High-temperature stabilized defect pyrochlore Bi2−xFexWO6 nanostructures and their effects on photocatalytic water remediation and photo-electrochemical oxygen evolution kinetics†
Abstract
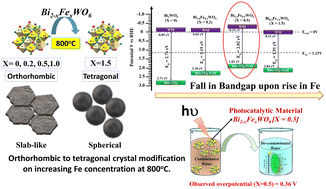
Fe3+ ion-substituted Bi2WO6 nanostructures have been fabricated by a facile co-precipitation technique with Fe concentrations varying from X = 0, 0.2, 0.5, 1.0, and 1.5. X-Ray diffraction analysis revealed the orthorhombic to tetragonal crystal structure transformation for the Fe-rich composition also known as the high-temperature phase. Optical property analysis by the UV-DRS technique showcased a reduction in the band gap upon Fe substitution, ideally due to an altered crystal field due to the hybridization of the Fe 3d-orbital and O 2p orbital, enhancing the efficacy of charge transfer. Band edge analysis through UPS disclosed that the valance band edge is closer to the Fermi level for X = 0.5 nominal compositions. The respective photocatalytic degradation of rhodamine B and tetracycline hydrochloride showed an increase of 11 times and 5.5 times the rate constant of degradation for Bi1.5Fe0.5WO6 (X = 0.5) as compared to Bi2WO6 (X = 0). Photoelectrochemical analysis for the oxygen evolution reaction (OER) was performed in the applied potential range of 0–1.5 V vs. Ag/AgCl. In comparison with different Fe compositions, Bi1.5Fe0.5WO6 (X = 0.5) exhibited a minimal onset potential for the OER with η = 0.36 V, and a 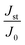 ratio of 5.28, proving an enhanced rate of hole transfer. The Tafel plot for the OER under illumination revealed a slope of 49.6 mV dec−1, implying a higher reaction rate. The improved photocatalytic and photo-electrochemical kinetics are accredited to the optimal Fe concentration in the BWO frame network leading to a hybridized band-edge position assisting in restricting the recombination of the excitons.
ratio of 5.28, proving an enhanced rate of hole transfer. The Tafel plot for the OER under illumination revealed a slope of 49.6 mV dec−1, implying a higher reaction rate. The improved photocatalytic and photo-electrochemical kinetics are accredited to the optimal Fe concentration in the BWO frame network leading to a hybridized band-edge position assisting in restricting the recombination of the excitons.


 Please wait while we load your content...
Please wait while we load your content...