis using J-Science MICRO CORDER JM10, (1Au: anal. calcd for C14H12N8O2FeAu2: C, 21.72%; H, 1.56%; N, 14.48%. Found: C, 21.67%; H, 1.64%; N, 14.49%, 1Ag and 1Ag·0.25G: anal. calcd for C14H12N8O2FeAg2 (1Ag): C 28.21%, H 2.03%, N 18.81%. anal. calcd for C14H12N8O2FeAg2·0.25(C5H6N2O) (1Ag·0.25G): C 29.38%, H 2.18%, N 19.10%. Found: 28.71%, H 2.23%, N 18.94%, 2Au·0.25G: anal. calcd for C14H12N8O2FeAu2·0.25(C6H8N2O2): C, 23.00%; H, 1.74%; N, 14.72%. Found: C, 23.02%; H, 1.92%; N, 14.68%. 2Ag·0.25G: anal. calcd for C14H12N8O2FeAg2·0.25(C6H8N2O2): C, 29.50%; H, 2.24%; C, 18.87%. Found: C, 29.61%; H, 2.38%; N, 18.97%). This complex was identified by TG analysis using a Hitachi High-Technologies Corporation TG/DTA6200 (Fig. S1–S4†) and powder X-ray diffraction measurement using a Rigaku Co. X-ray diffractometer or MAC Science M03XHF22 (Fig. S5–S8†). The presence of 1Ag in the 1Ag·0.25G powder was confirmed via powder X-ray diffraction measurement (Fig. S6†).
The superconducting quantum interference device (SQUID) method from Quantum Design MPMS - 5S was used to measure the magnetic susceptibility of the complexes in the temperature range of room temperature to 4 K under a 0.1 T field and a cooling rate of 1 K min−1. The sample used for measurement was filled in a gelatine capsule, which was filled with a plastic straw. The magnetic susceptibility of 1Ag was measured using a pure single crystal, while that of the other samples was measured using a powder sample. We measured the magnetic susceptibility of the powder sample of 1Ag·0.25G twice, where the repeated measurement result is shown in Fig. S13;† however, we could not measure this property of the pure crystal of 1Ag·0.25G due to the worldwide helium shortage.
The crystal structure of the synthesized complexes was determined by single-crystal X-ray diffraction (XRD) using a Bruker SMART diffractometer having a Mo-Kα line at 298 K (HS state), 190 K (intermediate state), and 150 K (LS state). The structure was solved using the SHELX 2014 software.24,25
Results and discussion
Fig. 1 and 2 show the magnetic susceptibilities of 1Au, 1Ag, 1Ag·0.25G, and 2Au·0.25G. Comparing these complexes, 1Au and 1Ag did not demonstrate the SCO phenomenon. 1Ag·0.25G showed a three-step spin transition between 70 and 250 K, where the 1st step is abrupt, and 2nd and 3rd steps are gradual. 2Au·0.25G showed an abrupt one-step spin transition between 150 and 180 K, and half the magnetic susceptibility, 2Ag·0.25G showed a relatively gradual one-step spin transition between 180 and 260 K and its magnetic susceptibility was halved. These results suggest that 1Au and 1Ag do not exhibit the SCO phenomenon, and all the Fe sites show the HS state below room temperature. The other samples showed the SCO phenomenon, where all the Fe sites in complex 1Ag·0.25G changed to the LS state, while only half of the Fe site in complex 2Au·0.25G changed to the LS state. In the case of 1Au, its magnetic susceptibility is ∼4 cm3 mol K−1 above 100 K, while that of 1Ag is ∼3.8 cm3 mol K−1 above 100 K. These values are higher than the calculated spin only octahedral magnetic susceptibility of the high spin Fe site (S = 2) of ∼3.0 cm3 mol K−1, which can be explained by the spin–orbital coupling.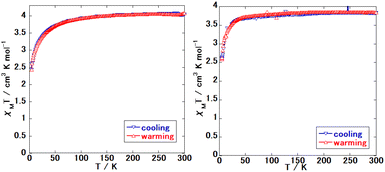 | ||
| Fig. 1 Magnetic susceptibility of the synthesized complex. (Left: 1Au and right: 1Ag (pure crystal).) | ||
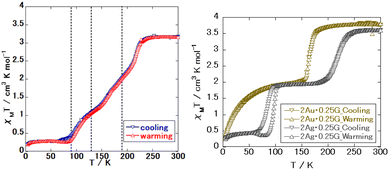 | ||
| Fig. 2 Magnetic susceptibility of guest-clathrate complex. (Left: 1Ag·0.25G (powder) and right: 2Au·0.25G and 2Ag·0.25G.) | ||
In the case of 1Ag·0.25G, its magnetic susceptibility at 298 K was 3.15 cm3 mol K−1, at 190 K it was 2.03 cm3 mol K−1, at 130 K it was 1.13 cm3 mol K−1, and at 50 K it was 0.28 cm3 mol K−1. This shows that this complex has all four states, i.e., all HS, ∼2/3 HS, ∼1/3 HS, and all LS states. For 2Au·0.25G, its magnetic susceptibility at 298 K was 3.77 cm3 mol K−1, while that at 140 K is 1.99 cm3 mol K−1. This shows this complex has two spin states, i.e., all HS and 1/2 HS. In the case of 2Ag·0.25G, its magnetic susceptibility at 298 K is 3.6 cm3 mol K−1, at 150 K, it was 1.94 cm3 mol K−1, and at 50 K it was 0.43 cm3 mol K−1. This shows three spin states, i.e., all HS and 1/2 HS states, which are similar to that of 2Au·0.25G, and all the LS states may exist below 50 K.
