Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Influence of alkali metal cations on the formation of the heterobimetallic actinide tert-butoxides [AnM3(OtBu)7] and [AnM2(OtBu)6] (AnIV = Th, U; MI = Li, Na, K, Rb, Cs)†
Andreas
Lichtenberg
 a,
Markus
Zegke
a,
Markus
Zegke
 a,
Gary S.
Nichol
a,
Gary S.
Nichol
 b,
Aida
Raauf
b,
Aida
Raauf
 *a and
Sanjay
Mathur
*a and
Sanjay
Mathur
 *a
*a
aInstitute of Inorganic Chemistry, University of Cologne, 50939 Cologne, Germany. E-mail: aida.raauf@uni-koeln.de; sanjay.mathur@uni-koeln.de
bSchool of Chemistry, The University of Edinburgh, David Brewster Road EH9 3FJ, Edinburgh, Scotland, UK
First published on 4th January 2023
Abstract
Heterobimetallic tert-butoxides of alkali metal cations with tetravalent actinide centers exhibit two distinctive structural motifs, [AnM2(OtBu)6] and [AnM3(OtBu)7] (AnIV = Th, U and MI = Li, Na, K, Rb, Cs), evidently governed by the size of the alkali metal ions. Both [AnM3(OtBu)7] AnM3 (AnIV = U, MI = Li; AnIV = Th, MI = Li, Na) and [AnM2(OtBu)6] AnM2 (AnIV = U, MI = Na–Cs; AnIV = Th, MI = K–Cs) compounds are obtained in nearly quantitative yields by reacting actinide and alkali metal silyl amides with an excess of tert-butyl alcohol. The AnM3 complexes form a cubane-type coordination motif, whereas the AnM2 complexes display a geometry resembling two face-shared bipyramids. The sodium derivatives of thorium and uranium (ThNa3 and UNa2) allow the determination of the structural transition threshold as a function of the ratio of the ionic radii ri(AnIV)/ri(MI). The AnM3 complexes are formed for ratios above 0.92 and the AnM2 type is formed for ratios below 0.87. All compounds are unambiguously characterized in both solution and solid states by NMR and IR spectroscopic studies and single crystal X-ray diffraction analyses, respectively.
Introduction
Metal alkoxides1 have found a broad range of applications, for instance in catalysis2–4 and material synthesis.5–11 There is relatively little information on actinide alkoxides, and the published data on uranium alkoxides relate to the original contributions of Henry Gilman and Don Bradley, which were instrumental in the design of this study. In addition to new synthetic routes, this work focuses on the solid-state structural chemistry of actinide alkoxides and the flexibility of their coordination sphere to accommodate different ligands and co-ligands as well as their propensity to coordinate to other metal alkoxides to form heterobimetallic frameworks.12–23 The latter, due to renewed interest in various generation IV nuclear reactor concepts, where new real and simulated fuels will be prepared for studies of properties required for certification, could be prepared by alkoxide based sol–gel processing.24–27 In this context, the preparation of heterometallic actinide alkoxides containing metals, found as fission products in nuclear reactors (e.g. cesium), is of interest.The influence of the ionic size upon the formation of novel heterometallic actinide complexes provides new opportunities for the design and synthesis of new compounds and shows the effect upon the precise constituents, including the basic octahedral coordination environment of the actinide alkoxide. Besides the investigation of the molecular structure and the coordination chemistry of the actinides, the examination of the systematic variation of the ion size provides a fundamental understanding of the functional and specific properties of novel actinide materials.28–31 For instance, a recently reported study shows that the hydration enthalpy of heterometallic actinide compounds correlates with the ionic size of the heterocation.32
An informative overview of the formation of hetero (bi- and tri) metallic alkoxide derivatives was provided in the review of Veith and Mathur et al. including several alkoxide structure-types upon various valences of the metal centres.33 The review of Sattelberger et al. additionally provides an insight into the field of actinide alkoxide chemistry.22 However, the authors warn the reader that some of the early works on actinide alkoxides referenced in the review may not be totally reliable. Nevertheless, some more valid structures have been published afterwards.
Monovalent alkali metal alkoxides of thorium and uranium with a trinuclear framework have been reported, such as [U2K(OtBu)9]34 (U2K) or [Th2Na(OtBu)9]35 (Th2Na) and [ULi2(OtBu)6(THF)2]23 (ULi2-THF). The heterobimetallic AnIV–MI compounds (An = Th, M = Na; An = U, M = Li, K) were obtained either through a salt metathesis reaction of UCl4 and 6 eq. of Li(OtBu) in THF23 or by Lewis acid–base interactions among [An2(OtBu)8(HOtBu)] (An = Th, U) and M(OtBu) (M = Na, K).21,35 Attempts to increase (>2) the alkali metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) actinide stoichiometry to obtain the tetranuclear species AnM3 have been made; however, such compounds could not be isolated and structurally investigated.23
actinide stoichiometry to obtain the tetranuclear species AnM3 have been made; however, such compounds could not be isolated and structurally investigated.23
The influence of the ionic size of the metal cations is a key feature of the structural chemistry of metal alkoxides with a series of alkali metal tert-butoxides [MI(OtBu)]n delivering a prominent example.11,36,37 Lithium tert-butoxide (MI = Li) for instance is found to be octameric (n = 8),38 however the larger sodium forms a hexameric (n = 6) framework.39 The nuclearity is found to decrease for larger cations (K, Rb, Cs) with potassium, rubidium or cesium tert-butoxides crystallizing in a tetrameric cubane-type structure (n = 4).36 Other examples are our recently reported iron-lanthanide alkoxides, where the size of the Ln3+ cations was found to affect the Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe ratio in the molecular structure.11 Such structural transformations are mainly affected by the ionic radii of the constituting metals and the steric requirement of the applied alkoxide ligands.
Fe ratio in the molecular structure.11 Such structural transformations are mainly affected by the ionic radii of the constituting metals and the steric requirement of the applied alkoxide ligands.
