 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Chemical space: limits, evolution and modelling of an object bigger than our universal library
Guillermo
Restrepo
*
Max Planck Institute for Mathematics in the Sciences, Inselstraße 22, 04103 Leipzig, Germany. E-mail: restrepo@mis.mpg.de; Fax: +49 341 9959 658; Tel: +49 341 9959 601
First published on 18th January 2023
Abstract
Correction for ‘Chemical space: limits, evolution and modelling of an object bigger than our universal library’ by Guillermo Restrepo et al., Digital Discovery, 2022, 1, 568–585, https://doi.org/10.1039/D2DD00030J.
The originally published version of this article included errors, the corrections for which are summarised below.
The first paragraph of Section 2.1 should read:
“It has been estimated that the number of particles in the universe is about 1080,28–30** which amounts to 7 × 1076 atoms.†† A first approach to estimating the possible number of substances is determining the theoretical number of collections of atoms held together by chemical bonds. The number of such possible atomic ensembles is given by  , where
, where 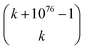 is the number of ways of selecting k atoms from a collection of 1076 atoms, such that order is not important and repetitions are allowed.32 So, here we are counting mono-, di, tri-, …, n-atomic ensembles up to the ultimate largest compound made of all 1076 atoms in the universe.‡‡ As usual in chemistry, we do not require the simultaneous “existence” of those substances, but the mere theoretical possibility of their existence and, importantly, of recording it.§§”
is the number of ways of selecting k atoms from a collection of 1076 atoms, such that order is not important and repetitions are allowed.32 So, here we are counting mono-, di, tri-, …, n-atomic ensembles up to the ultimate largest compound made of all 1076 atoms in the universe.‡‡ As usual in chemistry, we do not require the simultaneous “existence” of those substances, but the mere theoretical possibility of their existence and, importantly, of recording it.§§”
Footnote ‡‡ should read:
“This material upper bound requires further adjustments to touch physical and, above all, chemical reality. It requires taking some few atoms out of the 1076 to account for the synthesiser of the largest compound, which may be either a human or a robot. Besides the constraints discussed in note ‖, energetic conditions constitute the key factors determining whether an atomic ensemble is chemically feasible or not. This requires determining the suitable conditions of pressure and temperature holding together the given atoms by electrostatic interactions. Although the chemical space has been traditionally regarded at ambient conditions (see Section 4), there is uncharted land at extreme conditions.33”
In footnote ‖, the sentence beginning “An account of the 19th-century...” should read:
“An account of the 19th-century evolution of similarities among chemical elements is found in ref. 27.”
In the PDF version of footnote ‡‡‡‡‡, two expressions have been typeset incorrectly. The relevant sentences should read:
“The number of disjoint parts of subsets for sets of size k is given by 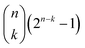 . Finally, the total number of disjoint pairs of subsets for any size k ≤ n is given by
. Finally, the total number of disjoint pairs of subsets for any size k ≤ n is given by  .”
.”
In the caption for Fig. 3 and the first sentence of Section 5.1, the expression for st should read st = 51.85e0.04324(t−1800).
In Section 5.1, the expression for St should read St = 1310.29e0.04305(t−1800), and the equality should read 10200 = e0.04305(t−1800).
In footnote ¶¶¶¶¶¶, the sentence beginning “In the directed hypergraph…” should read:
“In the directed hypergraph model, the density of the chemical network by year t (dt), consisting of st substances and rt reactions, is given by rt/st, where rt corresponds to the amount of directed hyperedges.”
In the first paragraph of Section 6, the expression for ![[capital script C]](https://www.rsc.org/images/entities/char_e522.gif) should read
should read  .
.
In Section 6, the sentence beginning “The charm of chemistry…” should read:
“The charm of chemistry is finding the function f(t) mapping ![[Doublestruck C]](https://www.rsc.org/images/entities/char_e166.gif) to
to ![[Doublestruck I]](https://www.rsc.org/images/entities/char_e16c.gif) (t).”
(t).”
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2023 |