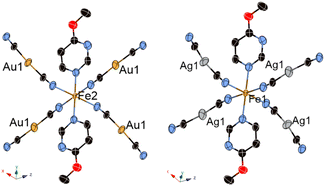
Fig. 3 and 4 show the crystal structure of 1Au and 1Ag, respectively. These complexes show a single-layer Soma–Iwamoto type structure without aurophilic or argentophilic interaction. The space group of both complexes was determined to be P21/n at RT and low temperature. The detailed crystallographic data is available in Tables S1–S4.† Comparing the RT and low temperature data, no bond length change was observed (Table 1). The insignificant change in the crystal structure between RT and low temperature supports that 1Au and pure 1Ag do not show the SCO phenomenon by magnetic measurements.
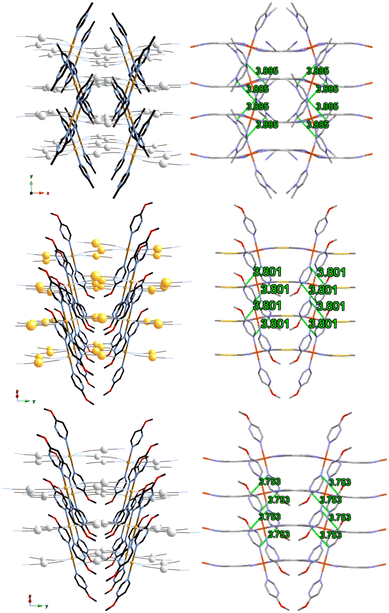 | ||
| Fig. 4 Packing structure of {Fe(4-methylpyrimidine)2[Ag(CN)2]2}16 (top, along the c axis), 1Au (middle, along the [1 0 −1] axis) and 1Ag (bottom, along the [1 0 −1] axis). (Gray: C, blue: N, red: N, orange: Fe, silver: Ag, yellow: Au.) | ||
| Average bond lengths/Å | ||||
|---|---|---|---|---|
| 1Au (M = Au) | 1Ag (M = Ag) | |||
| T/K | 296(2) | 100(2) | 296(2) | 90(2) |
| Fe–Nligand | 2.229(3) | 2.223(3) | 2.241(2) | 2.2363(14) |
| Fe–Ncyano | 2.159(3) | 2.155(3) | 2.1579(19) | 2.1532(14) |
| M–C | 1.976(4) | 1.983(3) | 2.059(2) | 2.0613(17) |
| M–Nligand | 3.435 | 3.373 | 3.125 | 2.988 |
| M–M | 4.780 | 4.658 | 4.729 | 4.604 |
The Ag–Nligand distance in 1Ag is closer than the Au–Nligand distance in 1Au by more than 0.3 Å (Table 1). This means that the Ag–N interaction is slightly stronger than the Au–N interaction. This can be explained by the relativistic effect.16,23 Furthermore, this trend was observed in previously reported Au- and Ag-type complexes using 4-methylpyrimidine.15,16
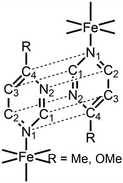
Comparing 1Au and 1Ag with {Fe(4-methylpyrimidine)2[Au(CN)2]2}15 and {Fe(4-methylpyrimidine)2[Ag(CN)2]2},16 respectively, 1Au shows a single-layered Soma–Iwamoto-type structure without Au–Au interaction, while {Fe(4-methylpyrimidine)2[Au(CN)2]2} shows a double-layered Soma–Iwamoto-type structure with Au–Au interaction. Also, 1Ag does not show the SCO phenomenon, while {Fe(4-methylpyrimidine)2[Ag(CN)2]2} shows the SCO phenomenon even though both complexes possess a Soma–Iwamoto-type single layer with Ag–N interaction. This result can be explained by the difference in the coordination field due to the different π–π interactions (Table 2) and electron density. The former difference led to a difference in the stacking of the pyrimidine ligands. The stacking of the pyrimidine ligands of 1Au and 1Ag were alternately arranged in a V-shape, whereas that of {Fe(4-methylpyrimidine)2[Ag(CN)2]2} crossed and arranged in an X-shape (Fig. 4). The latter difference can be explained by the greater electron-donating ability of the methoxy group compared to that of the methyl group. This led to the greater electron density and lower electrophilicity of 4-methoxypyrimidine than that of 4-methylpyrimidine, and thus 4-methoxypyrimidine forms a weaker coordination field than 4-methylpyrimidine. Consequently, 1Au and 1Ag show the HS state even at very low temperatures.