In this work, we have investigated the structural diversity in a series of alkali metal actinide alkoxides based on the complexation of tert-butoxides of alkali metal cations and tetravalent uranium and thorium. The heterobimetallic actinide-alkali metal alkoxides have been obtained by a ligand exchange reaction starting from the AnIV metallacycle compound [AnN′N′′2] (AnIV = Th, U, N′ = {(CH2SiMe2)N(SiMe3)}, N′′ = {N(SiMe3)2}) and monovalent alkali metal silylamides with tert-butyl-alcohol in a nonpolar hydrocarbon solvent.
Results and discussion
Synthesis of the AnIV–MItert-butoxide compounds
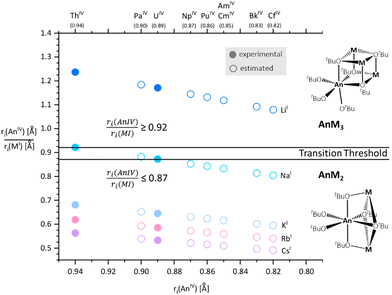
The AnIV–MItert-butoxides of the general formula [AnM3(OtBu)7] (AnM3-type: ULi3 (CCDC: 2152019), ThLi3 (CCDC: 2152017), ThNa3 (CCDC: 2152018)) and [AnM2(OtBu)6] (AnM2-type: UNa2 (CCDC: 2152025), UK2 (CCDC: 2152024), ThK2 (CCDC: 2152021), URb2 (CCDC: 2152026), ThRb2 (CCDC: 2152022), UCs2 (CCDC: 2152023), ThCs2 (CCDC: 2152020))† were synthesized through a reaction of 1 eq. of [AnN′N′′2] (AnIV = Th, U) and 2 or 3 eq. of [MN′′] (MI = Li, Na, K, Rb, Cs) with 6 or 7 eq. of HOtBu (Scheme 1).The targeted formation of the AnM3 and AnM2 structures was controlled by the ratio of the ionic radii of the AnIV and MI centers. For instance, the smallest of the alkali metal cations, lithium (0.76 Å), yielded the AnM3 structural types for both uranium (0.89 Å) and thorium (0.94 Å), whereas the formation of AnM2 compounds was favored with increasing ionic radii of potassium (1.38 Å), rubidium (1.52 Å) and cesium (1.67 Å). The sodium (1.02 Å) derivatives represent a borderline case with both structure types ThNa3 and UNa2 observed as thermodynamically favored arrangements of cations.40
Calculating the ratio of the ionic radii ri(AnIV)/ri(MI) (for C.N. 6) allowed us to define the threshold of the structural transition in the M(I)–An(IV) series of mixed-metal tert-butoxides. The AnM3 structures were typically obtained for a ri(AnIV)/ri(MI) ratio of 0.92 or higher. Herein, three smaller alkali metals can bind towards the tert-butoxide octahedron surrounding the AnIV center. The AnM2 structure is formed below a ratio of 0.87 that reasonably explains the increased space requirement for the larger alkali metal cations. The transition threshold for both structures is apparently located between a ratio of 0.87 and 0.92, which corroborates the formation of UNa2 and ThNa3, respectively.
[AnM3(OtBu)7] derivatives (AnM3)
The molecular structures of ThLi3, ULi3 and ThNa3 display the AnIV center to be in a distorted octahedral coordination environment formed by six tert-butoxide groups out of which three bind terminally to the AnIV center, while the remaining three take a triply bridging position (μ3-fashion) interconnecting the AnIV center to two of the MI cations (Fig. 1). The seventh tert-butoxide ligand coordinates only to the three MI centers in a μ3-capping mode. The structure exhibits a distorted cubane-type [AnM3O4] framework, with the four metal centers and four μ3-bridged alkoxide ligands occupying the corners of a cube that is distorted due to discrepancy in the ionic size of LiI (0.76 Å) and ThIV/UIV (0.94/0.89 Å).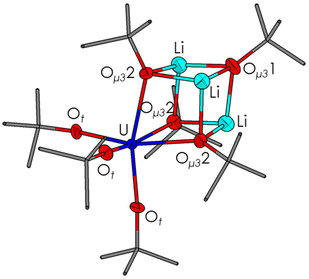 | ||
| Fig. 1 Molecular structure of [ULi3(OtBu)7] (ULi3). Thermal ellipsoids are shown at the 50% probability level and hydrogen atoms have been omitted for clarity. | ||
Selected bond lengths, angles and metal–metal distances for ULi3, ThLi3 and ThNa3 are comparable to the previously reported tert-butoxides [ULi2(OtBu)6(THF)2]23ULi2-THF, [U2Li(OtBu)9(THF)]23U2Li-THF and [Th2Na(OtBu)9]35Th2Na as listed in Table S3.† The AnIV–Ot and AnIV–Oμ3 bond lengths are in good agreement with the literature reports for AnIV (Th, U) tert-butoxides. The An–M distances in the AnM3 complexes were found to be increased (ca. 0.3 Å) when compared to the reported tetravalent actinide (Th, U) alkali metal tert-butoxides. This elongation can be attributed to the presence of the cubane-type geometry and an accompanying increased steric repulsion of the metal centers and alkoxo-ligands in the molecular structure of [AnM3(OtBu)7] (AnM3), causing an increase of the An–M distances.
Fig. 2 shows the structural changes in the actinide–oxygen–alkali metal frameworks as a function of the metal ion size within the series of [AnM3(OtBu)7] derivatives ULi3, ThLi3 and ThNa3. Increasing the ionic radius within the actinide series from ThIV to UIV only slightly affects the An–Li interatomic distance (ULi3: 3.243(7) Å, ThLi3: 3.29(2) Å), the Li–Li contact (ULi3: 2.460(9) Å; ThLi3: 2.43(2) Å) and slightly increases the An–Ot (ULi3: 2.120(3) Å; ThLi3: 2.193(6) Å) and An–Oμ32 bond lengths (ULi3: 1.945(6) Å; ThLi3: 1.99(2) Å). In contrast, a significant metal–metal contact and M–O bond length change are observed upon varying the alkali metal ions. For the Li and Na derivatives ThLi3 and ThNa3, the An–M distance (ThLi3: 3.29(2) Å; ThNa3: 3.606(3) Å), the M–M contact (ThLi3: 2.43(2) Å; ThNa3: 3.091(3) Å) and all M–O bonds were found to be elongated by ∼0.3–0.4 Å. Additionally, the tert-butoxide octahedron surrounding the AnIV centers exhibited a higher degree of distortion for the smaller alkali metal ions, indicated by the trans Ot–An–Oμ32 bond angle (ThLi3: 163.0(3)°; ThNa3: 166.6(1)°).
