Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Current trends in the detection and removal of heavy metal ions using functional materials
Meng
Li
 *a,
Quanyu
Shi
a,
Ningxin
Song
a,
Yumeng
Xiao
a,
Lidong
Wang
*a,
Quanyu
Shi
a,
Ningxin
Song
a,
Yumeng
Xiao
a,
Lidong
Wang
 a,
Zhijun
Chen
a,
Zhijun
Chen
 *b and
Tony D.
James
*b and
Tony D.
James
 *cd
*cd
aHebei Key Lab of Power Plant Flue Gas Multi-Pollutants Control, Department of Environmental Science and Engineering, North China Electric Power University, Baoding, 071003, P. R. China. E-mail: mlincepu@hotmail.com
bKey Laboratory of Bio-based Material Science and Technology of Ministry of Education, Material Science and Engineering College, Northeast Forestry University, Hexing Road 26, Harbin 150040, P. R. China. E-mail: chenzhijun@nefu.edu.cn
cDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
dSchool of Chemistry and Chemical Engineering, Henan Normal University, Xinxiang 453007, P. R. China
First published on 2nd August 2023
Abstract
The shortage of freshwater resources caused by heavy metal pollution is an acute global issue, which has a great impact on environmental protection and human health. Therefore, the exploitation of new strategies for designing and synthesizing green, efficient, and economical materials for the detection and removal of heavy metal ions is crucial. Among the various methods for the detection and removal of heavy ions, advanced functional systems including nanomaterials, polymers, porous materials, and biomaterials have attracted considerable attention over the past several years due to their capabilities of real-time detection, excellent removal efficiency, anti-interference, quick response, high selectivity, and low limit of detection. In this tutorial review, we review the general design principles underlying the aforementioned functional materials, and in particular highlight the fundamental mechanisms and specific examples of detecting and removing heavy metal ions. Additionally, the methods which enhance water purification quality using these functional materials have been reviewed, also current challenges and opportunities in this exciting field have been highlighted, including the fabrication, subsequent treatment, and potential future applications of such functional materials. We envision that this tutorial review will provide invaluable guidance for the design of functional materials tailored towards the detection and removal of heavy metals, thereby expediting the development of high-performance materials and fostering the development of more efficient approaches to water pollution remediation.
Key learning pointsA. Introduction to the hazards of heavy metal ion pollution and the necessity of water treatment.B. Advantages and disadvantages of different materials for the detection and removal of heavy metal ions. C. Applications of functional materials for wastewater treatment. D. Current challenges and future developments. |
1. Introduction
With the advancement of industrialization, global demand for fresh water globally is expected to increase by 55% between 2000 and 2050. However, the contamination of water by heavy metal ions further exacerbates the current situation.1–3 For instance, mercury ions, a commonly occurring bioaccumulative heavy metal ion pollutant, pose a significant threat to human health and environmental ecology. Mercury is predominantly generated by various anthropogenic activities such as coal combustion, battery production, and waste incineration. Most of the harmful Hg2+ ions are distributed in aqueous solutions, and their excessive presence in fish and water leads to severe health problems, as demonstrated by the Minamata disease in Japan. Mercury ions significantly influence human mental and neurological functions by coordinating with thiol groups present in proteins,4,5 while the interaction of copper ions with proteins and enzymes in the body can lead to gastrointestinal problems, osteoporosis, and various diseases including Alzheimer's disease.6,7 Similarly, exposure to lead ions is linked with cardiovascular effects, increasing the blood pressure and raising hypertension rates of adults.8,9 Therefore, the development of real-time and highly efficient sensors capable of detecting and removing metal ions is of great importance. Organic molecular probes,10 biomolecules,11 inorganic materials,12 and a range of optical artificial systems have been used to construct functional materials for monitoring and removing heavy metal ions. These functional materials exhibit rapid recognition of heavy metal ions due to the mechanisms of photo-induced electron transfer (PeT), aggregation-induced emission (AIE), intramolecular charge transfer (ICT), Förster resonance energy transfer (FRET), inner filter effect (IFE), chelation-enhanced fluorescence (CHEF), and chelation quenched fluorescence (CHQF). Meanwhile, the porous structure and surface functional groups of these substrate materials play a critical role in the removal of heavy metal ions, leading to an excellent uptake ability for heavy metal ions. The specific mechanisms underlying detection and removal will be reviewed in the subsequent sections. Up to now, various techniques, including electrochemical analysis,13,14 inductively coupled plasma mass spectrometry (ICP-MS),15,16 atomic absorption spectroscopy,17,18 and optical detection, have been explored for the monitoring of heavy metal ions.10,19 Among the developed detection technologies, mass spectrometry (ICP-MS) and atomic absorption spectroscopy are considered as the main techniques for detecting heavy metal ions. However, these methods suffer from drawbacks such as high cost, complexity, and long analysis time, rendering them unsuitable for real-time online monitoring of heavy metal ions. Fluorescence detection methods, on the other hand, have emerged as a prominent approach for pollutant detection due to their ease of operation, excellent responsiveness, in situ monitoring capabilities, and high sensitivity.20,21In fact, traditional materials suffer from high cost, poor stability, low sensitivity, long response time, and low removal efficiency. Therefore, it is imperative to develop novel materials and strategies with outstanding performance to overcome these challenges. Functional materials that integrate detection and removal capabilities offer significant advantages in the identification and elimination of heavy metal ions in water. Most conventional materials exhibit limited functionality in wastewater treatment, as they can only achieve singular detection or adsorption of heavy metal ions, thereby restricting their widespread application. The use of functional materials for the simultaneous detection and removal of pollutants in water not only reduces treatment costs but also improves material performance. When the adsorbent contains a probe, it can enhance the mechanical properties of the adsorbent and stabilize the probe, thereby improving water treatment efficiency. So far, significant research has been devoted to establishing various functional materials, including nanomaterials,22,23 polymer materials,24,25 porous materials,26–28 and biomaterials,29,30 for the treatment of heavy metal ions with low detection limits, high sensitivity, high selectivity, and large adsorption capacity. Each functional material possesses unique advantages and holds great potential in addressing the issue of heavy metal ion pollution in practical applications. Firstly, nanomaterials such as metal nanoprobes and photoluminescent carbon dots (CDs) have attracted attention in functional system development due to their unique electronic, magnetic, and optical properties.31,32 Nanomaterials provide new strategies for constructing functional materials that can accurately, conveniently, and rapidly detect and remove heavy metal ions from water. Secondly, polymer materials possess abundant reactive groups and favorable structures, making them the preferred choice for constructing high performance chemical sensors. Undoubtedly, polymer probes exhibit outstanding recoverability, low cost, and advantages in green detection (the probe is linked with the polymer so it can be recycled and does not cause secondary contamination) when applied in real samples.33,34 In addition, mesoporous silica, metal–organic frameworks (MOFs), and covalent organic frameworks (COFs), among other porous materials, have emerged as popular systems for the detection and removal of heavy metal ions due to their significant advantages, including extended π-conjugated frameworks, large surface areas, tunable functionality, and inherent porous structures.35–38 Apart from the aforementioned three types of functional materials, biocompatible biomaterials have gained significant attention due to their multifunctionality and compatibility with living systems. Materials such as proteins and peptides exhibit unique advantages in constructing biosensors and medical devices.39,40
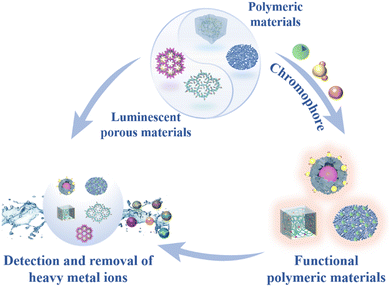
Currently, extensive developments have been made in functional materials to meet the growing demands of water pollution control. Considering the advantages of these functional materials, this review aims to summarize the latest progress in the detection and removal of heavy metal ions using different types of functional materials, including nanomaterials, polymers, porous materials, and biomaterials (Fig. 1). Additionally, the future prospects and challenges faced by these four material categories are discussed, with the hope of inspiring novel ideas in the detection and removal of heavy metal ions and fostering a better understanding of the future development in this research field.
 | ||
| Fig. 1 Schematic diagram of functional materials fabrication and their applications for detection and removal of heavy metal ions. | ||
2. Types of fluorescent probes for heavy metal ion sensing
There are several types of fluorescent probes for sensing heavy metal ions, such as molecular chromophores, nanomaterials, metal organic frameworks (MOFs), covalent organic frameworks (COFs), etc. Based on photophysical processes, fluorescent probes respond to heavy metal ions mainly through several signalling mechanisms including photo-induced electron transfer (PeT), aggregation induced emission (AIE), intramolecular charge transfer (ICT), Förster resonance energy transfer (FRET), inner-filter effect (IFE), chelation-enhanced fluorescence (CHEF), chelation-quenched fluorescence (CHQF) (Table 1).| Chromophores | Signal | Mechanism | Analytes | LOD (M) | Ref. |
|---|---|---|---|---|---|
| Pyrene-amino mercaptothiadiazole | Fluorescence quenching | Heavy atom effect | Hg2+ | 0.35 × 10−9 | 41 |
| TPE–An–Py | Fluorescence quenching | CHQF | Cu2+ | 2.36 × 10−7 | 45 |
| Schiff base | Fluorescence increase | ICT | Pb2+ | 1.24 × 10−3 | 46 |
| Tmbipe | Fluorescence increase | CHEF | Hg2+, MeHg+ | 6.30 × 10−10 | 47 |
| H2Pc, ZnPor | Ratiometric response | FRET | Pb2+ | 4.10 × 10−9 | 49 |
| Tetraphenylene, Rho | Ratiometric response | DTBET | Hg2+ | 1.50 × 10−9 | 50 |
2.1. Molecular chromophores
The addition of heavy metal ions into a solution containing a fluorescent probe frequently induces fluorescence quenching through mechanisms such as electron transfer, FRET, heavy atom effect, and spin–orbit coupling (Fig. 2). For example, Hg2+ ions can markedly quench the fluorescence of ligands. In 2017, John and co-workers designed a pyrene-amino mercapto thiadiazole fluorescent probe (Fig. 3a) for the detection of Hg2+ ions via a ‘‘turn-off” mode, exhibiting three emission peaks at 378, 388 and 397 nm.41 Significant fluorescence quenching (96%) could be observed after binding with Hg2+ ions (Fig. 3b), while other metal ions (e.g. Pb2+ and Fe3+) had little effect on the fluorescence intensity. This probe exhibited a good linear correlation with a wide range of Hg2+ concentrations, providing an impressive limit of detection (LOD) of 0.35 nM. The fluorescence quenching mechanism of this probe was attributed to the heavy atom effect induced by the Hg2+ ions. | ||
| Fig. 2 Chromophores with different fluorescence response for the detection of heavy metal ions. (a) Fluorescence quenching. (b) Fluorescence enhancement (c) ratiometric sensing. | ||
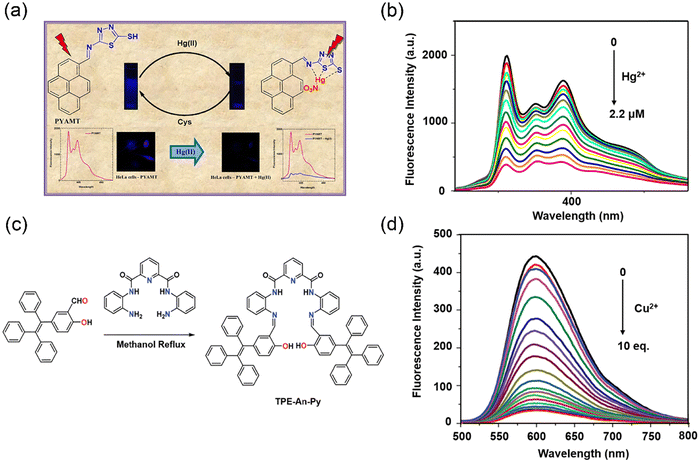 | ||
| Fig. 3 The synthesis of “turn-off” fluorescent probes and their fluorescence spectra for detecting heavy metal ions. (a) Pyrene-based fluorescent probe for detection of Hg2+ ions with turn-off response. (b) Fluorescence spectra of PYAMT in the presence of Hg2+. Reproduced from ref. 41 with permission from Elsevier B.V. Copyright 2017. (c) Synthetic route to probe TPE–An–Py. (d) Fluorescence response of TPE–An–Py on addition of Cu2+. Reproduced from ref. 45 with permission from Royal Society of Chemistry. Copyright 2020. | ||
The majority of organic fluorescent dyes or fluorophores exhibit strong fluorescence emissions in a solution state, whereas their fluorescence emission intensity is reduced when they are in a solid state or aggregated form.42 However, Tang et al. observed that some flexible molecular systems exhibit weak emission in the solution phase but strong emission at high concentrations or when aggregated.43 Such Aggregation-induced emission (AIE) molecular systems are now widely used as fluorescent sensors and optoelectronic materials.44 In 2020, Feng and co-workers developed a non-planar tetraphenylethylene-functionalized salicylaldehyde Schiff base fluorescent probe (TPE–An–Py) (Fig. 3c) with aggregation-induced enhanced emission (AIEE) properties via a classical Knoevenagel condensation reaction.45 Its fluorescence spectrum in tetrahydrofuran (THF) exhibited two emission bands at 477 nm and 598 nm corresponding to the enol and ketone forms, respectively. The probe can easily transfer from the enol form to the keto form via a photo-tautomerization process under photoexcitation via the excited-state intramolecular proton transfer (ESIPT). Based on a ligand-induced complexation mechanism, coordination between Cu2+ and the nitrogen and oxygen atoms in the Schiff base result a selective off response of TPE–An–Py towards Cu2+ in a mixture of THF and water (Fig. 3d). The detection limit of the probe for Cu2+ was determined to be 2.36 × 10−7 M based on the “turn-off” fluorescence detection. Meanwhile, this probe also provides a convenient “naked eye” detection method for Cu2+ ions, demonstrating its promising potential as a sensitive and selective sensing platform for heavy metal ions, which pave the way for the development of novel fluorescent probes with tunable properties for a wide range of applications in chemical and biological sensing.