| π–π interaction distance/Å | ||||
|---|---|---|---|---|
| Ref. 16 | 1Au | 1Ag | ||
|
N1–C4 | 3.885 | 3.801 | 3.753 |
| N2–C2 | 3.804 | 3.754 | 3.691 | |
| C1–C3 | 3.833 | 3.754 | 3.671 | |
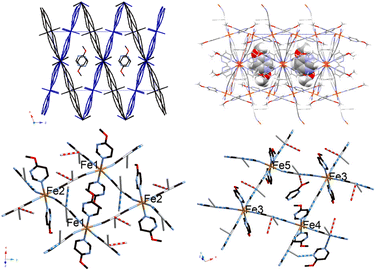
Fig. 5 shows the crystal structure of 1Ag·0.25G, which shows a double-layered penetrated Hofmann-type structure with five Fe sites. At 296 K, the layers intersect at an angle of 39.89°. Layer 1 consists of two nonequivalent Fe sites at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In contrast, layer 2 contains three non-equivalent Fe sites at a ratio of 2
1. In contrast, layer 2 contains three non-equivalent Fe sites at a ratio of 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Layers 1 and 2 are along the (1 0 0) and (−1 0 2) planes of the unit lattice, respectively. Additionally, an Ag–Ag interaction exists between layers 1 and 2. The space group was determined to be P21/c at 296 K, 190 K, 130 K and 87 K. The detailed crystallographic data is available in Tables S5–S8.†Table 3 shows the bond length of 1Ag·0.25G, which indicates that an argentophilic interaction exists between the two layers. At 190 K, half of the Fe site on layer 1 transforms to the LS state, and a quarter of that on layer 2 transforms to the LS state. Consequently, five-eighths of the Fe site remains in the HS state at 190 K. At 87 K, all of the Fe site on layer 1 is transformed to the LS state, while half of that on layer 2 is transformed to the LS state. Therefore, a quarter of the Fe site remains in the HS state at 87 K. Thus, 1Ag·0.25G shows three spin states, i.e., HS, HS5/8LS3/8, and HS1/4LS3/4 (Fig. 6). This result supports the multi-step SCO phenomenon in magnetic susceptibility. The two non-equivalent Soma–Iwamoto-type layers can explain this phenomenon. Also, 1Ag·0.25G may show completely spin transition states, e.g., all LS states, at very low temperatures due to its almost zero magnetic susceptibility at very low temperatures. Its crystal structure at 130 K is similar to that at 87 K even though the powder sample showed a complete transition to the LS at 87 K and this behaviour was reproduced by repeating the measurement (Fig. S13†). This suggests that the spin transition temperature of the single crystal is lower than that of the powder sample. An Ag–O interaction was also observed in 1Ag·0.25G, which can be stabilized in this structure. Penetrated structures are very interesting given that only a few Soma–Iwamoto-type complexes with penetrated structures have been reported. One of the examples of a penetrated Soma–Iwamoto-type structure is Mn(4-methylpyridine)2[Ag(CN)2]2.22 In contrast to the results of the Ag-type complex, the penetrated Au-type complex, i.e., complex 1Au·0.25G, was not obtained. This can be explained by the fact that the Au–N interaction is not strong enough to stabilize the penetrated structure. Comparing 1Ag·0.25G to 1Ag, many differences exist. That can mainly be explained by the fact that the O atoms in the methoxy group weakly coordinate to the Ag atom in the cyanometallate. This not only leads to the formation of a penetrated structure but also less electron density on 4-methoxypyrimidine than that of the non-coordinated one, creating a stronger coordination field. Thus, 1Ag·0.25G shows the SCO phenomenon, whereas 1Ag does not.
1. Layers 1 and 2 are along the (1 0 0) and (−1 0 2) planes of the unit lattice, respectively. Additionally, an Ag–Ag interaction exists between layers 1 and 2. The space group was determined to be P21/c at 296 K, 190 K, 130 K and 87 K. The detailed crystallographic data is available in Tables S5–S8.†Table 3 shows the bond length of 1Ag·0.25G, which indicates that an argentophilic interaction exists between the two layers. At 190 K, half of the Fe site on layer 1 transforms to the LS state, and a quarter of that on layer 2 transforms to the LS state. Consequently, five-eighths of the Fe site remains in the HS state at 190 K. At 87 K, all of the Fe site on layer 1 is transformed to the LS state, while half of that on layer 2 is transformed to the LS state. Therefore, a quarter of the Fe site remains in the HS state at 87 K. Thus, 1Ag·0.25G shows three spin states, i.e., HS, HS5/8LS3/8, and HS1/4LS3/4 (Fig. 6). This result supports the multi-step SCO phenomenon in magnetic susceptibility. The two non-equivalent Soma–Iwamoto-type layers can explain this phenomenon. Also, 1Ag·0.25G may show completely spin transition states, e.g., all LS states, at very low temperatures due to its almost zero magnetic susceptibility at very low temperatures. Its crystal structure at 130 K is similar to that at 87 K even though the powder sample showed a complete transition to the LS at 87 K and this behaviour was reproduced by repeating the measurement (Fig. S13†). This suggests that the spin transition temperature of the single crystal is lower than that of the powder sample. An Ag–O interaction was also observed in 1Ag·0.25G, which can be stabilized in this structure. Penetrated structures are very interesting given that only a few Soma–Iwamoto-type complexes with penetrated structures have been reported. One of the examples of a penetrated Soma–Iwamoto-type structure is Mn(4-methylpyridine)2[Ag(CN)2]2.22 In contrast to the results of the Ag-type complex, the penetrated Au-type complex, i.e., complex 1Au·0.25G, was not obtained. This can be explained by the fact that the Au–N interaction is not strong enough to stabilize the penetrated structure. Comparing 1Ag·0.25G to 1Ag, many differences exist. That can mainly be explained by the fact that the O atoms in the methoxy group weakly coordinate to the Ag atom in the cyanometallate. This not only leads to the formation of a penetrated structure but also less electron density on 4-methoxypyrimidine than that of the non-coordinated one, creating a stronger coordination field. Thus, 1Ag·0.25G shows the SCO phenomenon, whereas 1Ag does not.