The room temperature 1H and 7Li NMR spectra of the actinide-lithium derivatives ULi3 and ThLi3 recorded in benzene-d6 and toluene-d8 showed a high complexity, with regard to the assignment of the signals to ligands observed in the solid-state structures. The 1H NMR spectra showed 5 peaks in the range of −2.26 to 4.18 ppm for ULi3 and 1.26 to 1.56 ppm for ThLi3, while the 7Li NMR spectra exhibited 3 resonances in the range of −7.2 to 0.88 ppm for ULi3 and 0.59 to 0.88 ppm for ThLi3. This is possibly due to the solution dynamics of the heterobimetallic compounds containing the smaller lithium cations and likely formation of different isomers in solution. The complexity of the spectra could not be reduced by low temperature measurement. A similar solution behavior based on NMR data was described by Hayton et al. for the uranium-lithium derivatives [ULi2(OtBu)6(THF)2] (ULi2-THF) and [U2Li(OtBu)9(THF)] (U2Li-THF). In order to resolve the complex nature of the NMR spectra, a series of titration experiments with a varying amount of LiOtBu were carried out.23
However, the 1H NMR spectrum for the sodium derivative ThNa3 in toluene-d8 verified the cubane-type structure with two singlets at 1.19 and 1.42 ppm and an integrative ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6. The resonance at 1.19 ppm is assigned to the μ3-tert-butoxide group bridged among three sodium atoms, whereas the resonance at 1.42 ppm includes three tert-butoxides surrounding the Th center and three tert-butoxide group bridging Th and Na centers. A 23Na NMR spectrum recorded in toluene-d8 confirmed the structural integrity of ThNa3 in solution, with one resonance observed at 5.07 ppm for the three chemically equivalent μ3-bridged sodium cations.
6. The resonance at 1.19 ppm is assigned to the μ3-tert-butoxide group bridged among three sodium atoms, whereas the resonance at 1.42 ppm includes three tert-butoxides surrounding the Th center and three tert-butoxide group bridging Th and Na centers. A 23Na NMR spectrum recorded in toluene-d8 confirmed the structural integrity of ThNa3 in solution, with one resonance observed at 5.07 ppm for the three chemically equivalent μ3-bridged sodium cations.
[AnM2(OtBu)6] derivatives (AnM2)
When compared to the previously reported [ULi2(OtBu)6(THF)]23 (ULi2-THF), the alkali metal centers in this study are not coordinated by any additional Lewis basic ligand, such as THF (Fig. 3). The use of a coordinating solvent substantially influences the structural motif of the resulting complex both in the solid state and in solution, as the formation of the above-mentioned AnLi3 structure is apparently suppressed by the coordination of one THF molecule to both LiI-centers each.The actinide centers in the series of compounds UNa2, ThK2, UK2, ThRb2, URb2, ThCs2 and UCs2 were coordinated by six tert-butoxide ligands in a distorted octahedral fashion (Fig. 3), similar to the ULi2-THF complex.23 Two of these tert-butoxide ligands are bound terminally (Ot) to the actinide center, whereas the two monovalent alkali metals are coordinated by alkoxide groups bridging to the AnIV center in bidentate (2 × Oμ2) and tridentate (2 × Oμ3) modes. Thus, the structure exhibits a framework of two face-shared [AnMO3] trigonal bipyramids, with one actinide center, one alkali metal center, one μ2-bridged and two μ3-bridged tert-butoxide ligands occupying the corners of each tetrahedron.
The AnIV–Ot bond lengths are in good agreement with the respective bonds in reported AnIV (Th, U) tert-butoxide structures.23,34,35 A comparison of the uranium–alkali metal compounds UNa2–UCs2 and the known uranium-lithium derivative ULi2-THF23 revealed that the size of the alkali metal ion affected the An–M distance that showed an increasing trend with increasing size of the cations (2.87(1) Å ULi2-THF;23 3.193(2) Å UNa2; 3.525(1) Å UK2; 3.656(3) Å URb2; 3.8754(9) Å UCs2) and M–M distance (2.89(3) Å ULi2-THF;23 3.453(3) Å UNa2; 4.043(3) Å UK2; 4.188(7) Å URb2; 4.6564(9) Å UCs2) (Fig. 4).
 | ||
| Fig. 4 The AnM2O6 skeletons of [ULi2(OtBu)6(THF)2]23 (ULi2-THF) and [AnM2(OtBu)6] (UNa2, UK2, URb2, UCs2) with the corresponding An–M and M–M distances and the Ot–An–Oμ32 (trans) angles. Thermal ellipsoids are shown at the 50% probability level and all hydrogen and carbon atoms have been omitted for clarity. | ||
Moreover, the M–O bonds were found to be elongated with increasing size of the monovalent alkali metal ion (Table S4†). A comparison of the Oμ3–An–Oμ3trans bond angles indicated a higher distortion of the AnIV octahedron for derivatives with smaller alkali metals. A higher distortion goes along with a decrease of the An–Ot bond length, since more space is provided for the terminal tert-butoxide groups, and an increase of the An–Oμ3 bond length as a consequence of a higher steric repulsion of the alkoxide ligands. In the AnM2 molecular structures, a benzene ring was found to be located near one of the alkali metals that seems to be a solvent incorporation and no π-coordination based on the distances towards the alkali metal and the fact that it is only located towards one of two available alkali metals. However, the hydrogen atoms on a methyl group of the bidentate bridged tert-butoxides of compound ThK2 show agostic interactions (K–H distances of 1.925(7) Å and K–C distances of 2.3(7) Å) with the alkali metal without the localized benzene molecule, whereas anagostic interactions (K–H distances of 2.67(8) Å and K–C distances of 3.4(3) Å) with the alkali metal localized by a benzene molecule are present (ESI-Fig. S20†).