Nevertheless, since fluorescent sensors based on a quenching mechanism may provide false signals, they are less practically applicable. Compared with turn-off sensors, turn-on sensors exhibit higher accuracy in detecting heavy metal ions. In 2019, Patra and co-workers designed a bis-Schiff base chemosensor incorporating triazole moieties, which exhibits colorimetric and fluorescent response towards Pb2+ and Cu2+ ions (Fig. 4a) via a ‘‘turn-on” mechanism.46 The free triazole probe exhibited a weak fluorescence emission at 440 nm, which was attributed to a non-radiative PeT process from the nitrogen to the excited fluorophore. In the presence of Cu2+ or Pb2+ ions, a remarkable amplification of fluorescence intensity by approximately 15-fold and 17-fold, respectively, was observed at 440 nm (Fig. 4b), accompanied by a blue shift of the emission wavelength to 412 nm. In contrast, no significant spectral changes were observed upon the addition of other metal ions. The LOD for Pb2+ and Cu2+ ions were determined as 0.99 mM and 1.24 mM, respectively. The underlying mechanism for the observed enhancement was attributed to the coordination of Pb2+ or Cu2+ with the triazole moieties and imine unit, which impedes the PeT process and reinforce the ICT process. Subsequently, Yuan and co-workers reported a benzimidazole-modified 1,1,2,2-tetrakis[4-(3-methyl-1H-benzimidazol-1-yl)phenyl]ethylene tetraiodide (Tmbipe) probe (Fig. 4c) for the detection of Hg2+.47 The probe exhibits negligible fluorescence emission in aqueous media. Which was attributed to the C–C bonds between phenyl rings and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds and the C–N bonds between phenyl rings and benzimidazoles exhibiting high rotational freedom, which diminished the molecular planarity, while concurrently increasing the possibility of radiationless relaxation. As such the probe exhibited significant fluorescence enhancements with Hg2+ and organic mercury (MeHg+ and PhHg+) (Fig. 4d). It was proposed that Hg2+ can coordinate with two benzimidazole units to form a binuclear tetracarbene Hg2+ complex, which is similar to the reported Ag+ or Au+ tetracarbene complexes.48 Therefore, free rotation is restricted due to formation of a closed macrocyclic structure, which triggers the fluorescence enhancement. Dynamic light scattering (DLS) results indicated that the average size of the probe in solution increased dramatically from 1.3 nm to 1000 nm after the addition of Hg2+, confirming the formation of large aggregates.
C bonds and the C–N bonds between phenyl rings and benzimidazoles exhibiting high rotational freedom, which diminished the molecular planarity, while concurrently increasing the possibility of radiationless relaxation. As such the probe exhibited significant fluorescence enhancements with Hg2+ and organic mercury (MeHg+ and PhHg+) (Fig. 4d). It was proposed that Hg2+ can coordinate with two benzimidazole units to form a binuclear tetracarbene Hg2+ complex, which is similar to the reported Ag+ or Au+ tetracarbene complexes.48 Therefore, free rotation is restricted due to formation of a closed macrocyclic structure, which triggers the fluorescence enhancement. Dynamic light scattering (DLS) results indicated that the average size of the probe in solution increased dramatically from 1.3 nm to 1000 nm after the addition of Hg2+, confirming the formation of large aggregates.
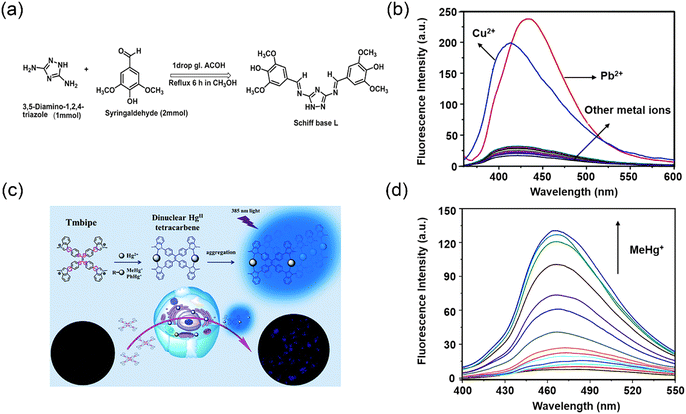 | ||
| Fig. 4 The synthesis of “turn-on” fluorescent probes and their fluorescence spectra for detecting heavy metal ions. (a) Synthesis of fluorescence probe L. (b) Fluorescence response of L after adding different metal ions. Reproduced from ref. 46 with permission from Royal Society of Chemistry. Copyright 2019. (c) Structure of Tmbipe and its application. (d) Fluorescence spectra of the Tmbipe probe in presence of MeHg+. Reproduced from ref. 47 with permission from Royal Society of Chemistry. Copyright 2019. | ||
The dual-channel signal based on the FRET effect provides more accurate and stable response when compared to single-channel signaling. Jiang and co-workers designed a phthalocyanine–porphyrin hetero-triad H2Pc-β-(ZnPor)2 probe for the ratiometric fluorescence detection of Pb2+ ions (Fig. 5a).49 The ratiometric fluorescence response relied on the coupled FRET and a metal-chelating induced fluorescence quenching. Specifically, the probe exhibited highly efficient intramolecular FRET from the two zinc–porphyrin (ZnPor) units to the metal-free phthalocyanine (H2Pc) unit. Selective binding of Pb2+ to H2Pc effectively quenched the emission of the phthalocyanine unit, and the emission of the ZnPor units recovered due to suppression of the intramolecular FRET process, resulting in an obvious ratiometric fluorescent response (Fig. 5b). The addition of Pb2+ resulted in a significant 82-fold increase in the emission intensity ratio of ZnPor and H2Pc, and a good linear relationship to Pb2+ concentrations over a range from 0–2.0 μM was observed, resulting in a LOD value of 4.1 nM that was not affected by other heavy metal ions. Tang and co-workers devised a dark through-bond energy transfer (DTBET) strategy for the development of high-performance ratiometric Hg2+ sensors (Fig. 5c).50 The system used a tetraphenylene (TPE) derivative with AIE characteristics as the dark donor to eliminate the leakage of donor emission. Energy transfer from the TPE derivative (dark donor) to the rhodamine unit (acceptor) proceeded with an energy transfer efficiency (ETE) of 99%. The through-bond energy transfer (TBET) mechanism was used due to the reduced sensitivity to spectral overlap than FRET, allowing for greater flexibility in the design of the system with large pseudo-Stokes shifts. The addition of Hg2+ results in two distinct effects: (i) the generation of a rhodamine core, leading to increased PL at 595 nm and decreased PL intensity of the TPE moiety due to rapid and efficient TBET processes; (ii) the reduction of sensor aggregation, resulting in a fluorescence decrease of the TPE unit (Fig. 5d). The sensor exhibited an ultra-high ratiometric enhancement and a very low detection limit of 0.3 ppb. Therefore, the combination of AIE and DTBET is a good design strategy for the development of high-performance sensors.
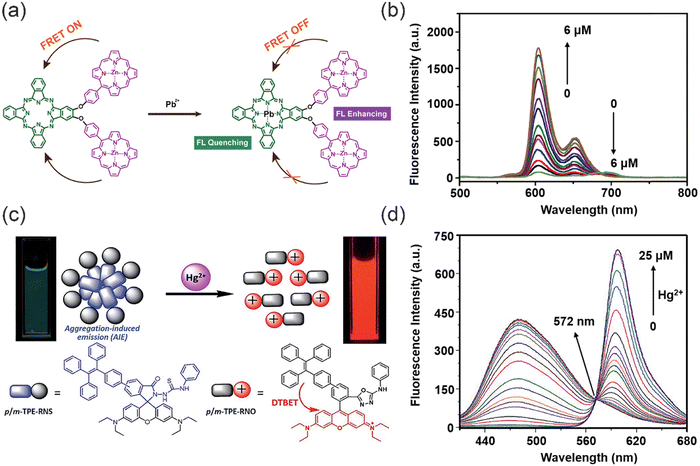 | ||
| Fig. 5 The synthesis of “ratiometric” fluorescent probes and their fluorescence spectra for detecting heavy metal ions. (a) The sensing mechanism of ratiometric probe for Pb2+. (b) fluorescence spectra of triad 1 upon addition of Pb2+. Reproduced from ref. 49 with permission from Elsevier Ltd. Copyright 2019. (c) The sensing mechanism of p/m-TPE–RNS for Hg2+. (d) Fluorescence spectra of 10 mM m-TPE–RNS under different concentrations of Hg2+. Reproduced from ref. 50 with permission from Royal Society of Chemistry. Copyright 2017. | ||
To improve the removal efficiency of heavy metal ions, molecular fluorescent probes have been incorporated into polymeric and porous materials. Such integration enables the simultaneous detection and removal of heavy metal ions, providing a comprehensive solution for environmental monitoring and remediation.
2.2. Nanomaterials
Nanomaterials are substances whose physical dimensions are within the nanoscale range, typically ranging from 1 to 100 nanometers, or those that possess a nanoscale internal structure or surface morphology. In general, common nanomaterials used in sensing heavy metal cations include metal nano materials,51,52 nano carbon materials,53,54 and nanofiber materials.55,56 Such nanomaterials exhibit special properties including surface effects, small size effects, quantum effects, and macro quantum tunnel effects.57 These properties contribute to their high sensitivity and selectivity. Consequently, a plethora of nanomaterials have been used for the purpose of detecting heavy metal ions in wastewater.58,59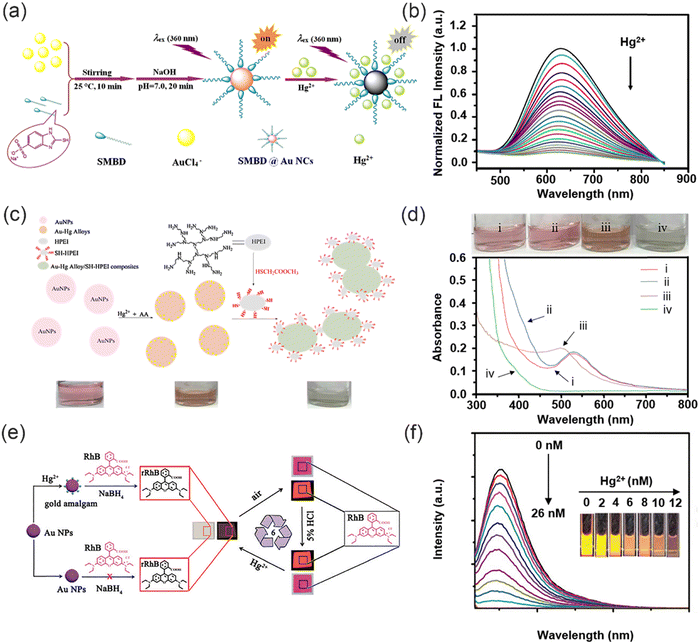 | ||
| Fig. 6 The synthesis of metal nanoprobes and their sensing mechanisms for heavy metal ions. (a) The synthesis of SMBD@AuNCs. (b) Fluorescence response of SMBD@AuNCs under different concentrations of Hg2+. Reproduced from ref. 66 with permission from Elsevier B.V. Copyright 2019. (c) Synthesis of SH-HPEI and the fluorescence response to Hg2+. (d) Photographs and UV-vis absorption spectra of AuNPs. Reproduced from ref. 67 with permission from Elsevier B.V. Copyright 2017. (e) The sensing mechanism of fluorescent probes for detection of Hg2+. (f) The fluorescence response of RhB with AuNPs and NaBH4 under different concentrations of Hg2+. Reproduced from ref. 68 with permission from Royal Society of Chemistry. Copyright 2017. | ||
Wang and co-workers used AuNPs as chemosensors for the dual signal amplification detection of Hg2+ (Fig. 6e).68 This simple method presents some notable advantages, including simple synthesis, rapid response, exceptional selectivity, and high sensitivity. The proposed system relies on the formation of gold amalgam and a gold amalgam-based reaction involving rhodamine B (RhB) and NaBH4, which exhibits fluorescence and colorimetric sensing functionalities. RhB was strategically selected as the visible signaling reporter due to its remarkable attributes, including long-wavelength absorption and emission, high absorption coefficient, exceptional quantum efficiency, and superior photostability. Notably, the strong and specific D10–D10 metallophilic interaction between Au+ and Hg2+ facilitates the catalytic reduction of RhB by the as-formed gold amalgam, leading to simultaneous changes in fluorescence and color of RhB (Fig. 6f), thus enabling dual signal amplification. Remarkably, the reduction product of RhB can be readily oxidized to regenerate RhB in air, allowing for repeatable and reusable utilization of the prepared sensor. To sum up, due to the specific interaction between metal nanomaterials and heavy metal ions, heavy metal ions can be identified precisely and sensitively in solution. Therefore, metal nanoparticles are promising practical tools for heavy metal ions detection.