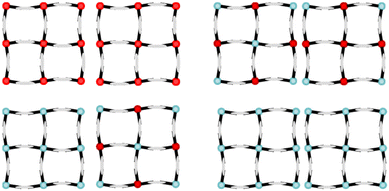 | ||
| Fig. 6 Soma–Iwamoto-type layer model of 1Ag·0.25G. (Upper left: RT, upper right: 190 K, lower left: 130 and 87 K, and lower right: lower temperature (predicted).) | ||
| Average bond lengths/Å | ||||
|---|---|---|---|---|
| T/K | 296(2) | 190(2) | 130(2) | 87(2) |
| Fe–Nligand | Layer 1: 2.210(10) | Layer 1: 2.223(9), 2.015(9) | Layer 1: 2.012(9) | Layer 1: 2.008(8) |
| Layer 2: 2.220(10) | Layer 2: 2.198(9), 2.022(9) | Layer 2: 2.210(9), 2.005(9) | Layer 2: 2.190(9), 2.003(8) | |
| Fe–Ncyano | Layer 1: 2.134(8) | Layer 1: 2.146(8), 1.948(8) | Layer 1: 1.952(8) | Layer 1: 1.944(8) |
| Layer 2: 2.155(7) | Layer 2: 2.140(8), 1.943(8) | Layer 2: 2.163(7), 1.940(7) | Layer 2: 2.145(7), 1.941(7) | |
| Ag–C | Layer 1: 2.072(10) | Layer 1: 2.070(10) | Layer 1: 2.073(9) | Layer 1: 2.068(9) |
| Layer 2: 2.055(9) | Layer 2: 2.067(10) | Layer 2: 2.072(9) | Layer 2: 2.068(9) | |
| Ag–Nguest | Layer 1: 3.181 | Layer 1: 2.984 | Layer 1: 3.012 | Layer 1: 2.968 |
| Layer 2: 3.186 | Layer 2: 3.053 | Layer 2: 2.963 | Layer 2: 2.944 | |
| Ag–Oguest | Layer 2: 3.041 | Layer 2: 2.946 | Layer 2: 2.902 | Layer 2: 2.874 |
| Ag–Ag | 3.1154(16) | 3.0832(14) | 3.0783(18) | 3.0599(11) |
Fig. 7 shows the crystal structure of 2Au·0.25G, possessing a two-layered penetrated Soma–Iwamoto-type structure with two Fe sites ion a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. These layers intersect at an angle of 41.14°. The Soma–Iwamoto-type layers are along two directions, i.e., (1 1 0) and (−1 1 0) planes of the unit lattice. Also, an Au–Au interaction exists between the two layers. The space group was determined to be C2/c at 296 K and 100 K. The detailed crystallographic data is available in Tables S9 and S10.†Table 4 shows the bond lengths of 2Au·0.25G, which indicate that an aurophilic interaction exists between the two layers. At 90 K, half of the Fe site remains in the HS state, and thus 2Au·0.25G shows two spin states, i.e., HS and HS0.5LS0.5 (Fig. 8). This corresponds to the magnetic measurement. The half-spin transition state was also observed in a previously reported complex, i.e., {Fe(4-methylpyrimidine)2[Au(CN)2]2}. The half-spin transition state is widely observed in complex 2Au·0.25G at a lower temperature range. In contrast, the half-spin transition state in {Fe(4-methylpyrimidine)2[Au(CN)2]2} is simply an intermediate state and was observed in a narrow temperature range. This can be explained by the fact that 2Au·0.25G contains two different types of Fe sites at all temperature ranges, whereas {Fe(4-methylpyrimidine)2[Au(CN)2]2} contains only one type of Fe site except in the intermediate state area. Also, the pyrimidine ligands coordinated to the Fe2 sites are disordered in two positions by a 0.522(13)
1 ratio. These layers intersect at an angle of 41.14°. The Soma–Iwamoto-type layers are along two directions, i.e., (1 1 0) and (−1 1 0) planes of the unit lattice. Also, an Au–Au interaction exists between the two layers. The space group was determined to be C2/c at 296 K and 100 K. The detailed crystallographic data is available in Tables S9 and S10.†Table 4 shows the bond lengths of 2Au·0.25G, which indicate that an aurophilic interaction exists between the two layers. At 90 K, half of the Fe site remains in the HS state, and thus 2Au·0.25G shows two spin states, i.e., HS and HS0.5LS0.5 (Fig. 8). This corresponds to the magnetic measurement. The half-spin transition state was also observed in a previously reported complex, i.e., {Fe(4-methylpyrimidine)2[Au(CN)2]2}. The half-spin transition state is widely observed in complex 2Au·0.25G at a lower temperature range. In contrast, the half-spin transition state in {Fe(4-methylpyrimidine)2[Au(CN)2]2} is simply an intermediate state and was observed in a narrow temperature range. This can be explained by the fact that 2Au·0.25G contains two different types of Fe sites at all temperature ranges, whereas {Fe(4-methylpyrimidine)2[Au(CN)2]2} contains only one type of Fe site except in the intermediate state area. Also, the pyrimidine ligands coordinated to the Fe2 sites are disordered in two positions by a 0.522(13)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.478(13) rate. These positions are vertical, and only one position has a clathrate guest molecule. The result that not all the Fe2 sites face the same direction can be explained by the fact that the steric hindrance of the two methyl groups prevents the pyrimidine ligands from facing the same direction (Fig. 9).