The 1H NMR spectra of the UM2-type structures in benzene-d6 showed high complexity due to the paramagnetism of UIV. The recorded spectra showed several sharp singlets in the range of −3.01 to 17.39 ppm and one broad signal at 1.67 ppm. Attempts to use another deuterated non-polar hydrocarbon solvent were not conclusive. For instance, the 1H NMR spectrum of UNa2 in toluene-d8 merely showed two signals at −3.03 and 17.36 ppm, which are also observed in the spectrum recorded in benzene-d6; however the broad signal at 1.67 ppm was not observed. Although UNa2 revealed one resonance in the 23Na NMR spectra in toluene-d8 at 32.89 ppm, supporting the existence of the UM2-type structure with two chemically equivalent μ3-bridged NaI-centers, the 1H NMR signals and their integrative ratios were not assignable to the UM2-type structure.
However, the 1H NMR spectrum of the diamagnetic thorium derivatives ThK2, ThRb2 and ThCs2 in benzene-d6 revealed a single resonance at 1.51, 1.55 and 1.58 ppm, respectively, which were unambiguously assigned to the six tert-butoxide ligands surrounding the thorium center, namely the Ot terminal, Oμ2 μ2-bridged and Oμ3 μ3-bridged tert-butoxide ligands.
Furthermore, both structures AnM2 and AnM3 were investigated by IR spectroscopy that exhibited mainly comparable peak patterns in both cases; however, the IR spectra of AnM2 and AnM3 differentiated in one additional IR band observed at ∼670–690 and ∼450–480 cm−1, respectively (see ESI, Fig. S18 and S19†).
Conclusions
A general synthetic strategy for heterobimetallic AnIV–MItert-butoxides (AnIV = Th, U; MI = Li, Na, K, Rb, Cs) was established through simple ligand exchange of alkali metals and actinide silyl amides in tert-butyl alcohol in non-polar hydrocarbon solvents. The targeted formation of [AnM3(OtBu)7] (AnM3-type: ULi3, ThLi3, ThNa3) and [AnM2(OtBu)6] (AnM2-type: UNa2, UK2, ThK2, URb2, ThRb2, UCs2, ThCs2) was simply controlled by the variation of the AnIV and MI ionic radii and was found to be independent of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 or 1
3 or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (AnIV
2 (AnIV![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MI) stoichiometry in the reactions. The ratio of the ionic radii40ri(AnIV)/ri(MI) allowed us to estimate the threshold values, limiting the formation of structures with 1
MI) stoichiometry in the reactions. The ratio of the ionic radii40ri(AnIV)/ri(MI) allowed us to estimate the threshold values, limiting the formation of structures with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (AnM2) or 1
2 (AnM2) or 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (AnM3) ratios. A ratio of 0.92 or higher was found to favor the AnM3 framework, whereas the AnM2 structure was obtained for values below 0.87. The transition threshold for both structures is located in between the ratio of 0.87 and 0.92, which are the corresponding ratio values of UNa2 and ThNa3, respectively. The respective ratios were calculated for all tetravalent actinides against all monovalent alkali metals as depicted in Fig. 5.
3 (AnM3) ratios. A ratio of 0.92 or higher was found to favor the AnM3 framework, whereas the AnM2 structure was obtained for values below 0.87. The transition threshold for both structures is located in between the ratio of 0.87 and 0.92, which are the corresponding ratio values of UNa2 and ThNa3, respectively. The respective ratios were calculated for all tetravalent actinides against all monovalent alkali metals as depicted in Fig. 5.
These values deliver predictions if the AnM2 or AnM3 structure-type is obtained when an equivalent of actinide precursor [AnN′N′′] (AnIV = Th, Pa, U, Np, Am, Cm, Bk, Cf) is treated with 2 or 3 eq. or an excess of [MN′′] (MI = Li, Na, K, Rb, Cs) and HOtBu for the available tetravalent actinides and monovalent alkali metals.
Experimental section
All reactions were performed under inert conditions in a glovebox with an argon atmosphere and less than 0.1 ppm H2O and O2 or using standard Schlenk techniques. Chemicals were obtained from Sigma-Aldrich Chemical Co., Acros Organics, Alfa Aesar, VWR, Fischer Scientific and Strem Chemical Co. Solvents and alcohols were dried over sodium and distilled prior to use. After degassing with the freeze–pump–thaw technique, it was brought into the glovebox and stored over dried molecular sieves (3 Å). The metal silyl amides [MN′′] (MI = Li,41 Rb,42 Cs43) and the metallacycle [AnN′N′′] (AnIV = Th, U)44 were synthesized according to the literature methods. The [MN′′] (MI = Na, K) derivatives were purchased from Sigma Aldrich and purified by sublimation under reduced pressure (10−3 mbar for MI = Li, Na, K, Rb, Cs; 10−6 mbar for AnIV = Th, U) prior to use.SXRD data were obtained by mounting a suitable single crystal on a MiTiGen Microloop™ and attaching this to the goniometer head of an SC-XRD Bruker D8 Venture. The crystal was cooled to 100–120 K using an Oxford Cryostream low temperature device. The full dataset was recorded and the images were processed using APEX2. Structure solution by direct methods was achieved using SHELXS programs, and the structural model was refined by full matrix least squares on F2 using SHELX97. Molecular graphics were plotted using Diamond. Editing of CIFs and construction of tables and bond lengths and angles was achieved using PLATON and Olex2.
The NMR spectra were recorded on a Bruker Avance 300 in benzene-d6 at 298 K. Additional NMR spectra were recorded on a Bruker Avance II 300, Bruker Avance 400 or Bruker Avance III 500 spectrometer. The 1H (300.1 MHz), 1H (400.1 MHz), 1H (500.1 MHz), 7Li (116.6 MHz), 23Na (132.3 MHz) chemical shifts are reported in parts per million (ppm) relative to external tetramethylsilane and are referenced internally to the proton impurity of the solvent. For characterization of the observed signal multiplicities, the following abbreviations were used: s (singlet) as well as br (broad). The NMR spectra were analyzed with the software Bruker Topspin 4.1.1.
Infrared spectra were obtained using a Platinum ATR Spectrometer on a crystal plate with samples analyzed using OPUS software. The spectrometer is placed in an argon glovebox.