Currently, CDs can be synthesized using two strategies, a top-down or bottom-up approach (Fig. 7).80 The top-down method involves the fragmentation of a wider range of carbon-based materials (graphite, carbon black, graphene oxide, etc.) into quantum-sized particles by chemical oxidation or electrical discharge, laser ablation, and acid exfoliation under harsh synthetic conditions. In 2021, Carbone and co-workers synthesized nitrogen-doped carbon quantum dots (N-CQDs) (Fig. 8a) by a top-down method using fullerene as the raw material.81 Since the opening of the fullerene rings requires a mixture of hydrogen peroxide (H2O2) and ammonia (NH4OH), which generates oxygenated and nitrogenated functional groups. The process of hydroxyl-radical-induced opening of fullerene results in the modification of the surface of the resulting carbon quantum dots (CQDs) with various functional groups including hydroxyl (–C–OH), carboxyl (–CHO, –COOH), ether and/or epoxy (–C–O–C–), amine (–C–NH2), and amide (–CO–NHx, x = 1, 2) moieties. XPS and Fourier transform infrared spectroscopy (FTIR) confirmed that the surface of the synthesized carbon dots was rich in reactive groups (hydroxyl, carboxyl and amino groups), which could provide sufficient reactive sites for heavy metal ion chelation. When Cu2+ and Cr3+ are added, the fluorescence intensity of the CDs was reduced by 44% and 60%, respectively (Fig. 8b). However, the high price of raw materials, complex preparation process, and relatively low yield results in a the high cost for the preparation of these CDs.82 Therefore, alternative approaches are required to improve the synthetic methods towards such CDs. The top-down methods for the synthesis of carbon dots typically require harsh conditions and high costs. In most cases, this method of synthesizing CDs is challenging and time-consuming.
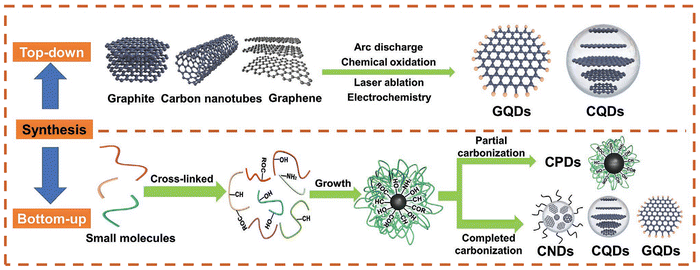 | ||
| Fig. 7 Schematic illustration of the synthesis of CDs via top-down and bottom-up methods. Reproduced from ref. 80 with permission from Wiley-VCH. Copyright 2021. | ||
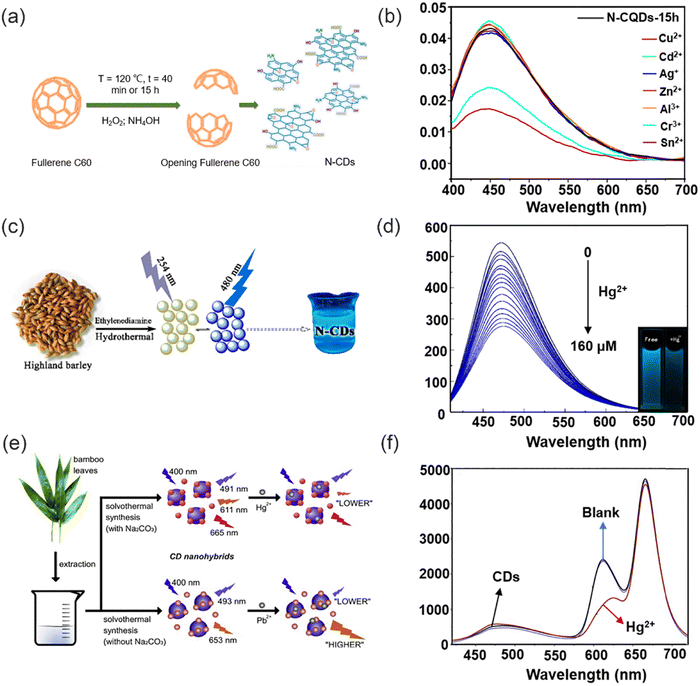 | ||
| Fig. 8 The synthesis of CDs via top-down (or bottom-up) and their fluorescence response to heavy metal ions. (a) Preparation of fluorescent N-CQDs. (b) The fluorescence spectra of N-CQDs-15 h in the presence of different metal ions. Reproduced from ref. 81 with permission from Multidisciplinary Digital Publishing Institute Copyright 2021. (c) The preparation of the N-CDs. (d) The fluorescence response of the N-CDs under different concentrations of Hg2+. Reproduced from ref. 88 with permission from Multidisciplinary Digital Publishing Institute. Copyright 2019. (e) Synthesis of dual- and three-emission CD nanohybrids for ratiometric fluorescent sensing of Pb2+ and Hg2+, respectively. (f) Fluorescence spectra of the three-emission CD nanohybrids before and after its application for the analysis of a blank sample. Reproduced from ref. 89 with permission from Elsevier B.V. Copyright 2019. | ||
Compared to top-down strategies, bottom-up approaches have significant advantages such as mild reaction conditions, rich carbon resources and better material morphology.83 This method is also known to be a green method of synthesizing CDs. Since, many renewable green resources are used to prepare CDs such as lemons, honey, flowers, and silk.84 Bottom-up approaches for the preparation of CDs include small molecule condensation, cross-linking or carbonization by hydrothermal methods, pyrolysis and microwave-assisted synthesis.85–87 These methods are simple, low cost, and eco-friendly, resulting in the green synthesis of CDs. In 2019, Liu and co-workers synthesized fully water-soluble nitrogen-doped carbon dots (N-CDs) using barley as the carbon source and ethylenediamine as the nitrogen source for the detection of Hg2+ (Fig. 8c).88 XPS and FTIR spectra confirmed that N-CDs carry a large number of reactive hydrophilic groups (oxygen and nitrogen containing functional groups) on their surface. The remarkable fluorescence quenching observed in the N-CDs is attributed to the robust chelating ability of Hg2+ ions towards the carboxylic functional groups on the surface of N-CDs, as depicted in Fig. 8d. This interaction leads to the formation of a non-fluorescent complex, facilitating non-radiative electron/hole annihilation through an efficient electron transfer process. Therefore, the fluorescence of N-CDs is effectively quenched through a static quenching mechanism. In addition, the N-CDs are environmentally friendly since the N-CDs were prepared using natural biomass as the raw materials. Similarly, Zhang and co-workers have successfully developed two new dual-emission and triple-emission CD nanohybrid ratiometric fluorescent nanosensors for the simple, sensitive and specific detection of Pb2+ and Hg2+ in complex environmental water samples, respectively (Fig. 8e).89 These two novel CD hybrids were prepared by solvent heat treatment of the same bamboo leaf extract. This method is characterized by its simplicity, cost-effectiveness, environmental sustainability, and practicality, as it circumvents the need for post-modification of CDs or co-incorporation with other fluorescent probes. Notably, these newly developed nanosensors exhibit built-in correction capabilities owing to their dual emission behavior, enabling detection limits as low as 0.14 nM and 0.22 nM for Pb2+ and Hg2+ respectively (Fig. 8f). The system was particularly sensitive for the selective detection of these analyte ions, indicating the unlimited possibilities of the nano-probe in chemical analysis of real samples.
In order to meet the demand for both detection and removal of heavy metal ions, researchers have explored the integration of nanomaterials into polymer matrices. The incorporation of nanomaterials within polymer matrices results in excellent functional materials capable of the simultaneous detection and removal of heavy metal ions.
2.3. DNA or RNA Biomaterials
The interaction of heavy metal ions with DNA, RNA and other biomolecules provides biomaterials with a remarkable advantage in terms of selectivity, thus facilitating the recognition and elimination of heavy metal ions. Specific aptamer-target interactions (T–Hg2+–T) in the presence of Hg2+ and aptamer make biomaterials functional for the detection of Hg2+. Wu and co-workers developed a Hg2+ sensing platform based on T–Hg2+–T pairing using a single-channel recording technique for the detection of Hg2+.90 The presence of Hg2+ can be confirmed at around 7 nM within 30 min. The sensor is manufactured from off-the-shelf materials, does not require synthesis, purification, or probe fabrication processes, and is highly selective for Hg2+ without interference from other metal ions. This sensing strategy opens new possibilities for the detection of many types of analytes that have specific interactions with DNA molecules.In recent years, significant advancements have been made in enhancing the sensitivity of sensors for Hg2+. In 2017, Chen and co-workers reported a versatile and sensitive colorimetric sensor for Hg2+ based on aptamer-target specific binding and target-mediated growth of AuNPs.91 T bases were used to detect Hg2+ by T–Hg2+–T coordination (Fig. 9a) and a detection limit of 9.6 × 10−9 M (Fig. 9b) was determined, different lengths of aptamer effected the sensitivity of Hg2+ detection. Starting with the 15-mer aptamer, the DNA sequences were extended and truncated to produce 25-, 59- and 8-mer (8T) sequences, and it was found that the detection performance of the 25-mer and 59-mer aptamers was greater than that of the 15-mer aptamer. In the presence of Hg2+, T–Hg2+–T coordination makes the T-base sequence detach from the AuNP surface, while the additional A base sequence remains adsorbed on the AuNP surface, thus differences in the number of DNA strands adsorbed may lead to morphological changes in the grown AuNP. This also indicates that the increased sensitivity due to prolonging aptamer strands can result in enhanced LOD. In 2021, Liu and co-workers developed a simple method to covalently incorporate unmodified DNA oligonucleotides to hydrogel nanoparticles and monoliths using A5 as an anchoring block (Fig. 9c).92 Various functional DNA sequences were ligated by designing DNA containing A5 blocks. The DNA was folded into a hairpin shape after Hg2+ mediated T–Hg2+–T formation. The DNA functionalized hydrogels were utilized to detect Hg2+ ions, demonstrating a remarkable detection limit of 10 nM (Fig. 9d). The thymine interaction with Hg2+ is particularly important for sensing applications. All the methods based on this interaction show excellent selectivity over other heavy-metal ions due to the high specificity and strength of the complex formed with Hg2+, which ensures that such biomaterials are ideal for heavy metal ions sensing. A similar approach was used in 2022 to fabricate supramolecular polymers with high efficiency for the detection and removal of Ag+ ions.93 Through, the incorporation of functional macrocycles and fluorescent molecules into the supramolecular polymers, an ideal material for detection and removal applications could be developed, offering new avenues for pollutant monitoring and environmental remediation. This innovative strategy holds great potential in the field of pollution detection and environmental management.