0.478(13) rate. These positions are vertical, and only one position has a clathrate guest molecule. The result that not all the Fe2 sites face the same direction can be explained by the fact that the steric hindrance of the two methyl groups prevents the pyrimidine ligands from facing the same direction (Fig. 9).
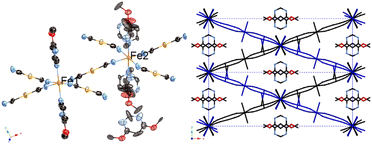 | ||
| Fig. 7 Structure of 2Au·0.25G. (Left: ORTEP structure and right: packing structure along the Z axis (ligand molecules are omitted).) | ||
| Average bond lengths/Å | |||||
|---|---|---|---|---|---|
| 2Au·0.25G (M = Au) | 2Ag·0.25G (M = Ag) | ||||
| T/K | 296(2) | 90(2) | 296(2) | 150(2) | 90(2) |
| Fe–Nligand | Fe1: 2.199(11) | Fe1: 2.208(10) | Fe1: 2.237(6) | Fe1: 2.235(4) | Fe1: 2.209(9) |
| Fe2: 2.20(4) | Fe2: 2.006(10) | Fe2: 2.204(8) | Fe2: 2.007(5) | Fe2: 1.998(9) | |
| Fe–Ncyano | Fe1: 2.160(9) | Fe1: 2.159(11) | Fe1: 2.149(5) | Fe1: 2.144(5) | Fe1: 2.134(10) |
| Fe2: 2.160(10) | Fe2: 1.934(12) | Fe2: 2.133(6) | Fe2: 1.936(5) | Fe2: 1.929(10) | |
| M–C | 1.985(13) | 1.984(14) | 2.058(5) | 2.054(6) | 1.984(14) |
| M–Nguest | 3.750 | 3.549 | 3.697 | 3.496 | 3.475 |
| M–Oguest | 3.466 | 3.329 | 3.331 | 3.252 | 3.213 |
| M–M | 3.2056(7) | 3.1291(9) | 3.1569(11) | 3.0940(9) | 3.0807(13) |
In the case of 2Au·0.25G, the 4,6-dimethoxypyrimidine guest is parallel to the 4-methoxypyrimidine ligand. That suggests that a π–π interaction exists between the host structure and guest molecule.
Fig. 10 shows the crystal structure of 2Ag·0.25G, where the pyrimidine ligands coordinated to Fe2 atoms are disordered to two positions and isostructural to 2Au·0.25G by a 0.482(7)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.518(7) rate, and Table 4 also shows the bond lengths of 2Ag·0.25G. The results indicate that an argentophilic interaction occurs between the two layers, and the Soma–Iwamoto-type layers intersect at an angle of 43.23°. The Soma–Iwamoto-type layers are along two directions, i.e., the (1 1 0) and (−1 1 0) planes of the unit lattice. At 150 and 90 K, half of the Fe site remains in the HS state, and thus 2Ag·0.25G shows two spin states, i.e., HS and HS0.5LS0.5 (Fig. 11). This corresponds to the magnetic measurement. Also, 2Ag·0.25G may show complete spin transition states, e.g., all LS states, at very low temperatures due to its almost zero magnetic susceptibility at very low temperatures. The space group was determined to be C2/c at 296, 150 and 90 K. The detailed crystallographic data is available in Tables S11–S13.† In the unit cell of complex 2Ag·0.25G, β is very close to 90°. However, this is monoclinic given that no symmetry elements on the a and c axis were detected by the ADDSYM search in PLATON.
0.518(7) rate, and Table 4 also shows the bond lengths of 2Ag·0.25G. The results indicate that an argentophilic interaction occurs between the two layers, and the Soma–Iwamoto-type layers intersect at an angle of 43.23°. The Soma–Iwamoto-type layers are along two directions, i.e., the (1 1 0) and (−1 1 0) planes of the unit lattice. At 150 and 90 K, half of the Fe site remains in the HS state, and thus 2Ag·0.25G shows two spin states, i.e., HS and HS0.5LS0.5 (Fig. 11). This corresponds to the magnetic measurement. Also, 2Ag·0.25G may show complete spin transition states, e.g., all LS states, at very low temperatures due to its almost zero magnetic susceptibility at very low temperatures. The space group was determined to be C2/c at 296, 150 and 90 K. The detailed crystallographic data is available in Tables S11–S13.† In the unit cell of complex 2Ag·0.25G, β is very close to 90°. However, this is monoclinic given that no symmetry elements on the a and c axis were detected by the ADDSYM search in PLATON.