Elemental analyses were carried out on a HEKAtech CHNS Euro EA 3000. The sample preparation was performed in a glove box, whereas the reweighing of the cartridges was done outside under environmental atmosphere.
According to the German legislation, natural uranium/thorium, used in this work, is not classified as “radioactive”, but merely as a chemical element in its natural isotopic composition, since the total inventory of natural uranium and thorium in the laboratory does not exceed 100 grams and 200 grams, respectively. Hence, no additional precautions were necessary, and the uranium/thorium precursor was handled and stored taking similar precautions to those applicable to any hazardous heavy metal compound.
General procedure for [AnM3(OtBu)7] (AnM3)
A mixture of 1.0 eq. of [An{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2] and 3.0 eq. of [M{N(SiMe3)2}] in benzene was treated with 7.0 eq. of tert-butanol. The mixture was stirred for 1 d at room temperature. After slow evaporation of all volatiles at room temperature, crystals of [AnM3(OtBu)7] were obtained.Synthesis of [ULi3(OtBu)7] (ULi3)
15.0 mg (20.9 μmol) of [U{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 10.5 mg (62.7 μmol) of [Li{N(SiMe3)2}] and 10.8 mg (146.2 μmol) of tert-butanol were reacted to obtain [ULi3(OtBu)7] (ULi3) in the form of bright blue crystals in a nearly quantitative yield of 15.6 mg (96%). 1H NMR (500 MHz, 25 °C, C6D6): δ −2.22 (s), δ 1.19 (s), δ 1.28 (s), δ 3.36 (s), δ 4.17 (s). 1H NMR (500 MHz, 25 °C, C7D8): δ −2.23 (s), δ 1.21 (s), δ 1.31 (s), δ 3.41 (s), δ 4.19 (s). 7Li NMR (117 MHz, 25 °C, C6D6): δ −7.20 (s), δ −6.21 (s), δ 0.88 (s). IR (cm−1): 2966 (m), 2867 (w), 1470 (w), 1385 (w), 1357 (m), 1208 (m), 1188 (s), 972 (s), 946 (s), 839 (w), 761 (m), 582 (m), 498 (s), 480 (s). Anal. calcd ULi3O7C28H63: C 43.64, H 8.24. Found: C 44.13, H 7.97.Synthesis of [ThLi3(OtBu)7] (ThLi3)
15.0 mg (21.1 μmol) of [Th{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 10.6 mg (63.2 μmol) of [Li{N(SiMe3)2}] and 10.9 mg (147.4 μmol) of tert-butanol were reacted to obtain [ThLi3(OtBu)7] (ThLi3) in the form of colorless crystals in a nearly quantitative yield of 15.8 mg (98%). 1H NMR (500 MHz, 25 °C, C6D6): δ 1.28 (s), δ 1.33 (s), δ 1.49 (s), δ 1.54 (s), δ 1.56 (s). 1H NMR (500 MHz, 25 °C, C7D8): δ 1.26 (s), δ 1.30 (s), δ 1.46 (s), δ 1.52 (s), δ 1.53 (s). 7Li NMR (117 MHz, 25 °C, C6D6): δ 0.59 (s), δ 0.73 (s), δ 0.88 (s, Li–OC(CH3)3). IR (cm−1): 2970 (m), 2931 (m), 2865 (w), 1472 (w), 1385 (w), 1359 (m), 1209 (s), 1190 (s), 967 (s), 946 (s), 901 (m), 757 (m), 579 (m), 494 (s), 480 (s). Anal. calcd ThLi3O7C28H63: C 43.98, H 8.30. Found: C 44.89, H 8.94.Synthesis of [ThNa3(OtBu)7] (ThNa3)
15.0 mg (21.1 μmol) of [Th{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 11.6 mg (63.2 μmol) of [Na{N(SiMe3)2}] and 10.9 mg (147.4 μmol) of tert-butanol were reacted to obtain [ThNa3(OtBu)7] (ThNa3) in the form of colorless crystals in a nearly quantitative yield of 16.5 mg (96%). 1H NMR (500 MHz, 25 °C, C7D8): δ 1.29 (μ3-bridged Na3–OC(CH3)3, s, 9H), δ 1.42 (μ3-bridged U–Na2–OC(CH3)3 & terminal U–OC(CH3)3, s, 54H). 23Na NMR (132 MHz, 25 °C, C7D8): δ 5.05 (μ3-bridged Na, s, 3Na). IR (cm−1): 2967 (w), 2860 (w), 1474 (w), 1382 (w), 1353 (m), 1195 (s), 948 (s), 833 (w), 761 (m), 542 (w), 481 (s), 435 (m). Anal. calcd ThNa3O7C28H63: C 41.38, H 7.81. Found: C 42.62, H 7.59.General reaction procedure for [AnM2(OtBu)6] (AnM2)
A mixture of 1.0 eq. of [An{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2] and 2.0 eq. of [M{N(SiMe3)2}] in benzene was treated with 6.0 eq. of tert-butanol. The mixture was stirred for 1 d at room temperature. After slow evaporation of all volatiles at room temperature, crystals of [AnM3(OtBu)7] were obtained.Synthesis of [UNa2(OtBu)6] (UNa2)
15.0 mg (20.9 μmol) of [U{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 7.7 mg (41.8 μmol) of [Na{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [UNa2(OtBu)6] (UNa2) in the form of blue crystals in a nearly quantitative yield of 16.2 mg (97%). 1H NMR (300 MHz, 25 °C, C6D6): δ −3.02 (s), δ 0.10 (s), δ 1.38 (s), δ 1.67 (br), δ 17.36 (s). 1H NMR (500 MHz, 25 °C, C7D8): δ −3.04 (s), δ 17.34 (s). 23Na NMR (132 MHz, 25 °C, C7D8): δ 32.89 (μ3-bridged Na, s, 2Na). IR (cm−1): 2960 (m), 2905 (w), 2868 (w), 1472 (w), 1380 (w), 1355 (m), 1221 (m), 1192 (s), 1008 (w), 948 (s), 899 (m), 761 (m), 692 (m), 478 (s). Anal. calcd UNa2O6C30H60: C 45.00, H 7.55. Found: C 43.86, H 8.01.Synthesis of [UK2(OtBu)6] (UK2)