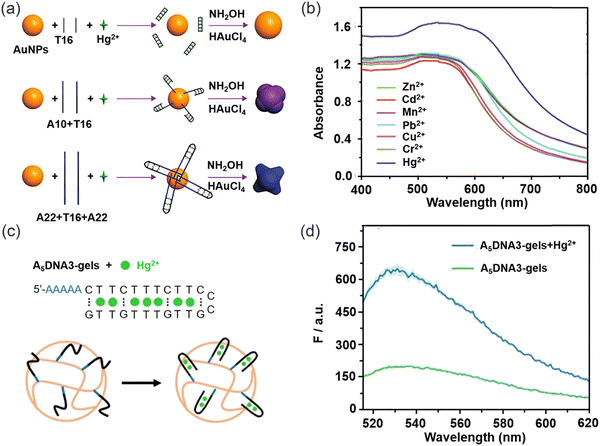 | ||
| Fig. 9 The specific receptors with strong metal binding ability of DNA or RNA biomaterials to facilitate the detection of Hg2+. (a) The sensing strategy for the colorimetric detection of Hg2+ based on the growth of AuNPs induced by different amounts of bases (adenine and thymine). (b) Absorption spectra of AuNPs grown in the presence of Hg2+ and other interfering heavy metal ions. Reproduced from ref. 91 with permission from Wiley-VCH. Copyright 2017. (c) The sequence of the A5DNA3 and Hg2+ binding to A5DNA3 containing hydrogel nanoparticles. (d) Fluorescence spectra of Hg2+ binding to A5DNA3 hydrogels. Reproduced from ref. 92 with permission from Wiley-VCH. Copyright 2021. | ||
3. Chromophore incorporated Polymeric Materials
Polymeric sensing materials, consisting of fluorescent probes and a hydrophilic polymer matrix, have drawn significant attention due to their remarkable ion-specific selectivity and exceptional sensitivity.10,94 A hydrophilic polymer is preferred, since while conventional hydrophobic polymers are more effective for heavy metal ion removal, they exhibit limited detection ability in waste water. While, fluorescent probes are difficult to remove from the sample for recycling, which limits their application in real water samples or wastewater.95 To tackle these problems, hydrophilic functional polymer materials with good sensing and removal abilities have been constructed (Fig. 10). Notably, the advantages of polymer-based functional systems are as follows: (i) additional adventitious properties are provided by functional polymer materials (e.g. good mechanical properties, pore size structure, hydrophilicity and reusability, etc.); (ii) larger reactive regions and more binding sites available on functional polymers;96 (iii) the loading and encapsulation of fluorescent materials using polymers can improve the stability and enable them to be used for a variety of environmental applications; (iv) some polymers can be designed to be degradable, which allows them to break down into harmless substances in the environment, reducing their impact. In addition, the mechanical properties of polymers can also impart fluorescent materials with good processability. It should be noted that the advantages of polymer-based functional systems are not limited to the polymers themselves. In many cases, these systems can be carefully designed to possess additional functionalities, such as optical, electrical, or magnetic properties. As a result, polymer-based functional systems have broad applications in various fields, including environmental remediation, and also energy storage.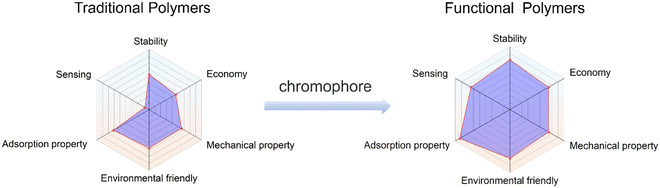 | ||
| Fig. 10 A comparison of the strengths and weaknesses of the traditional polymers and functional polymers. | ||
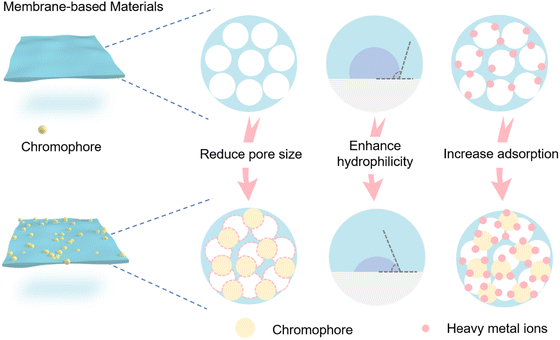
3.1. Membrane-based materials
Membrane filtration is an effective separation technique that uses the selective permeability of a membrane under the influence of a driving force, such as pressure, to effectively separate substrates. Polymeric membranes exhibit great promise for heavy metal ion removal due to their ease of processing, high mechanical strength and acceptable separation performance. Nevertheless, while conventional polymeric membranes exhibit good retention ability for heavy metal ions, the ability to identify heavy metal ions requires improvement. As such polymer membranes can be modified to have the ability to both identify and remove heavy metal ions. The hydrophilicity and pore size of the modified polymer membrane will also be modified, potentially enhancing the retention capacity of the polymer membrane for heavy metal ions (Fig. 11). In addition, the polymer structure can provide a good carrier for chromophores, which enhances the stability and reusability of the chromophores.Chuang and co-workers designed a novel functional membrane material for the detection and removal of Cr(VI) (Fig. 12a).97 Phthalocyanine (Pc) derivatives and hyperbranched polyamidoamines (HPAMAM) were coupled to polytetrafluoroethylene (PTFE) membranes modified with furan groups via the Diels–Alder (DA) reaction. Fluorescence quenching occurs when Pc forms chelates with specific heavy metal ions (Fig. 12b). To mitigate the rapidly flow of detected heavy metal ions, hyperbranched poly(amidomethylamine) (PAMAM) can effectively sequester heavy metal ions through the formation of chelates. The intricate dendritic architecture of PAMAM, functionalized by numerous primary and tertiary amine groups, enables it to retain heavy metal ions through chelation.58,98–102 In addition, after modification by HPAMAM and Pc, the surface pore structure of the membrane is partially filled with a thick polymer layer, resulting in the average pore size of the membrane being reduced from 2.3 μm to 1.3 μm. Significantly, the 145.2 ± 0.17° contact angle of the extremely hydrophobic neat PTFE membrane was decreased to 57.7 ± 0.80° by the hydrophilic polymer HPAMAM. The membrane surface pore size was decreased by the polymer layer following HPAMAM/Pc modification, and the decreased hydrophobicity improved water permeation.103–105 Moreover, the reversible Diels–Alder (DA) reaction between furan and maleimide moieties, operating under thermal conditions, endows the membrane with selective regenerative properties. Hence, the integration of multiple functional materials within a single membrane holds immense potential for enabling synergistic effects for a diverse range of applications. However, this functional membrane material faces the difficulties of complicated preparation and does not readily biodegrade. In general, using natural substances (e.g. cellulose, chitosan and lignin etc.) to synthesize polymers can fully reduce the secondary pollution caused by the polymer adsorbents to the environment. Li and co-workers devised a biodegradable fluorescent cellulose membrane by direct dip-coating of graphene oxide (GO) with a naphthalimide fluorescence probe (Fig. 12c).106 GO can be immobilized directly onto a cellulose surface via hydrogen bonding. The resulting composite material exhibits a significantly enhanced surface area, as confirmed by the Brunauer–Emmett–Teller (BET) measurement, which reveals an increase from 4.813 m2 g−1 to 12.660 m2 g−1. This marked increase in surface area provides a larger reaction interface, thereby facilitating a 300% increase in available binding sites for efficient removal of Cu2+. Meanwhile, when Cu2+ coordinates with the nitrogen on the naphthalimide the fluorescence of the probe molecule changes (Fig. 12d). The detection limit and removal limit for Cu2+ were 7.3 × 10−7 M and 100 ppm. Notably, immersion of a cellulose film in the GO suspension engenders not only enhanced binding between the cellulose film and fluorescent molecules through augmented stacking, but also modulates the surface wettability. Following GO modification of the cellulose membrane, the contact angle between water drops and the film was observed to decrease from 81.0 ± 0.31° to 54.2 ± 0.21°, reflecting a heightened hydrophilicity. EDS-mapping revealed that Cu2+ ions were uniformly distributed within the cellulose layer subsequent to filtration, indicating that Cu2+ removal was achieved via absorption. Furthermore, the even dispersion of Cu2+ throughout the membrane further suggests an effective adsorption process. In addition, the recyclability of the membrane materials indicates that the GO membranes exhibit good Cu2+ removal capacity even after several recycling cycles. This study presents a rapid and cost-effective methodology for fabricating fluorescent cellulose materials through doping with fluorophores. The resulting materials can be utilized for both heavy metal ion removal and sensing applications, which represents a convenient and versatile solution.
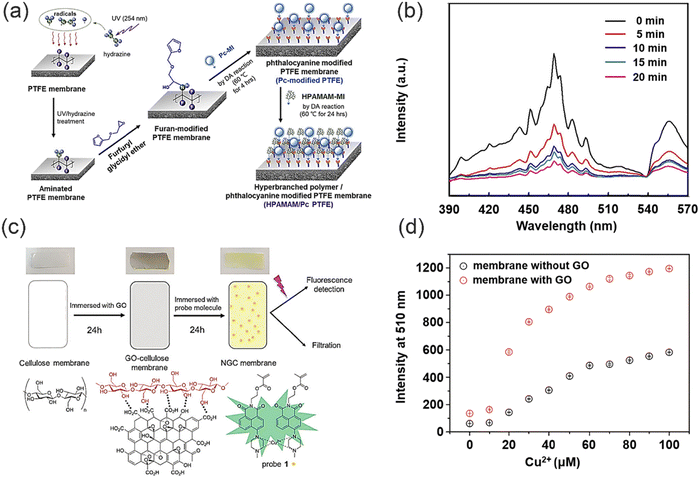 | ||
| Fig. 12 The preparation of functional polymer membranes and their sensing and removal for heavy metal ions. (a) The preparation of HPAMAM/Pc PTFE membrane. (b) The fluorescence spectra of HPAMAM/Pc PTFE membrane with Cr(VI) ions adsorbed. Reproduced from ref. 97 with permission from Elsevier Ltd. Copyright 2019. (c) The comparison of the cellulose membrane, GO-cellulose membrane and modified NGC membrane. (d) Fluorescence intensity of probe 1 attached to the cellulose membrane with and without a GO coating. | ||
Dong and co-workers have successfully fabricated self-supporting flexible nanofiber membranes (NMs) composed of amidated polyacrylonitrile (aPAN) and branched polyethyleneimine (BPEI) via a facile electrospinning technique combined with a subsequent hydrothermal process (Fig. 13a),107 in which NMs not only serve as strips for visual detection of Cu2+, but also demonstrate excellent performance as adsorbents for the efficient removal of Cu2+ from water. The Cu2+ ions are captured by the aPAN/BPEI NMs, where they interact with the abundant amino groups in polyethyleneimine, resulting in a noticeable colour change in the NMs. This unique property allows for simultaneous detection and removal of Cu2+ with an impressive filtration capacity of 209.53 mg g−1. The filtration process also facilitates preconcentration of Cu2+, thereby further enhancing the sensitivity and adsorption capacity of the aPAN/BPEI NMs. Notably, the aPAN/BPEI NMs exhibit exceptional selectivity and recovery, accompanied by a simple treatment process. Furthermore, in contrast to powdered materials, the flexible and bulk self-supporting membranous structure of aPAN/BPEI NMs offers a significantly enlarged specific surface area, which facilitates the detection and absorption of Cu2+, and allows for easy separation from aqueous solution, thereby greatly enhancing the operability of the materials and minimizing the risk of secondary environmental pollution. Nevertheless, further improvement in the sensitivity of aPAN/BPEI NMs is required. In 2019, Li and co-workers developed a new “cellulose spacer” strategy.108 Specifically, when modifying the membrane with fluorescent dyes, conventional fluorophores are prone to experiencing aggregation-caused quenching (ACQ), which can potentially undermine their effectiveness in sensing applications. However, fluorescent solid nanomaterials can be prepared by assembling nanocellulose with coumarin based probe molecules (Fig. 13b and c). Nanocellulose exhibits hydrogen bonding interactions with hydroxyl-containing coumarin, which serves as a spacer that prevents the π–π stacking of coumarin. The membrane exhibits excellent sensing and filtration performance for Hg2+, along with excellent recovery and biocompatibility. Therefore, the utilization of the “cellulose spacer” strategy for the assembly of fluorescent materials with sensing and removal capabilities holds great promise in various applications, particularly in the field of heavy metal ion sensing and removal. This approach offers a versatile and effective means to create fluorescent materials that possess desired properties, allowing for precise control over their performance. Such an approach spans a wide range of applications, making it a compelling method for addressing challenges in water treatment and heavy metal ion sensing.
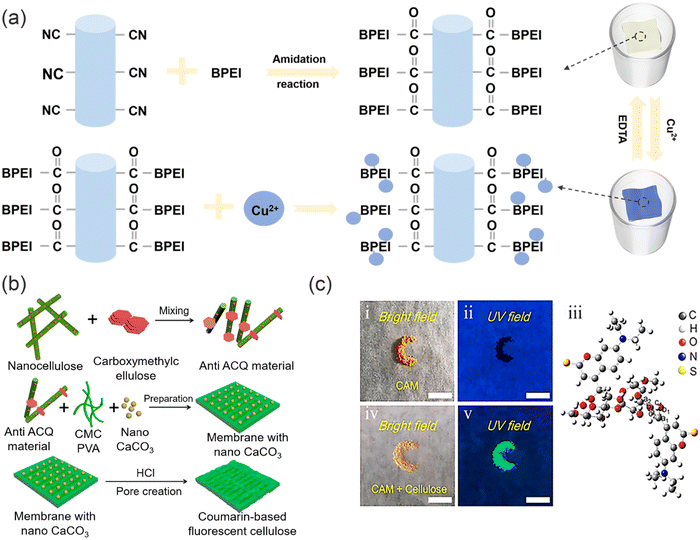 | ||
| Fig. 13 The sensing and removal mechanism of functional polymer membranes for heavy metal ions in. (a) The preparation of aPAN/BPEI NMs and detection/removal of Cu2+. Reproduced from ref. 107 with permission from American Chemical Society. Copyright 2021. (b) The preparation of anti-ACQ materials. (c) The optical image of CAM under natural light and ultraviolet light and the optical image of anti-ACQ material under natural light and ultraviolet light. | ||
The above composite membrane materials used for the effective detection and removal of heavy metal ions can be obtained by modifying simple membrane materials with fluorescent dyes and other small molecules. However, the ability of the membrane for the removal of heavy metal ions needs to be improved when compared with other polymeric materials.
