Large changes in hydricity as a function of charge and not metal in (PNP)M–H (de)hydrogenation catalysts that undergo metal–ligand cooperativity†
Abstract
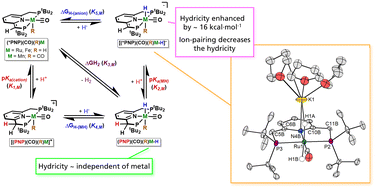
Pincer-ligated catalysts that can undergo metal–ligand cooperativity (MLC), whereby H2 is heterolytically cleaved (with proton transfer to the ligand and hydride transfer to the metal), have emerged as potent catalysts for the hydrogenation of CO2 and organic carbonyls. Despite the plethora of systems developed that differ in metal/ligand identity, no studies establish how variation of the metal impacts the pertinent thermochemical properties of the catalyst, namely the equilibrium with H2, the hydricity of the resulting hydride, and the acidity of the ligand. These parameters can impact the kinetics, scope, and mechanism of catalysis and hence should be established. Herein, we describe how changing the metal (Co, Fe, Mn, Ru) and charge (neutral vs. anionic) impacts these parameters in a series of PNP-ligated catalysts (PNP = 2,6-bis[(di-tert-butylphosphino)methyl]pyridine). A linear correlation between hydricity and ligand pKa (when bound to the metal) is found, indicating that the two parameters are not independent of one another. This trend holds across four metals, two charges, and two different types of ligand (amine/amide and aromatization/de-aromatization). Moreover, the effect of ligand deprotonation on the hydricity of (PNP)(CO)(H)Fe–H and (PNP)(CO)(H)Ru–H is assessed. It is determined that deprotonation to give anionic hydride species enhances the hydricity by ∼16.5 kcal mol−1 across three metals. Taken together, this work suggests that the metal identity has little effect on the thermodynamic parameters for PNP-ligated systems that undergo MLC via (de)aromatization, whilst the effect of charge is significant; moreover, ion-pairing allows for further tuning of the hydricity values. The ramifications of these findings for catalysis are discussed.
- This article is part of the themed collection: Emerging Investigator Series


 Please wait while we load your content...
Please wait while we load your content...