15.0 mg (20.9 μmol) of [U{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 8.3 mg (41.8 μmol) of [K{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [UK2(OtBu)6] (UK2) in the form of blue crystals in a nearly quantitative yield of 17.1 mg (98%). 1H NMR (300 MHz, 25 °C, C6D6): δ −1.55 (s), δ 1.68 (s), δ 2.04 (s), δ 2.56 (s), δ 3.12 (s), δ 14.42 (s). IR (cm−1): 2960 (w), 2899 (w), 2864 (w), 1482 (w), 1380 (w), 1353 (m), 1215 (m), 1193 (s), 1008 (w), 949 (s), 764 (w), 691 (m), 478 (s), 409 (w). Anal. calcd UK2O6C30H60: C 43.26, H 7.26. Found: C 43.91, H 7.02.Synthesis of [URb2(OtBu)6] (URb2)
15.0 mg (20.9 μmol) of [U{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 10.3 mg (41.8 μmol) of [Rb{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [URb2(OtBu)6] (URb2) in the form of blue crystals in a nearly quantitative yield of 19.0 mg (98%). 1H NMR (300 MHz, 25 °C, C6D6): δ −2.71 (s), δ −1.79 (s), δ 1.67 (s), δ 2.61 (s), δ 4.07 (s), δ 16.20 (s). IR (cm−1): 2953 (w), 2899 (w), 2865 (w), 1484 (w), 1460 (w), 1380 (w), 1353 (w), 1193 (s), 1010 (w), 946 (s), 760 (w), 689 (m), 476 (s), 408 (w). Anal. calcd URb2O6C30H60: C 38.92, H 6.53. Found: C 37.89, H 6.75.Synthesis of [UCs2(OtBu)6] (UCs2)
15.0 mg (20.9 μmol) of [U{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 12.2 mg (41.8 μmol) of [Cs{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [UCs2(OtBu)6] (UCs2) in the form of blue crystals in a nearly quantitative yield of 21.3 mg (96%). 1H NMR (400 MHz, 25 °C, C6D6): δ −6.48 (s), δ −5.08 (s), δ 1.18 (s), 2.11 (s), 5.51 (s), 5.91 (s). IR (cm−1): 2966 (m), 2930 (w), 2907 (w), 2874 (w), 1465 (w), 1383 (w), 1359 (m), 1224 (m), 1184 (s), 980 (m), 945 (s), 900 (s), 837 (w), 766 (m), 692 (w), 494 (s), 475 (s). Anal. calcd UCs2O6C33H63: C 37.40, H 5.99. Found: C 36.87, H 6.32.Synthesis of [ThK2(OtBu)6] (ThK2)
15.0 mg (21.1 μmol) of [Th{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 8.4 mg (42.2 μmol) of [K{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [ThK2(OtBu)6] (ThK2) in the form of colorless crystals in a nearly quantitative yield of 16.9 mg (97%). 1H NMR (300 MHz, 25 °C, C6D6): δ 1.51 (μ3-bridged OC(CH3)3 & μ2-bridged OC(CH3)3 & terminal U-OC(CH3)3, s, 54H). IR (cm−1): 2960 (w), 2899 (w), 2861 (w), 1484 (w), 1457 (w), 1379 (w), 1353 (m), 1196 (s), 1013 (w), 954 (s), 755 (w), 691 (m), 478 (s). Anal. calcd ThK2O6C30H60: C 43.57, H 7.31. Found: C 44.74, H 6.67.Synthesis of [ThRb2(OtBu)6] (ThRb2)
15.0 mg (21.1 μmol) of [Th{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 10.4 mg (42.2 μmol) of [Rb{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [ThRb2(OtBu)6] (ThRb2) in the form of colorless crystals in a nearly quantitative yield of 18.4 mg (95%). 1H NMR (300 MHz, 25 °C, C6D6): δ 1.55 (μ3-bridged OC(CH3)3 & μ2-bridged OC(CH3)3 & terminal U–OC(CH3)3, s, 54H). IR (cm−1): 2953 (s), 2895 (w), 2864 (w), 1484 (w), 1460 (w), 1380 (w), 1353 (m), 1195 (s), 1011 (w), 953 (s), 755 (w), 687 (m), 476 (s). Anal. calcd ThRb2O6C30H60: C 39.18, H 6.58. Found: C 38.73, H 6.34.Synthesis of [ThCs2(OtBu)6] (ThCs2)
15.0 mg (21.1 μmol) of [Th{(CH2SiMe2)N(SiMe3)2}{N(SiMe3)2}2], 12.4 mg (42.2 μmol) of [Cs{N(SiMe3)2}] and 9.3 mg (125.3 μmol) of tert-butanol were reacted to obtain [ThCs2(OtBu)6] (ThCs2) in the form of colorless crystals in a nearly quantitative yield of 21.0 mg (98%). 1H NMR (300 MHz, 25 °C, C6D6): δ 1.58 (μ3-bridged OC(CH3)3 & μ2-bridged OC(CH3)3 & terminal U–OC(CH3)3, s, 54H). IR (cm−1): 2966 (m), 2933 (w), 2871 (w), 1470 (w), 1382 (w), 1357 (m), 1191 (s), 952 (s), 934 (s), 839 (m), 790 (m), 767 (m), 689 (w), 585 (w), 480 (s). Anal. calcd ThCs2O6C30H60: C 35.51, H 5.96. Found: C 34.98, H 5.71.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors cordially thank Dr Carsten Lenczyk and Dr Tobias Stürzer, both Bruker AXS Karlsruhe, for additional crystallographic advice and support, providing us with preliminary models for the modulated dataset URb2, the inversion twin dataset of ThNa3 and the merohedral twin dataset of ThCs2. We also thank Mr Dirk Pullem, University of Cologne, for CHNS measurements.References
- D. C. Bradley, Metal alkoxides and dialkylamides, in Advances in inorganic chemistry and radiochemistry, Elsevier, 1972, vol. 15, pp. 259–322 Search PubMed.
- S. M. Guillaume, Recent advances in ring-opening polymerization strategies toward α, ω-hydroxy telechelic polyesters and resulting copolymers, Eur. Polym. J., 2013, 49(4), 768–779 CrossRef CAS.