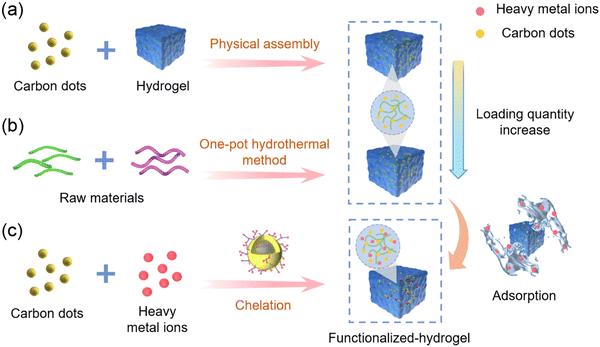
3.2. Hydrogel-based materials
The distinct physical and chemical properties of hydrogels, including their intricate three-dimensional porous structure, abundant functional groups, binding sites, inherent hydrophilicity, exceptional swelling capacity, and facile modification, have sparked significant attention towards the advancement and utilization of novel hydrogel materials for the treatment of wastewater. Hydrogels have demonstrated remarkable efficacy in the adsorptive elimination of diverse inorganic pollutants, including heavy metal ions, as well as organic pollutants, such as toxic dyes.109 Nevertheless, hydrogels crosslinked through physical means using natural polysaccharides such as chitosan or cellulose suffer from insufficient mechanical strength, instability in acidic environments, and limited active site diversity, thereby constraining their potential as versatile adsorbents.110Significantly, the mechanical strength of a hydrogel can be improved by doping with nanomaterials (CDs, Fe3O4), and in addition additional functional groups from the CDs nanomaterials can provide additional binding sites for the adsorption of heavy metal ions. Notably, the three-dimensional mesh structure of the hydrogel can immobilize CDs, which makes them recoverable and as such they will not cause secondary contamination to the system during the detection and removal of heavy metal ions. Therefore, making the system cost-effective for the simultaneous detection and adsorption of heavy metal ions.111,112 As such, the application of functionalized-hydrogels in the detection and removal of heavy metal ions has attracted much recent attention. For example, Wei and co-workers reported a novel carbon dot (CD-Fla) based system for the detection of toxic Pb2+ metal ions using a one-pot hydrothermal method using Ginkgo biloba flavonoid extracts as the raw material (Fig. 14a).113 CD-Fla could be doped into an agarose hydrogel using H-bonding and dipole–dipole interactions. The as-prepared CD-Fla-doped hydrogel facilitated the simultaneous visual fluorescence detection and removal of Pb2+ from water. The prepared CD-Fla exhibited excellent biocompatibility and strong blue light emission and was selectively quenched by Pb2+ (Fig. 14b) enabling the quantitative detection of Pb2+ over a range from 0.1–20.0 nM. Meanwhile, the XPS and FTIR spectra indicted that the surface of the CD-Fla was rich in hydroxyl groups, which provided more adsorption sites for the removal of Pb2+. The maximum adsorption capacity was determined to be 0.35 mg Pb2+ per milligram of CD-Fla. Notably, the fluorescent hydrogel could be regenerated using HCl solution after treatment the hydrogel could be re-used multiple times. Similarly, Wu and co-workers have developed a novel fluorescent nanocellulose hydrogel using the high ratio of cellulose nanofilaments (CNF) and high-performance luminescence of CDs, which serves as an effective adsorbent for heavy metal ion removal and an optical sensor for heavy metal ion detection (Fig. 14c).114 The modification of CNF with CDs not only facilitates the hydroxyl-induced aggregation of heavy metal ions, enhancing the adsorption capacity, but also enables rapid visual response to heavy metal ions improving the stability of the fluorescence signal and sensitivity for determining heavy metal ions concentrations.115,116 The CDs that are enveloped within intricate three-dimensional network structures have been employed as the fluorescent source for facilitating prompt visual detection of heavy metal ions. Subsequent to the adsorption and entrapment of heavy metal ions within the distinct structure of the synthesized fluorescent hydrogel, the fluorescence quenching effect of the heavy metal ions is enabled. Moreover, the three-dimensional network structure of the hydrogel promotes accelerated diffusion and aggregation of the heavy metal ions, facilitated by the high density of amide, hydroxyl, and carboxyl groups, thereby allowing efficient accumulation and adsorption of heavy metal ions. These properties highlight the potential of the developed fluorescent nanocellulose hydrogels as a promising platform for the sensitive and rapid detection of heavy metal ions in environmental and analytical applications. Remarkably, the maximum adsorption capacities of the fluorescent hydrogels for Fe3+, Ba2+, Pb2+, and Cu2+ were determined to be 769, 212, 2056, and 1246 mg g−1, respectively (Fig. 14d). This innovative approach, combining the unique properties of CNF and CDs, results in a multifunctional nanocellulose hydrogel with enhanced performance for heavy metal ion removal and detection, offering novel insights into the design and fabrication of advanced materials for addressing pressing environmental challenges. Meanwhile, in 2020, a novel fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots was successfully synthesized for the efficient detection and removal of Cr(VI) (Fig. 14e).117 Compared with the normal chitosan hydrogel without carbon dots, the fluorescent chitosan-based hydrogel exhibited sensitive detection and enhanced adsorption capacity for Cr(VI) (detection limit of 8.5 mg L−1 and maximum adsorption capacity of 228.2 mg g−1), owing to the abundant amino and hydroxyl groups of the CDs and the high surface area of titanate providing additional binding sites for the removal of heavy metal ions. Moreover, the mechanical properties of the fluorescent chitosan-based hydrogels were enhanced after doping with titanate and CDs. The crosslinking reaction of chitosan and glutaric dialdehyde occurs to form a novel chitosan-based hydrogel in the presence of CDs and titanate nanofibers which were connected to the chitosan network by hydrogen bonding. The resulting hydrogel exhibited an interconnected network structure, facilitated by hydrogen bonding between the chitosan chains and the nanofibers. Notably, the amino groups on the chitosan chains facilitated the adsorption of Cr(VI) ions onto the surface of the hydrogel through electrostatic interactions. Moreover, the hydrogel exhibited a porous structure that served as an efficient mass transfer channel, enabling rapid movement of Cr(VI) ions from the surface to the interior of the hydrogel. This unique characteristic was attributed to the porous nature of the fluorescent chitosan-based hydrogel, which enabled efficient internalization of the adsorbed Cr(VI) ions. Additionally, the CDs contained within the porous structures of the fluorescent chitosan-based hydrogel served as optical sensors for Cr(VI). Cr(VI) ions that were rapidly adsorbed on to the CDs due to electrostatic interactions, which resulted in fluorescence quenching. The fluorescent chitosan-based hydrogel exhibited a visual response for the highly efficient detection of Cr(VI) (Fig. 14f). The synthesis of this chitosan-based hydrogel with its unique structure, including the presence of CDs, titanate nanofibers, and a porous structure, represents a promising avenue for potential applications in areas such as environmental remediation and controlled release systems, owing to its efficient adsorption and mass transfer properties. These findings highlight the potential of these fluorescent chitosan-based hydrogels as promising materials for various practical applications and generating a significant contribution to the field of materials science and environmental chemistry.
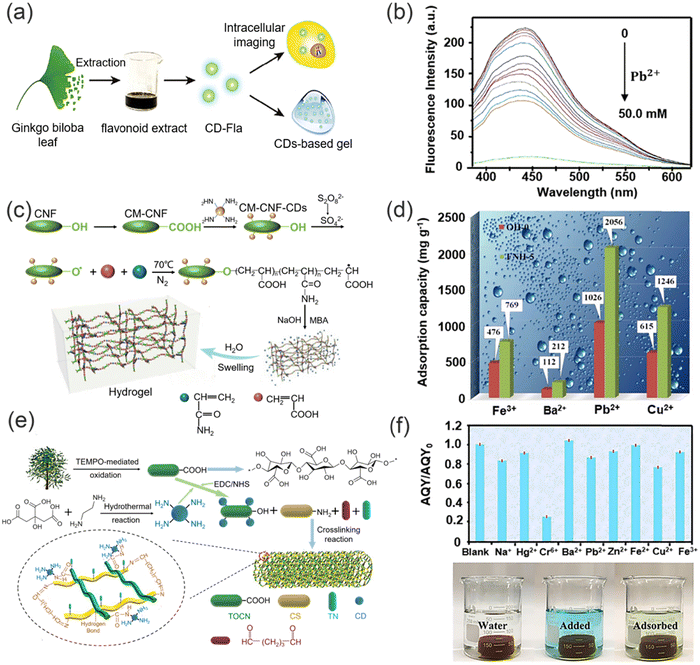 | ||
| Fig. 14 The synthesis of green functional hydrogel polymers and their sensing and removal abilities for heavy metal ions. (a) The synthesis of CD-Fla hydrogel and its applications. (b) Fluorescence spectra of CD-Fla under different concentrations of Pb2+. Reproduced from ref. 113 with permission from Springer Nature. Copyright 2018. (c) Fabrication of the fluorescent nano-cellulosic hydrogel. (d) The comparison of adsorption of heavy metal ions by synthetic hydrogel and original hydrogel. Reproduced from ref. 114 with permission from Royal Society of Chemistry Copyright 2019. (e) Synthesis of chitosan-based hydrogel. (f) Variations in absolute quantum yield (AQY) of the chitosan-based hydrogel in presence of different heavy metal ions. Reproduced from ref. 117 with permission from Elsevier B.V. Copyright 2021. | ||
Compared to single-signal fluorescence sensing, ratiometric sensing is more accurate and sensitive. In 2021, Li and co-workers designed a novel cellulose-based ratiometric fluorescent hydrogel (CDs-Rho). The ratiometric fluorescent probe was prepared using an amidation reaction between CDs as energy donor and rhodamine (Rho) as energy acceptor, and then the probe was loaded into a hydrogel to prepare a ratiometric fluorescent hydrogel.111 CDs-Rho acts as a fluorescent unit, and the amino groups on the surface chelate with Hg2+, leading to fluorescence quenching. Meanwhile, the carboxyl-rich CDs-Rho provides sites for Hg2+ chelation. In addition, the incorporation of CDs-Rho can effectively enhance the mechanical strength and improve the elasticity of the hydrogel. This fluorescent hydrogel was able to efficiently detect and remove Hg2+ from contaminated water. The probe exhibited a sensitive linear response to Hg2+ over a range from 0–100 μM, with a lower limit of detection of 2.19 × 10−9 M and exhibited higher selectivity towards Hg2+ than other cations. In addition, the fluorescent hydrogel displayed a removal efficiency of ∼95% for Hg2+, which was higher than for traditional hydrogel materials. Importantly, the fluorescent hydrogel maintained a high adsorption efficiency after five consecutive cycles of use and does not cause secondary contamination. Therefore, this system provides a promising strategy for the effective identification and removal of heavy metal ions. Most fluorescent hydrogels are formed by first preparing the CDs and then integrating them into the hydrogel to form a fluorescent hydrogel that can simultaneously detect and adsorb heavy metal ions (Fig. 15a). However, fluorescent hydrogels obtained through non-covalent binding face problems such as low loading efficiency and poor stability in complex environments. To overcome the challenge of low loading efficiency in functional materials prepared by immersion, which leads to reduced sensitivity and stability. Wu and co-workers employed cellulose nanofibers as the raw material and employed a one-pot hydrothermal method for the single-step fabrication of fluorescent hydrogels with in situ formation of carbon dots (Fig. 15b).118 The resultant functional material exhibited remarkable capability for the detection and removal of Hg2+. The fluorescent hydrogel synthesized via this approach not only exhibited good mechanical strength, but also exhibited outstanding fluorescence properties and adsorption capacity. By integrating the synthesis of probes and substrates within a unified process, the one-pot hydrothermal method both circumvented the need for separate preparation steps and significantly enhanced the detection sensitivity and stability of the functional materials. This expedient and efficient synthetic strategy offers significant promise for advancing the development of sustainable sensing and detection materials and systems. Similarly, Zhang and co-workers directly employed nitrogen-doped carbon dots (N-CDs) synthesized by low-temperature sintering to form hydrogels upon coordination with heavy metal ions (Fig. 15c).119 Specifically, due to the abundant –COO–, –CO–, NH–, –NH–, and –OH groups on N-CDs, negatively charged N-CDs bind to various positively charged heavy metal ions (Pb2+, Cu2+, Ni2+, Co2+, Cd2+) in water through electrostatic interaction and coordination, forming hydrogels and removing heavy metal ions from water. Fluorescence and UV spectroscopy analyses show that N-CDs can sensitively detect Pb2+ in water, with a detection limit of 3 ppb. The rapidly formed hydrogel can effectively remove a variety of heavy metal ions and can be easily separated from treated wastewater over multiple cycles. The simple and creative method of using a hydrogel formation process to remove heavy metal ions directly is particularly attractive and worthy of additional investigation.
 | ||
| Fig. 15 The preparation of functional hydrogels using different methods. (a) Impregnation method. (b) One-pot hydrothermal process. (c) Interaction between fluorescent probes and heavy metal ions. | ||
Although fluorescent hydrogels exhibit excellent capabilities in the detection and removal of heavy metal ions. The metal ions take a long time to diffuse to the adsorption sites, which limits further application of this type of fluorescent hydrogels.