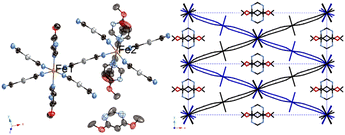 | ||
| Fig. 10 Structure of 2Ag·0.25G. (Left: ORTEP structure and right: packing structure along the Z axis (ligand molecules are omitted).) | ||
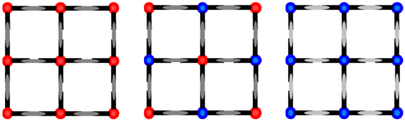 | ||
| Fig. 11 Soma–Iwamoto-type layer model of 2Ag·0.25G. (Left: RT, center: 150 and 90 K, and right: low temperature (predicted).) | ||
Comparing 2Au·0.25G and 2Ag·0.25G, the Ag–N distance in 2Ag·0.25G is closer than the Au–N distance in 2Au·0.25G by less than 0.16 Å, which is a smaller difference than the non-guest-type structure. The shorter M–N distance suggests that the more electron-deficient pyrimidine ring has a stronger coordination interaction of the pyrimidine N atom to Ag or Au atom in the bridging ligand. That leads to a stronger coordination field. In fact, 2Ag·0.25G showed a higher transition temperature than 2Au·0.25G, and additionally all LS state by magnetic measurement.
Due to the limitation of the cryostat, it was impossible to observe all the LS states in 1Ag·0.25G and 2Ag·0.25G at very low temperatures using single-crystal XRD measurement.
Comparing the non-guest complexes, 1Au and 1Ag, and the guest clathrate complexes, 1Ag·0.25G and 2Au·0.25G, respectively, 2Ag·0.25G. 1Au and 1Ag show normal Soma–Iwamoto-type structures, whereas the other is penetrated. This behaviour is similar to the case of [Cd(py)2{Ag(CN)2}2] and [Cd(py)2{Ag(CN)2}2]·Guest (Guest = benzene or pyrrole), where the former shows a single-layered Soma–Iwamoto-type structure, while the latter shows a penetrated structure.
The crystals of 1Ag·0.25G, 2Au·0.25G, and 2Ag·0.25G were obtained as twin crystals. The twin matrix is shown in Fig. 12.
These complexes can be easily twinned as another layer grows beyond one layer with little lattice misalignment due to twinning.
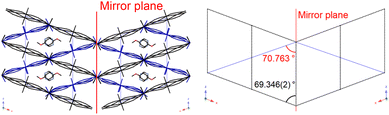
In 1Ag·0.25G, the angle formed by the (−1 0 2) plane and (0 0 1) plane is near to 180° − β (69.346(2)°), and thus this complex can be twinned by the (−1 0 2) plane (Fig. 13).
In 2Ag·0.25G and 2Au·0.25G, the β angle of these complexes is close to 90°, and thus their single crystals can be easily twinned by the (1 0 0) or (0 0 1) plane.
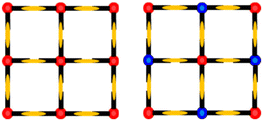
Comparing 1Ag·0.25G with 2Ag·0.25G (and 2Au·0.25G), it was observed that the former complex has two inequivalent layers and gradual three-step SCO phenomenon, whereas the latter complex contains two equivalent layers and one- or two-step SCO phenomenon. This difference can be explained by the symmetry of the guest molecule (Fig. 14). In 1Ag·0.25G, 4-methoxypyrimidine does not have a mirror plane orthogonal to the pyrimidine ring, and thus the two Soma–Iwamoto-type layers of 1Ag·0.25G are inequivalent. In contrast, that of 2Ag·0.25G is equivalent because 4,6-dimethoxypyrimidine has a mirror plane orthogonal to the pyrimidine ring. This leads to a difference in the number of inequivalent Fe sites and intermediate states.
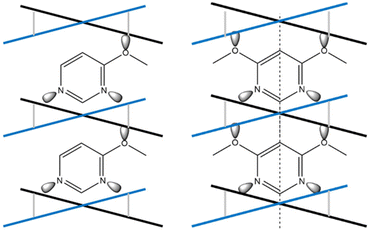 | ||
| Fig. 14 Comparison of the symmetry of the penetrated Soma–Iwamoto-type layer and guest molecule between 1Ag·0.25G and 2Au·0.25G. | ||
In the 4,6-dimethoxyprrimidine clathrate complex, i.e., 2Au·0.25G and 2Ag·0.25G, a penetrated structure was not only observed in the Ag-type complex but also the Au-type complex, where the two methoxy groups can explain the additional M–O interaction.