- M. Hatano and K. Ishihara, Lanthanum(III) catalysts for highly efficient and chemoselective transesterification, Chem. Commun., 2013, 49(20), 1983–1997 RSC.
- A. Tsubouchi, D. Muramatsu and T. Takeda, Copper(I)–Catalyzed Alkylation of Aryl–and Alkenylsilanes Activated by Intramolecular Coordination of an Alkoxide, Angew. Chem., Int. Ed., 2013, 52(48), 12719–12722 CrossRef CAS PubMed.
- T. D. Manning, Y. F. Loo, A. C. Jones, H. C. Aspinall, P. R. Chalker, J. F. Bickley, L. M. Smith and G. W. Critchlow, Deposition of LaAlO3 films by liquid injection MOCVD using a new [La–Al] single source alkoxide precursor, J. Mater. Chem., 2005, 15(33), 3384–3387 RSC.
- S. Mathur, M. Veith, V. Sivakov, H. Shen, V. Huch, U. Hartmann and H. B. Gao, Phase–Selective Deposition and Microstructure Control in Iron Oxide Films Obtained by Single–Source CVD, Chem. Vap. Deposition, 2002, 8(6), 277–283 CrossRef CAS.
- A. Jamil, J. Schlafer, Y. Gonullu, A. Lepcha and S. Mathur, Precursor-Derived Rare Earth Metal Pyrochlores: Nd2Sn2O7 Nanofibers and Thin Films As Efficient Photoabsorbers, Cryst. Growth Des., 2016, 16(9), 5260–5267 CrossRef CAS.
- S. Mathur, S. Barth, U. Werner, F. Hernandez-Ramirez and A. Romano-Rodriguez, Chemical Vapor Growth of One–dimensional Magnetite Nanostructures, Adv. Mater., 2008, 20(8), 1550–1554 CrossRef CAS.
- S. Mathur, T. Ruegamer and I. Grobelsek, Phase–Selective CVD of Vanadium Oxide Nanostructures, Chem. Vap. Deposition, 2007, 13(1), 42–47 CrossRef CAS.
- A. Raauf, J. Leduc, M. Frank, D. Stadler, D. Graf, M. Wilhelm, M. Grosch and S. Mathur, Magnetic Field-Assisted Chemical Vapor Deposition of UO2 Thin Films, Inorg. Chem., 2021, 60(3), 1915–1921 CrossRef CAS PubMed.
- A. Raauf, J. Schläfer, I. Gessner, A. Lichtenberg, M. Zegke, T. Fischer and S. Mathur, Homo-and heteroleptic lanthanide-iron alkoxides as precursors in materials synthesis, J. Indian Chem. Soc., 2022, 100347 CrossRef CAS.
- D. Bradley, A. K. Chatterjee and A. K. Chatterjee, Sexavalent compounds of uranium—I: Uranyl alkoxides and uranium hexa-alkoxides, J. Inorg. Nucl. Chem., 1959, 12(1–2), 71–78 CrossRef CAS.
- D. Bradley and H. Holloway, Metal oxide alkoxide polymers: part V. The hydrolysis of some alkoxides of tin(IV), CERIUM(IV), AND URANIUM(V), Can. J. Chem., 1962, 40(6), 1176–1182 CrossRef CAS.
- D. Bradley, R. Kapoor and B. Smith, 30. Organosiloxy-derivatives of metals. Part II. Trialkylsilyloxides of quinquevalent and sexivalent uranium, J. Chem. Soc., 1963, 204–207 RSC.
- D. Bradley, R. Kapoor and B. Smith, Alkoxides of uranium(IV), J. Inorg. Nucl. Chem., 1962, 24(7), 863–867 CrossRef.
- R. Jones, G. Karmas, G. Martin Jr. and H. Gilman, Organic compounds of Uranium. II. Uranium(IV) amides, alkoxides and mercaptides, J. Am. Chem. Soc., 1956, 78(17), 4285–4286 CrossRef CAS.
- R. Jones, E. Bindschadler, D. Blume, G. Karmas, G. Martin Jr., J. Thirtle and H. Gilman, Organic Compounds of Uranium. V. Derivatives of Uranium(V) Alkoxides, J. Am. Chem. Soc., 1956, 78(23), 6027–6030 CrossRef CAS.
- R. Jones, E. Bindschadler, G. Karmas, G. Martin Jr., J. Thirtle, F. Yoeman and H. Gilman, Organic compounds of uranium. IV. Uranium(V) alkoxides, J. Am. Chem. Soc., 1956, 78(17), 4289–4290 CrossRef CAS.
- R. Jones, E. Bindschadler, D. Blume, G. Karmas, G. Martin Jr., J. Thirtle, F. Yeoman and H. Gilman, Organic compounds of uranium. VI. Uranium(VI) alkoxides, J. Am. Chem. Soc., 1956, 78(23), 6030–6032 CrossRef CAS.
- R. Jones, E. Bindschadler, G. Martin Jr., J. Thirtle and H. Gilman, Organic Compounds of Uranium. VII. Uranyl Alkoxides and Dithiocarbamates, J. Am. Chem. Soc., 1957, 79(18), 4921–4922 CrossRef CAS.
- W. G. Van der Sluys, A. P. Sattelberger and M. W. McElfresh, Uranium alkoxide chemistry V. Synthesis, characterization and interconversion of uranium(IV) tert-butoxide complexes, Polyhedron, 1990, 9(15–16), 1843–1848 CrossRef CAS.
- W. Van der Sluys and A. Sattelberger, Actinide alkoxide chemistry, Chem. Rev., 1990, 90(6), 1027–1040 CrossRef CAS.
- S. Fortier, G. Wu and T. W. Hayton, Synthesis and Characterization of Three Homoleptic Alkoxides of Uranium:[Li (THF)] 2 [UIV (O t Bu) 6],[Li (Et2O)][UV (O t Bu) 6], and UVI (O t Bu) 6, Inorg. Chem., 2008, 47(11), 4752–4761 CrossRef CAS PubMed.
- T. Monde, H. Kozuka and S. Sakka, Superconducting oxide thin films prepared by sol–gel technique using metal alkoxides, Chem. Lett., 1988, 17(2), 287–290 CrossRef.