3.3. Aerogel-based materials
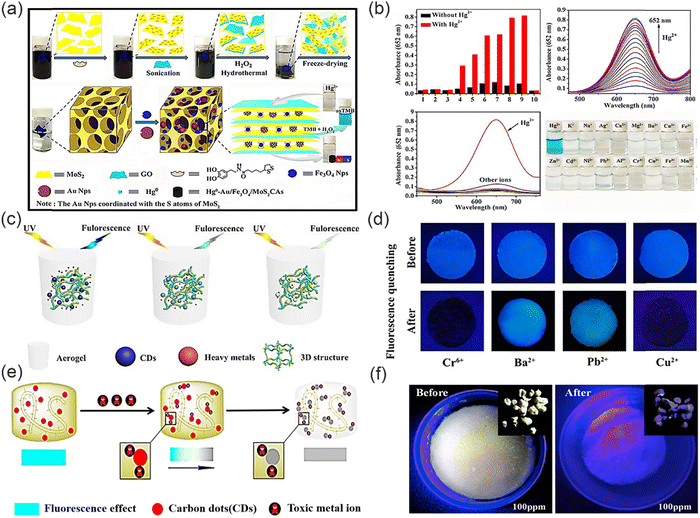
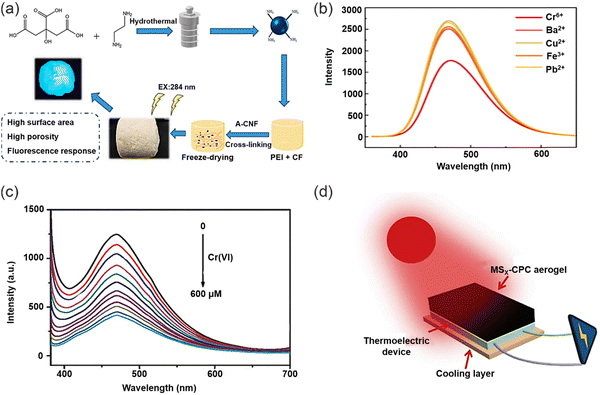
Aerogels represent a class of nanostructured, low-density bulk materials with rigid skeletal frameworks, porous structures and open pores, which can be meticulously tuned at the nanoscale. These unique characteristics provide aerogels exceptional physical properties, including ultra-high specific surface areas and unprecedented adsorption capacities. As a result, aerogels have emerged as highly promising materials for a wide spectrum of applications, including sensors,120 chemical adsorption,121 catalyst supports,122 biomedical devices,123 thereby propelling advancements in the field of environmental protection. In addition, by modifying aerogels with nanomaterials (CDs, AuNPs and Fe3O4), aerogels can attain even more properties, for example, aerogels doped with CDs can gain the ability to detect and adsorb heavy metal ions simultaneously, and can exhibit enhanced mechanical stability compared with pure aerogels, as well as displaying greater efficiency for heavy metal ion removal. Meanwhile, the three-dimensional skeleton of an aerogel can immobilize CDs, which improves their fluorescence stability and reusability, which enhances the efficiency and environmental friendliness of CDs for heavy metal ion detection.Wang and co-workers reported a novel porous MoS2-based composite aerogel (Fig. 16a).124 Specifically, the composite aerogel was prepared from graphene oxide (GO) and molybdenum disulfide flakes, and then gold and iron tetroxide nanoparticles (NPs) were embedded within the GO-doped molybdenum disulfide flakes, respectively, thereby obtaining porous Au/Fe3O4/MoS2CAs aerogels. In this system, the AuNPs as a fluorescence source can specifically bind to Hg2+ to form a gold amalgam, thus detecting and removing Hg2+ (Fig. 16b). Meanwhile, the aerogel has a porous structure and thus enhanced binding sites, which can provide sufficient binding sites for Hg2+. Moreover, the morphology and pore size of the aerogel can be adjusted by changing the doping amount of GO, which can effectively improve the mechanical properties of the aerogel. The detection limit of the functional aerogel for Hg2+ was 3.279 nM, and the adsorption capacity is about 1527 mg g−1. Such a low detection limit and high adsorption capacity make the functional aerogel outstanding amongst similar materials. In addition, because of the magnetic properties of the Fe3O4 nanoparticles, the composite aerogels were easily magnetically separated after doping with Fe3O4, which is beneficial for the recovery and multiple repeated uses of the aerogels. Significantly, the Au/Fe3O4/MoS2CAs aerogels still exhibited high removal efficiency (>95%) after 10 times of continuous recycling. However, although the composite aerogel has excellent heavy metal ions detection and removal capabilities, the preparation process is costly and requires the introduction of additional metal ions (from the aerogel), which may cause secondary contamination. Therefore, the development of aerogels that are cheap and easily available and environmentally friendly are in demand. In 2020, Wu and co-workers developed and prepared a novel fluorescent aerogel with a three-dimensional meshwork structure (Fig. 16c) using highly photoluminescent CDs and renewable natural carboxymethylated cellulose nanofilaments (CM-CNF).125 Fluorescent carbon dots were obtained by hydrothermal reaction, and then the fluorescent carbon dots were soaked and integrated into the CM-CNF aerogel by hydrogen bonding to obtain a composite aerogel for the simultaneous detection and removal of Cr(VI) (CDs-CM-CNF). The fluorescent CDs encapsulated within the novel aerogel network structures exhibit abundant amino, carboxyl, and hydroxyl groups. This unique composition enables these CDs to serve as a highly sensitive and rapid visual source for the detection of Cr(VI) ions, as demonstrated in Fig. 16d. Furthermore, the abundance of adsorption sites on the aerogel surface facilitates the rapid aggregation of Cr(VI) ions, driven by electrostatic interactions with the positively charged amino groups. This phenomenon promotes the diffusion of anionic Cr2O72− ions to the surface of the synthesized aerogel, effectively enhancing the efficiency for Cr(VI) adsorption. The maximum adsorption amount reached was 433.5 mg g−1. This architecture and composition of the aerogel-encapsulated CDs offer a promising approach for the efficient detection and removal of Cr(VI) ions, with potential implications in environmental and analytical applications. Similarly, Guo and co-workers developed a novel carbon quantum dot (CQDs)/nanocellulose (NFC) composite aerogel prepared using a green chemical synthetic method and applied it for the adsorption and detection of heavy metal ions (Cr3+) in water (Fig. 16e).126 CQDs and NFCs are directly bonded to each other by intermolecular forces or hydrogen bonds, without the addition of additional cross-linking agents which is adventitious since those reagents could result in contamination. The composite aerogel formed this way does not change the original structure of CQDs and NFCs. However, in the presence of Cr3+, CQD exhibits excellent fluorescence response (Fig. 16f). In addition, the presence of abundant amino and hydroxyl groups on the surface of the CQDs provides additional adsorption sites for the removal of Cr3+, which enhances the removal efficiency of the composite aerogel. The three-dimensional structure of the NFC aerogel provided a good carrier for CQDs, which made it possible to repeat the detection and removal of Cr3+ repeatedly. The synergistic effect of the CQDs and aerogel enhanced the detection and removal efficiency for heavy metal ions, and in addition the composite aerogel does not undergo toxic chemical reactions or produce toxic substances during the whole process of adsorption of Cr3+ pollutants in water, which enhances the environmental friendliness of this composite material. This study provides a new method for the preparation of green adsorbents with the synergistic effect of adsorption and fluorescence. In 2022, Han and co-workers successfully fabricated multifunctional aerogels by employing waste collagen, polyethyleneimine (PEI), and carbon dots, which are cross-linked with aldehyde cellulose nanofibers, for the purpose of detecting and adsorbing Cr(VI) ions. This approach demonstrates the utilization of sustainable materials and advanced nanotechnology for environmental applications (Fig. 17a).127 With this system, CDs are attached to the aerogel network by hydrogen bonding. Upon immersion of the aerogel in a solution containing Cr(VI) ions, rapid adsorption of the ions onto the surface of the aerogel, enriched with numerous amino groups, occurs due to strong electrostatic interactions with the positively charged amino groups on surface of CDs (Fig. 17b). The resulting aerogel skeleton, featuring a three-dimensional network structure, serves as an efficacious adsorbent and trapping agent for heavy metal ions.128 Notably, fixing the CDs with high quantum yield within the 3D framework enhances the stability of the fluorescence signal.125 While the synergistic effects of the CDs and aerogel enhances the removal efficiency for Cr(VI). Stress–strain curves indicated that the aerogel containing CDs exhibited good mechanical strength compared with the aerogel without CDs. In addition, the aerogel loaded with CDs could be recycled several times and maintained good removal efficiency. The resulting aerogels exhibit exceptional performance in terms of both detection sensitivity and adsorption capacity, making them promising candidates for efficient and sustainable remediation of Cr(VI) contamination.
 | ||
| Fig. 16 Synthesis of functional fluorescent aerogels and their mechanism of sensing and removing heavy metal ions. (a) The detection mechanism of Au/Fe3O4/MoS2CAs for Hg2+. (b) The fluorescence spectra and adsorption properties of Au/Fe3O4/MoS2C for Hg2+. Reproduced from ref. 124 with permission from American Chemical Society. Copyright 2016. (c) The mechanism of the adsorption and fluorescence sensing of Cr(VI). (d) The fluorescence quenching image in presence of different heavy metal ions. Reproduced from ref. 125 with permission from Royal Society of Chemistry. Copyright 2020. (e) The mechanism of detection and removal of heavy metal ions by CQDs/NFC composite aerogel. (f) The optical image of fluorescent aerogel under ultraviolet light before and after adsorption. Reproduced from ref. 126 with permission from Elsevier B.V. Copyright 2020. | ||
 | ||
| Fig. 17 The sensing and removal of functional aerogels for heavy metal ions and their resource utilization. (a) The synthesis of fluorescent aerogel. (b) Fluorescence spectra of fluorescent aerogel in presence of different heavy metal ions. Reproduced from ref. 127 with permission from Elsevier B.V. Copyright 2022. (c) Fluorescence spectra of the CPC aerogel under different concentrations of Cr(VI). (d) Schematic diagram of a rotating motor driven using the solar-powered thermoelectric generator. | ||
However, most composite aerogels used for detection and removal of heavy metal ions are simply discarded after multiple uses, which may cause damage to the environment. How to dispose of the used composite aerogels is a problem that needs to be solved. Towards solving this important problem, Li and co-workers fabricated porous fluorescent aerogels, denoted as CPC aerogels, through the immersion of amino-functionalized carbon dots (CDs-NH2) into a three-dimensional network of PEI and carboxymethylated cellulose (CMC) aerogels. This aerogel allowed for simultaneous detection and adsorption of Cr(VI) ions.129 The CPC aerogels exhibited a linear response to Cr(VI) over a concentration range from 0–600 × 10−6 M (Fig. 17c). Moreover, the aerogel can effectively remove Cr(VI) ions by electrostatic and chelating effects, with a maximum adsorption capacity of 354.61 mg g−1 for Cr(VI). Remarkably, following the adsorption of Cr(VI), the CPC aerogel can be vulcanized (MSx-CPC gel), resulting in the formation of MSx-CPC gel, which can be utilized for solar thermoelectric power generation to generate electricity (Fig. 17d). In addition, the MSx-CPC gel demonstrates a remarkable evaporation rate of approximately 1.31 kg m−2 h−1 under one sun solar irradiation, making it an ideal candidate for solar steam generation. This approach provides new uses for aerogels after adsorption of heavy metal ions. Therefore, providing a new paradigm for researchers working towards multiple use aerogels that can be up-cycled.