Conclusions
Using the 4-methoxypyrimidine ligand, 1Au, 1Ag, 1Ag·0.25G, 2Au·0.25G, and 2Ag·0.25G, we synthesized a novel Soma–Iwamoto-type complex. We showed via magnetic susceptibility that 1Ag·0.25G and 2Au·0.25G exhibit the SCO phenomenon. 1Ag·0.25G exhibits a gradual three-step SCO phenomenon, 2Au·0.25G exhibits an abrupt one-step half SCO phenomenon, and 2Ag·0.25G exhibits an abrupt two-step SCO phenomenon. In the crystal structure of all the synthesized complexes, Ag–N or Au–N interaction exists. Moreover, 1Au and 1Ag are isostructural as well as 2Au·0.25G and 2Ag·0.25G. 1Au and 1Ag show only the HS state. 1Ag·0.25G shows three spin states, i.e., HS, HS5/8LS3/8, and HS1/4LS3/4. 2Au·0.25G and 2Ag·0.25G show two spin states, i.e., HS and HS1/2LS1/2. 1Ag·0.25G and 2Ag·0.25G exhibit all the LS states at very low temperatures. Complexes 1Ag·0.25G, 2Au·0.25G, and 2Ag·0.25G show penetrated Soma–Iwamoto-type structures, which are interesting given that only a few of these structures has been reported to date. Comparing the Ag-type complex 1Ag and 2Ag·0.25G and Au-type complex 2Au and 2Au·0.25G, the Ag–N distance is closer than the Au–N distance. This can be explained by the fact that the Ag–N interaction is stronger than the Au–N interaction.Conflicts of interest
There are no conflicts to declare.References
- K. A. Hofmann and F. Z. Küspert, Verbindungen von Kohlenwasserstoffen mit Metallsalzen, Z. Anorg. Allg. Chem., 1897, 15, 204 CrossRef.
- T. Kitazawa, Y. Gomi, M. Takahashi, M. Takeda, M. Enomoto, A. Miyazaki and T. Enoki, Spin-crossover behaviour of the coordination polymer FeII(C5H5N)2NiII(CN)2, J. Mater. Chem., 1996, 6, 119 RSC.
- V. M. Hiiuk, S. Shova, A. Rotaru, A. A. Golub, I. O. Fritsky and I. A. Gural'skiy, Spin crossover in 2D iron(II) phthalazine cyanometallic complexes, Dalton Trans., 2020, 49, 5302–5311 RSC.
- L. Piñeiro-López, M. Seredyuk, M. C. Muñoz and J. A. Real, Effect of Guest Molecules on Spin Transition Temperature in Loaded Hofmann-Like Clathrates with Improved Porosity, Eur. J. Inorg. Chem., 2020, 764–769 CrossRef.
- R. Li, G. Levchenko, F. J. Valverde-Muñoz, A. B. Gaspar, V. V. Ivashko, Q. Li, B. Liu, M. Yuan, H. Fylymonov and J. A. Real, Pressure Tunable Electronic Bistability in Fe(II) Hofmann-like Two-Dimensional Coordination Polymer [Fe(Fpz)2Pt(CN)4]: A Comprehensive Experimental and Theoretical Study, Inorg. Chem., 2021, 60, 16016–16028 CrossRef CAS PubMed.
- F. J. Valverde-Muñoz, R. Kazan, K. Boukheddaden, M. Ohba, J. A. Real and T. Delgado, Downsizing of Nanocrystals While Retaining Bistable Spin Crossover Properties in Three-Dimensional Hofmann-Type {Fe(pz)[Pt(CN)4]}− Iodine Adducts, Inorg. Chem., 2021, 60, 8851–8860 CrossRef PubMed.
- I. S. Kuzevanova, O. I. Kucheriv, V. M. Hiiuk, D. D. Naumova, S. Shova, S. I. Shylin, V. Kotsyubynsky, A. Rotaru, I. O. Fritsky and I. A. Gural'skiy, Spin crossover in iron(II) Hofmann clathrates analogues with 1,2,3-triazole, Dalton Trans., 2021, 50, 9250–9258 RSC.
- D. J. Mondal, A. Mondal, A. Paul and S. Konar, Guest-Induced Multistep-to-One-Step Reversible Spin Transition with Enhanced Hysteresis in a 2D Hofmann Framework, Inorg. Chem., 2022, 61, 4572–4580 CrossRef CAS PubMed.
- T. Kosone, R. Kosuge, M. Tanaka, T. Kawasaki and N. Adachi, New family of Hofmann-like coordination polymers constructed with imidazole ligands and associated with spin crossover and anisotropic thermal expansions, New J. Chem., 2022, 46, 10540–10544 RSC.
- V. Niel, J. M. Martínez-Agudo, M. C. Muñoz, A. B. Gaspar and J. A. Real, Cooperative Spin Crossover Behavior in Cyanide-Bridged Fe(II)−M(II) Bimetallic 3D Hofmann-like Networks (M = Ni, Pd, and Pt), Inorg. Chem., 2001, 40, 3838–3839 CrossRef CAS PubMed.
- Y.-Y. Peng, S.-G. Wu, Y.-C. Chen, W. Liu, G.-Z. Huang, Z.-P. Ni and M.-L. Tong, Asymmetric seven-/eight-step spin-crossover in a three-dimensional Hofmann-type metal–organic framework, Inorg. Chem. Front., 2020, 7, 1685–1690 RSC.
- K.-P. Xie, Z.-Y. Ruan, X.-X. Chen, J. Yang, S.-G. Wu, Z.-P. Ni and M.-L. Tong, Light-induced hidden multistability in a spin crossover metal–organic framework, Inorg. Chem. Front., 2022, 9, 1770–1776 RSC.