- J. Livage and C. Sanchez, Sol-gel chemistry, J. Non-Cryst. Solids, 1992, 145, 11–19 CrossRef CAS.
- K. Murty and I. Charit, Structural materials for Gen-IV nuclear reactors: Challenges and opportunities, J. Nucl. Mater., 2008, 383(1–2), 189–195 CrossRef CAS.
- P. Makowski, X. Deschanels, A. Grandjean, D. Meyer, G. Toquer and F. Goettmann, Mesoporous materials in the field of nuclear industry: applications and perspectives, New J. Chem., 2012, 36(3), 531–541 RSC.
- G. L. Murphy, E. M. Langer, O. Walter, Y. Wang, S. Wang and E. V. Alekseev, Insights into the structural chemistry of anhydrous and hydrous hexavalent uranium and neptunium dinitrato, trinitrato, and tetranitrato complexes, Inorg. Chem., 2020, 59(10), 7204–7215 CrossRef CAS PubMed.
- G. Murphy, B. J. Kennedy, B. Johannessen, J. A. Kimpton, M. Avdeev, C. S. Griffith, G. J. Thorogood and Z. Zhang, Structural studies of the rhombohedral and orthorhombic monouranates: CaUO4, α-SrUO4, β-SrUO4 and BaUO4, J. Solid State Chem., 2016, 237, 86–92 CrossRef CAS.
- G. L. Murphy, P. Kegler, Y. Zhang, Z. Zhang, E. V. Alekseev, M. D. de Jonge and B. J. Kennedy, High-pressure synthesis, structural, and spectroscopic studies of the Ni–U–O system, Inorg. Chem., 2018, 57(21), 13847–13858 CrossRef CAS PubMed.
- G. L. Murphy, C.-H. Wang, Z. Zhang, P. M. Kowalski, G. Beridze, M. Avdeev, O. Muransky, H. E. Brand, Q.-F. Gu and B. J. Kennedy, Controlling oxygen defect formation and its effect on reversible symmetry lowering and disorder-to-order phase transformations in nonstoichiometric ternary uranium oxides, Inorg. Chem., 2019, 58(9), 6143–6154 CrossRef CAS PubMed.
- G. B. Jin, J. Lin, S. L. Estes, S. Skanthakumar and L. Soderholm, Influence of countercation hydration enthalpies on the formation of molecular complexes: a Thorium–nitrate example, J. Am. Chem. Soc., 2017, 139(49), 18003–18008 CrossRef CAS PubMed.
- M. Veith, S. Mathur and C. Mathur, New perspectives in the tailoring of hetero (bi-and tri-) metallic alkoxide derivatives, Polyhedron, 1998, 17(5–6), 1005–1034 CrossRef CAS.
- F. A. Cotton, D. O. Marler and W. Schwotzer, Dinuclear uranium alkoxides. Preparation and structures of KU2 (OCMe3) 9, U2 (OCMe3) 9, and U2 (OCHMe2) 10, containing [uranium(IV), uranium(IV)],[uranium(IV), uranium(V)], and [uranium(V), uranium(V)], respectively, Inorg. Chem., 1984, 23(25), 4211–4215 CrossRef CAS.
- D. L. Clark and J. G. Watkin, Synthesis and characterization of thorium tert-butoxide complexes: X-ray crystal structures of Th (O-tert-Bu) 4 (py) 2 and NaTh2 (O-tert-Bu) 9, Inorg. Chem., 1993, 32(9), 1766–1772 CrossRef CAS.
- E. Weiss, H. Alsdorf, H. Kühr and H.-F. Grützmacher, Röntgenographische, NMR-und massenspektrometrische Untersuchungen der tert.-Butylate des Kaliums, Rubidiums und Caesiums, Chem. Ber., 1968, 101(11), 3777–3786 CrossRef CAS.
- A. Jamil, J. Schläfer, Y. Gönüllü, A. Lepcha and S. Mathur, Precursor-derived rare earth metal pyrochlores: Nd2Sn2O7 nanofibers and thin films as efficient photoabsorbers, Cryst. Growth Des., 2016, 16(9), 5260–5267 CrossRef CAS.
- J. F. Allan, R. Nassar, E. Specht, A. Beatty, N. Calin and K. W. Henderson, Characterization of a kinetically stable, highly ordered, octameric form of lithium tert-butoxide and its implications regarding aggregate formation, J. Am. Chem. Soc., 2004, 126(2), 484–485 CrossRef CAS PubMed.
- H. Nekola, F. Olbrich and U. Behrens, Kristall–und Molekülstrukturen von Lithium–und Natrium–tert–butoxid, Z. Anorg. Allg. Chem., 2002, 628(9–10), 2067–2070 CrossRef CAS.
- R. D. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A: Cryst. Phys., Diffr., Theor. Gen. Crystallogr., 1976, 32(5), 751–767 CrossRef.
- R. Shaw, D. Skovlin, B. Smith, J. Rosenthal and W. Jolly, Lithium Bis(trimethylsilyl)amide and Tris(trimethylsilyl)amine, 2007, pp. 19–22 Search PubMed.
- S. Krieck, P. Schüler, H. Görls and M. Westerhausen, Straightforward synthesis of rubidium bis (trimethylsilyl) amide and complexes of the alkali metal bis (trimethylsilyl) amides with weakly coordinating 2, 2, 5, 5-tetramethyltetrahydrofuran, Dalton Trans., 2018, 47(36), 12562–12569 RSC.
- A. I. Ojeda-Amador, A. J. Martínez-Martínez, A. R. Kennedy and C. T. O'Hara, Structural studies of cesium, lithium/cesium, and sodium/cesium bis (trimethylsilyl) amide (HMDS) complexes, Inorg. Chem., 2016, 55(11), 5719–5728 CrossRef CAS PubMed.
- A. Dormond, A. El Bouadili, A. Aaliti and C. Moise, Insertion of carbonyl compounds into actinide—carbon σ bonds: Reactivity of [(Me3Si)2)2N]2M·Ch2Si (Me) 2NSiMe3, J. Organomet. Chem., 1985, 288(1), C1–C5 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2152017–2152026. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d2dt01316a |
| This journal is © The Royal Society of Chemistry 2023 |