3.4. Other polymer materials
In addition to, hydrogel and aerogel polymers, other polymer-based materials have been developed to detect and remove heavy metal ions in various environments. For example, in 2017, Liu and co-workers developed a simple one-pot method for the synthesis of luminescent AuNPs with sponge-like networks (Fig. 18a).130 The luminescent AuNPs could be prepared using pentaerythritol tetrakis 3-mercaptopropionate (PTMP) as both reducing and surface coating ligand. The specific and strong D10–D10 interactions between AuNPs and Hg2+ resulted in sensitive and selectivity sensing of Hg2+ (Fig. 18b). The use of PTMP as a cross-linker in the formation of the three-dimensional sponge-like network of AuNPs not only promotes the structural integrity of the network, but also enhances the luminescent properties of the AuNPs. This innovative one-pot synthesis strategy circumvents the challenges associated with the incompatibility between the formation of sponge-like structures and the stability of AuNPs luminescence, which are commonly encountered in conventional methods. Furthermore, the unique combination of the highly porous sponge-like structure and the strong metallophilic Hg2+–Au+ interactions result in a remarkable saturation capacity of 2.48 g Hg2+ per gram of sorbent for the novel AuNPs-based sponge-like network, surpassing the capacities reported for typical mercury absorbents. This approach represents a significant advancement in the fabrication of highly efficient and stable mercury sorbents with potential implications in environmental remediation and pollution control. Yan and co-workers devised and fabricated a novel chitosan–gold nanocomposite, which has been integrated into functionalized paper strips for the purpose of visual sensing and removal of trace amounts of Hg2+ ions (Fig. 18c).131 Notably, this nanocomposite exhibits exceptional response towards Hg2+ ions in solution, as evidenced by the impressive detection limit of 3.2 × 10−9 M (Fig. 18d). This remarkable sensitivity is attributed to the reversible formation of gold amalgam between the gold nanoparticles and Hg2+ ions. Moreover, the gold nanochromophores were dispersed with minimal aggregation due to the chitosan and the filter paper, which effectively prevents the ACQ effect. Meanwhile, due to the multiple hydroxyl groups and free amino groups of the chitosan–gold nanocomplex, it was able to remove Hg2+ from solution. Furthermore, the fabrication process of chitosan–gold nanocomplexes is straightforward, making them suitable for repeated utilization in detecting trace amounts of Hg2+ in various environmental aqueous solutions as well as fruit or vegetable juice samples (Fig. 18e).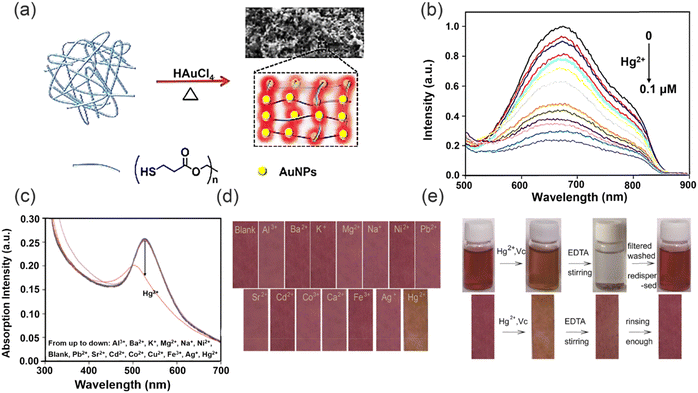 | ||
| Fig. 18 Synthesis of metal-based nanopolymers and its detection-removal for heavy metal ions. (a) The synthesis of the luminescent sponge-like network of AuNPs. (b) The fluorescence spectra of PTMP-AuNPs under different concentrations of Hg2+. Reproduced from ref. 130 with permission from Royal Society of Chemistry. Copyright 2017. (c) The selectivity of the proposed chitosan–AuNP system (d) the color change of chitosan–AuNP functionalized paper-strips. (e) The reversibility of chitosan–AuNP solution and its functionalized paper-strips. Reproduced from ref. 131 with permission from Royal Society of Chemistry. Copyright 2019. | ||
However, a crucial consideration in the utilization of metal nanoparticles for detecting heavy metal ions is the inevitable co-introduction of other metal ions. Additionally, the inherent limitations of high costs and low adsorption capacity pose significant challenges to the advancement of such metal nanomaterials. Addressing these issues is imperative for researchers working towards the treatment and recovery of heavy metal ions, and represents a crucial aspect that requires further improvement in order to enhance the efficacy of these materials.132,133 Xu and co-workers designed an environment-friendly fluorescent sensing platform towards Au3+ detection and removal (Fig. 19a).134 Versatile fluorescent microspheres, specifically melamine formaldehyde microspheres (MF), incorporating nitrogen and sulfur co-doped carbon dots (N,S-CDs), were synthesized via the MF pre-polymer incorporation process. The highly branched MF pre-polymer exhibited remarkable efficacy in efficiently integrating fluorophores under acidic catalysis and elevated heating conditions. Stable fluorescent microspheres (MF-CDs) can be obtained by doping the MF prepolymer with CDs. Fluorescence quenching of the N,S-CDs by Au3+ was observed (Fig. 19b), attributed to the non-radiative electron-transfer process from N,S-CDs to Au3+ ions, facilitated by the strong affinity between Au and the N/S species.135 This remarkable fluorescence sensing ability of the MF-CDs stemmed from the synergistic effect of the amplification of the MF substrate and the collective contribution of N, and S-CDs towards the fluorescence response of the MF-CDs.136–138 Specifically, in an acidic environment, Au3+ primarily exist as AuClxOH(4−x)− in solution while the MF was protonated, promoting electrostatic interactions between Au3+ and the MF matrix,139 in addition to the inherent affinity of gold for nitrogen.140 As a result, Au3+ is captured and enriched on the surface of the MF via enhanced local concentration around the nitrogen and N,S-CDs immobilized on the MF-CDs. Furthermore, the high density of N,S-CDs on the MF-CDs provides abundant binding sites for Au3+, facilitating the interaction of a single Au3+ ion with multiple N,S-CDs and promoting cooperative processes that strengthen fluorescence quenching. Additionally, the MF-CDs exhibit remarkable adsorption capacity for Au3+, with an adsorption capacity of up to 1 mmol g−1 when used as an adsorbent. Notably, the adsorption of Au3+ onto the MF-CDs results in an in situ conversion to AuNPs, leading to the formation of an immobilized nanocatalyst, denoted as MF-CDs-AuNP. This nanocatalyst exhibits remarkable recyclability and efficiently reduces 4-nitrophenol, showcasing its potential for sustainable catalytic applications. In addition, the selectivity of the fluorescence response towards Au3+ indicates superior performance compared to other assessed metal ions. The fluorescent CDs exhibit exceptional sensitivity and rapid response for the detection in real samples, without the use of organic solvents or harmful dyes, which is highly advantageous for wastewater purification, and holds great promise for environmentally sustainable applications in water treatment. Similarly, in 2021, Chai and co-workers developed a novel dual-function Fe3O4–Carbon dots nano-composite (Fe3O4/CDs) based magnetic fluorescent probe for the detection and recovery of Hg2+ through a hydrothermal method (Fig. 19c).141 The Fe3O4/CDs exhibited numerous advantageous features compared to existing single-function probes, including remarkable Hg2+ removal capability, reusability, excellent environmental tolerance, and cost-effectiveness. With this sensing system, Hg2+ can efficiently coordinate onto the surface of Fe3O4/CDs by means of electrostatic interactions and quench the fluorescence signal of Fe3O4/CDs as a result of fluorescence charge transfer (turn-off) (Fig. 19d), with a limit of detection of 0.3 nM. Notably, the inherent magnetic properties of Fe3O4/CDs enabled the removal of Hg2+ from contaminated water, thus conferring significant advantages for environmental remediation applications. Moreover, the Fe3O4/CDs could be recycled by the addition of EDTA solution, highlighting their potential for real sample analysis, thus underscoring the practical utility of this composite material in addressing the pressing issue of Hg2+ contamination in natural bodies of water.
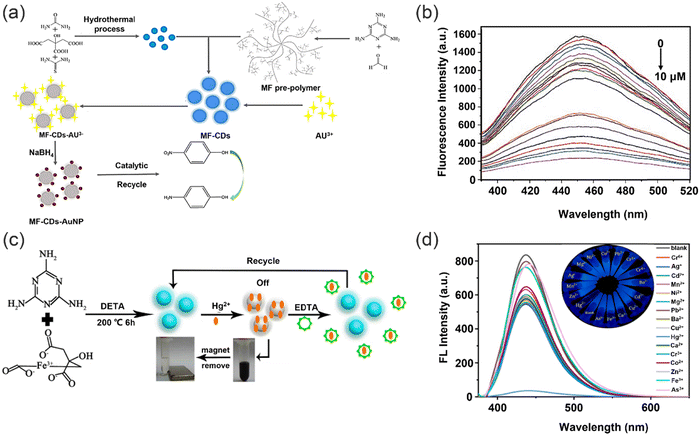 | ||
| Fig. 19 Preparation of polymer sensors and their detection and removal of heavy metal ions. (a) The synthesis and applications of fluorescent microspheres. (b) The effect of pH on the detection of Au3+. Reproduced from ref. 134 with permission from Elsevier B.V. Copyright 2021. (c) The synthesis Fe3O4/CDs and utility in sensing and removal of Hg2+. (d) Fluorescence spectra of probe in presence of different heavy metal ions. Reproduced from ref. 141 with permission from Elsevier Ltd. Copyright 2021. | ||
Moreover, supramolecular polymers containing DNA have been regarded as promising candidates for the selective sensing and efficient removal of heavy metal ions, thereby complementing the use of nanomaterial-based polymers. For example, in 2017, Wu and co-workers reported the construction of a supramolecular system generated by the self-assembly of thymine substituted copolymer aromatics and tetraphenylene (TPE) derivatives in the presence of Hg2+.142 The thymidine group of the copolymer tightly coordinates with Hg2+via the T–Hg2+–T pair. The interaction between the aromatics and the butyronitrile groups of the TPE derivatives forms a crisscrossed fluorescent network structure. In addition, AIE of the supramolecular polymers results in high fluorescence, facilitating the detection and removal of Hg2+ (Fig. 20a) with a limit of detection 2.3 μM, and stable fluorescence from Hg2+ in the presence of competing ions. Which is a testament to the exceptional selectivity of this supramolecular system towards Hg2+. The fluorescence due to Hg2+ was still evident in the presence of other competing ions, confirming remarkable selectivity of this supramolecular system for Hg2+. While the addition of Na2S to the supramolecular polymer allows for recovery of the polymer for re-use. The removal efficiency for Hg2+ can reach more than 96%. Significantly, the system exhibits almost no loss of activity when recycled for the sensing and trapping of Hg2+. In 2019, Yang and co-workers enhanced the properties of the supramolecular polymer, endowing it with rapid reaction rate and superior selectivity, whilst also achieving fast adsorption kinetics and high adsorption capacity towards the efficient removal of Hg2+. A fluorescent supramolecular polymer featuring AIE was fabricated using supramolecular host–guest interactions, which involved a biphenyl-extended pillar arene decorated with two thymine sites as arms (H) and a tetraphenylethylene (TPE)-bridged bis (quaternary ammonium) guests (G).143 Most notably, the method of combining AIE with a supramolecular strategy provides a suitable method for the selective detection (Fig. 20b) and rapid removal of Hg2+ and provides scope for the design of new adsorbent materials. Moreover, the strong bonding of the T–Hg2+–T pair between the thymine group and Hg2+ makes the supramolecular polymer easily generate a supercross-linked network, which results in a multifunctional Hg2+ absorbent. The material can reach more than 90% Hg2+ removal efficiency, and the emission increases linearly with Hg2+ concentration over a range from 0–15 μM. When the concentration of Hg2+ was added gradually, the emission intensity of the supramolecular polymer dramatically increased and the fluorescence was significantly enhanced under UV light irradiation (Fig. 20c) with a detection limit of 3 × 10−7 M.