- V. M. Hiiuk, S. I. Shylin, D. D. Barakhtii, D. M. Korytko, V. O. Kotsyubynsky, A. Rotaru, S. Shova and I. A. Gural'skiy, Two-Step Spin Crossover in Hofmann-Type Coordination Polymers [Fe(2-phenylpyrazine)2{M(CN)2}2] (M = Ag, Au), Inorg. Chem., 2022, 61, 2093–2104 CrossRef CAS PubMed.
- S. Wu, S. Bala, Z. Ruan, G. Huang, Z. Ni and M. Tong, Four-step spin-crossover in an oxamide-decorated metal-organic framework, Chin. Chem. Lett., 2022, 33, 1381–1384 CrossRef CAS.
- K. Kitase and T. Kitazawa, A novel two-step Fe–Au type spin-crossover behavior in a Hofmann-type coordination complex {Fe(4-methylpyrimidine)2[Au(CN)2]2}, Dalton Trans., 2020, 49, 12210–12214 RSC.
- K. Kitase, D. Akahoshi and T. Kitazawa, Effects of both methyl and pyrimidine groups in Fe–Ag spin-crossover Hofmann-type complex {Fe(4-methylpyrimidine)2[Ag(CN)2]2}, Inorg. Chem., 2021, 60, 4717–4722 CrossRef CAS PubMed.
- T. Soma, H. Yuge and T. Iwamoto, Three-Dimensional Interpenetrating Double and Triple Framework Structures in [Cd(bpy)2{Ag(CN)2}2] and [Cd(pyrz){Ag2(CN)3}{Ag(CN)2}], Angew. Chem., Int. Ed. Engl., 1994, 33, 1665–1666 CrossRef.
- T. Soma and T. Iwamoto, Variations of multi-dimensional supramolecular structures built of the two-dimensional [Cd(py)2{Ag(CN)2}2]n network: Three-dimensional textile structures of catena-poly[trans-bis(pyridine)cadmium(II)-di-μ-{dicyanoargentato(I)-N,N′}·G (G = benzene or pyrrole) and two-dimensional layer structure of catena-poly[trans-bis(pyridine)cadmium(II)-di-μ-{dicyanoargentato(I)-N,N′}], J. Inclusion Phenom. Macrocyclic Chem., 1996, 26, 161–173 CrossRef CAS.
- S. Nishikiori, T. Soma and T. Iwamoto, In-plane and Out-of-plane Motion of Benzene Trapped in a Cd(py)2{Ag(CN)2}2 Host as Studied by Deuterium NMR, J. Inclusion Phenom. Macrocyclic Chem., 1997, 27, 233–243 CrossRef CAS.
- J. A. Rodriguez-Velamazán, M. Castro, E. Palacios, R. Burriel, T. Kitazawa; and T. Kawasaki, A Two-Step Spin Transition with a Disordered Intermediate State in a New Two-Dimensional Coordination Polymer, J. Phys. Chem. B, 2007, 111, 1256–1261 CrossRef PubMed.
- T. Kosone, C. Kachi-Terajima, C. Kanadani, T. Saito and T. Kitazawa, Isotope Effect on Spin-crossover Transition in a New Two-dimensional Coordination Polymer [FeII(C5H5N)2][AuI(CN)2]2, [FeII(C5D5N)2][AuI(CN)2]2, and [FeII(C5H515N)2][AuI(CN)2]2, Chem. Lett., 2008, 37, 754–755 CrossRef CAS.
- T. Kosone, Y. Suzuki, C. Kanadani, T. Saito and T. Kitazawa, Structural Isomers of {MnII(L)2[AgI(CN)2]2} (L = 3-Methylpyridine or 4-Methylpyridine), Bilayer Structure with Binuclear Argentophilic Interaction and Interpenetrated Structure with 1D Chain Argentophilic Interaction; Synthesis, Crystal Structure, and Magnetic Properties, Bull. Chem. Soc. Jpn., 2009, 82(3), 347–351 CrossRef CAS.
- P. Pyykkö, Chem. Rev., 1988, 88, 563–594 CrossRef.
- G. M. Sheldrick, Acta Crystallogr., Sect. C: Struct. Chem., 2015, 71, 3–8 Search PubMed.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, 64, 112–122 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2191439–2191451. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt03716e |
| ‡ Crystal data of complex 1Au; C14H12N8O2FeAu2, M = 774.17 g mol−1, CCDC reference number: 2191439 (296 K) and 2191440 (100 K). 1Ag; C14H12N8O2FeAg2, M = 595.97 g mol−1, CCDC reference number: 2191441 (296 K) and 2191442 (90 K). 1Ag·0.25G; C14H12N8O2FeAg2·0.25(C5H6N2O), M = 623.50 g mol−1, CCDC reference number: 2191443 (296 K), 2191444 (190 K), 2191445 (130 K) and 2191446 (87 K). 2Au·0.25G; C14H12N8O2FeAu2·0.25(C6H8N2O2), M = 809.202 g mol−1, CCDC reference number: 2191447 (296 K) and 2191448 (90 K). 2Ag·0.25G; C14H12N8O2FeAg2·0.25(C6H8N2O2), M = 631.00 g mol−1, CCDC reference number: 2191449 (296 K), 2191450 (150 K) and 2191451 (90 K) |
| This journal is © The Royal Society of Chemistry 2023 |