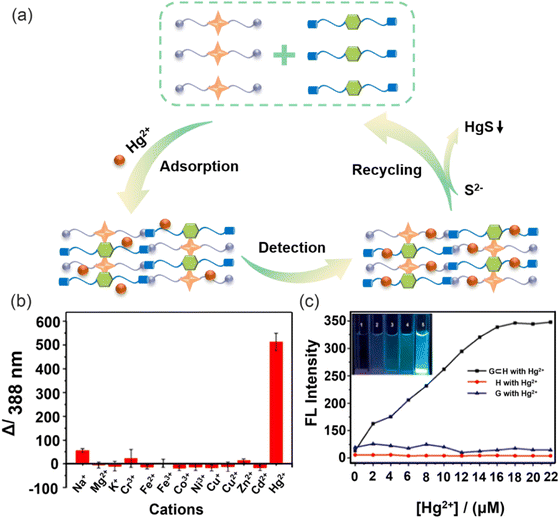 | ||
| Fig. 20 Supramolecular host–guest interactions of biomaterials for the detection and removal of heavy metal ions. (a) Mechanism of polymers for the detection and removal of heavy metal ions. (b) Fluorescence emission of G⊂H with the addition of different metal ions in aqueous solutions. (c) Variation of fluorescence intensity of G⊂H with the increasing concentration of Hg2+. Reproduced from ref. 143 with permission from American Chemical Society. Copyright 2019. | ||
4. Luminescent porous materials
Porous materials are polymer materials with high specific surface area containing networks of interconnected pores. Based on the size of the pores, porous materials can be divided into three categories, which includes micropore materials (less than 2 nm), mesoporous materials (around 2–50 nm), and macropore materials (more than 50 nm),144 which includes zeolites, metal organic frameworks (MOFs), covalent organic frameworks (COFs) and organic polymers.145 Since polymers have been introduced above, they will not be covered in this section. Porous materials exhibit good scalability, long-term stability, selectivity and high adsorption capacity, but also exhibit excellent processability and low cost.146,147 The advantages of porous materials make them suitable for the detection and adsorption of heavy metal ions in water. In the detection and adsorption process, the most significant advantage of COFs and MOFs as luminescent porous materials is that they provide the same interaction site with heavy metal ions, making their interaction with heavy metal ions more effective (Fig. 21).4.1. COF-based materials
Covalent organic frameworks (COFs) are a type of crystalline porous material constructed using organic building blocks.148,149 Because of their excellent chemical/thermal stability, porosity, and flexible topological connectivity, COFs are ideal materials with intrinsic “all-in-one” sensing functionalities for the detection and adsorption of heavy metal ions.150 The controllable pores of COF materials can act as active sites, which are capable of accommodating guest molecules, including heavy metal ions.151 Furthermore, the internal surface of the COF feature diverse functional groups, including amino, carboxyl, or hydroxyl groups,152,153 enabling synergistic interaction with heavy metal ions to form strong coordination bonds. This facilitates the specific and selective binding of heavy metal ions, endowing COF materials with the capability to detect and adsorb heavy metal ions.The synergistic effects of chelating groups in porous COFs helps with efficient detection and removal of heavy metal ions. The properties of COFs are improved by the rational placement of various functional groups in the COF framework. For example, Wang and co-workers designed a thioether-functionalized COF, COF-LZU8, for the selective detection and easy removal of Hg2+.154 Due to the porous structure of COFs, the thioether groups can be uniformly distributed in the pores and can interact with Hg2+ (Fig. 22a). The detection limit for Hg2+ was determined to be 25.0 ppb. The fluorescence spectra of COF-LZU8 in the solid state exhibits a maximum emission band at 460 nm and a quantum yield of 3.5% upon excitation at 390 nm. Since the structure of COFs can avoid the ACQ effect, COF-LZU8 exhibits high fluorescence (Fig. 22b). Moreover, the straight-through channel in the topology of COFs facilitates direct contact between S atoms and Hg2+, thus facilitating efficient heavy metal ion removal. For solutions containing very low concentrations of mercury, COFs can achieve removal rates of over 98%. The stable hydrazone bond in the structure of COF-LZU8 makes it very stable in standard organic solvents and water, which also ensures the effective recovery of COF-LZU8. Yuan and co-workers designed an allyl and hydroxyl functionalized hydrazone-linked AH-COF, for the exclusive detection and effective removal of Hg2+.155 The ordered pore structure of COFs allows the allyl and hydroxyl groups to be uniformly distributed in the COFs framework. In addition, allyl and hydroxyl groups as the exclusive reaction receptor sites for Hg2+ also ensure the detection and removal of Hg2+. Due to the extension of the π-conjugated structure in the AH-COF framework, AH-COF exhibits fluorescence (Fig. 22c) and the fluorescence spectra of AH-COF in acetonitrile solution can reach a fluorescence quantum yield of 3.8% at an excitation wavelength of 420 nm, compared with just 0.9% for the monomers and the corresponding model small-molecule compound. AH-COF was also selective and sensitive to Hg2+ in the presence of competing ions (Fig. 22d). Significantly, AH-COF can be easily recovered and recycled by adding NaBH4.
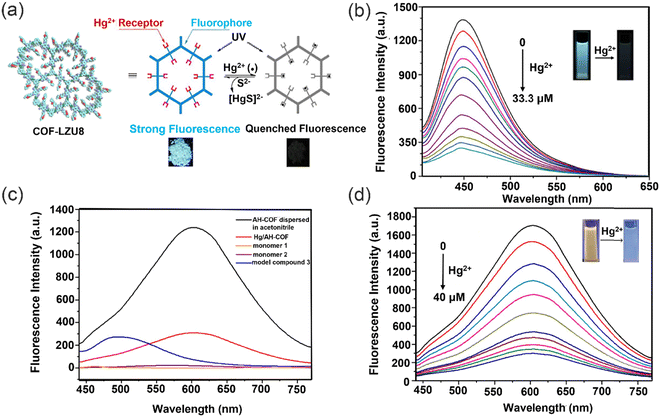 | ||
| Fig. 22 Synergistic effect of chelating groups in porous COFs for the detection and removal of heavy metal ions. (a) The sensing and removing mechanism of COF-LZU8 for Hg2+. (b) Fluorescence intensity of COF-LZU8 with the addition of Hg2+. Reproduced from ref. 154 with permission from American Chemical Society. Copyright 2016. (c) Fluorescence spectra of AH-COF (black) and Hg/AH-COF (red). (d) Fluorescence intensity of AH-COF dispersed in acetonitrile with Hg2+. Reproduced from ref. 155 with permission from Elsevier B.V. Copyright 2020. | ||
Disappointingly, the sensitivity and fluorescence efficiency of COFs in the above examples are relatively low, which can hinder the exploration of COFs for fluorescence sensing. To address this issue, more flexible linkers can be incorporated. In 2019, Qiu and co-workers developed a COF named TFPPy-CHYD by combining 1,3,6,8-tetrakis(4-formylphenyl)-pyrene (TFPPy) with a carbohydrazide (CHYD) linker with highly luminescent properties and a quantum yield of 13.6%.156 The detection limit for Hg2+ was 17 nM and the adsorption capacity for Hg2+ was 758 mg g−1 (Fig. 23a). The π–π stacking between COFs frameworks is affected by the CHYD-linked unit, which enhances the luminescence of TFPPy-CHYD. In addition, the homogeneous porous structure of the COFs allows the secondary amine groups to be abundantly present in the porous structure and to act as mercury receptors. Importantly, the response of TFPPy-CHYD towards Hg2+ is very fast and equilibrium is achieved within 2 s. The uniform channel structure of the COFs, results in rapid diffusion of Hg2+ and facilitates detection and removal of Hg2+. Thermogravimetric analysis and FT-IR spectra of the COFs indicated excellent thermal and chemical stability.
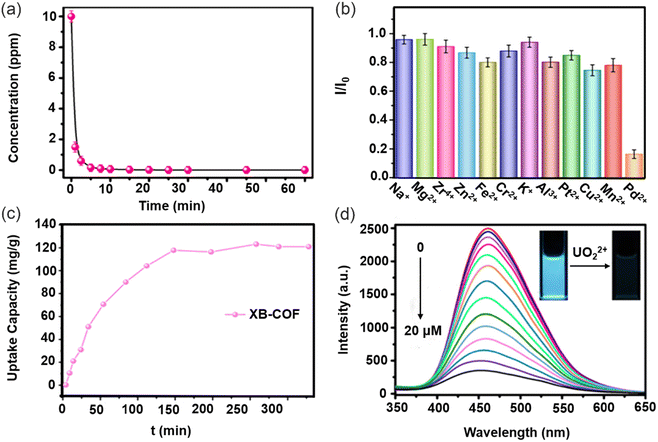 | ||
| Fig. 23 COFs linkers with high sensitivity and fluorescence efficiency. (a) Adsorption kinetics of Hg2+ with TFPPy-CHYD. Reproduced from ref. 156 with permission from American Chemical Society. Copyright 2020. (b) Fluorescence response of XB-COF in presence of different metal ions. (c) Adsorption curves of XB-COF for Pd2+. Reproduced from ref. 157 with permission from American Chemical Society. Copyright 2021. (d) Fluorescence spectra of TFPT–BTAN–AO under different concentrations of UO22+. Reproduced from ref. 159 with permission from Springer Nature. Copyright 2020. | ||
These unique COFs can also detect and remove other metal ions, such as Pb2+, Cr3+, Cu2+, etc. In 2021, Yuan and co-workers reported a new class of 2D COFs (XB-COFs) featuring allyl functionalized hydrazones for the selective and sensitive detection of Pd2+ ions through fluorescence signaling (Fig. 23b).157 The inherent stability of the hydrazone bond and the exceptional complexation ability of allyl towards Pd2+ are instrumental in the high-performance fluorescence sensing exhibited by XB-COFs. Notably, XB-COFs exhibit excellent sensing ability across a broad range of pH values, with Pd2+ adsorption capacity reaching an impressive 120 mg g−1 (Fig. 23c). An increase in fluorescence intensity for XB-COF in solution due to interaction of the Pd2+ ions with the allyl of XB-COF, results in high sensitivity with a limit of detection of 0.29 μM. In addition, due to the widespread use of nuclear power and the improper treatment of nuclear waste and accidents, large amounts of radioactive ions have escaped into the environment.158 Making the exploration of the detection and removal of radioactive ions by COFs an important area of research. In 2020, Qiu and co-workers first developed a cyano-based COF (TFPT-BTAN) by using 2,4,6-tris(4-formylphenyl)-1,3,5-triazine (TFPT) and 2,2′,2′′-(benzene-1,3,5-triyl)triacetonitrile (BTAN) and then integrated triazine-based building blocks with amidoxime (AO)-substituted linkers to obtain a fluorescent sp2 carbon-conjugated COF (TFPT–BTAN–AO) for detecting and removing UO22+.159 TFPT–BTAN–AO exhibited a UO22+ adsorption capacity of 427 mg g−1 and a fast response time with a UO22+ detection limit of 6.7 nM (Fig. 23d). This COF exhibited good luminescence and chemical and thermal stability. Furthermore, the COF exhibited a high selectivity for UO22+ because of the introduction of the amidoxime functional group in the open one-dimensional channel of the COF. More importantly, unlike previous COF adsorbents TFPT–BTAN–AO used a very stable carbon–carbon double bond, which ensures excellent stability of the COFs and the high adsorption capacity of TFPT–BTAN–AO for UO22+.
In conclusion, COFs materials are porous, stable, and have a large adsorption capacity since it is possible to easily introduce specific functional groups to interact with heavy metal ions. The aforementioned benefits render COFs a highly attractive candidate for heavy metal ions sensing and removal applications.148 In addition, COFs with ligand sites can serve as supramolecular ligands and carriers to further enhance the functions of COFs-based materials. However, the structure of 3D COFs is complex and the synthetic conditions of COFs are limited. Therefore, these characteristics need to be improved to further enhance the functionality of COF-based materials.
4.2. MOF-based materials
Metal organic frameworks (MOFs), a type of porous crystalline material that can be self-assembled through the coordination of metal ions or clusters with organic ligands, have attracted significant attention in recent years.160–162 MOFs have the advantages of abundant internal surface area, chemical tunability and selective adsorption of a large number of guests,163,164 allowing MOFs to simultaneously detect and adsorb heavy metal ions in water. For the adsorption of heavy metal ions, MOFs can be regulated by channel functionalization, using a variety of ligands to derive abundant adsorption sites, giving MOFs the capacity to selectively adsorb heavy metal ions in significant amounts. Similar to COFs, MOFs share the same interaction sites for heavy metal ions during the processes of detection and removal. Furthermore, by functionalizing their channels and tuning the pore sizes as well as utilizing diverse ligands, MOFs can acquire abundant adsorption sites and exhibit selective adsorption capabilities towards various heavy metal ions.165Effective fluorescence detection and adsorption of heavy metals ions depends on the selectivity and sensitivity of MOFs. The chelating sites are provided by the MOFs surface groups to facilitate the adsorption of heavy metal ions. For example, Li and co-workers designed and prepared a series of isoreticular luminescent metal–organic frameworks (LMOFs) with Zn-based architectures that incorporate a variety of co-linkers exhibiting diverse functionalities (Fig. 24a).166 Among them, LMOF-263 exhibits good water stability, high porosity and strong luminescence, with an impressive adsorption capacity for Hg2+ of 380 mg g−1, the detection limit for Hg2+ was determined to be 3.3 ppb and 19.7 ppb for Pb2+. LMOF-263 has a high quantum yield (89.2%) and a BET surface area of 1004 m2 g−1, indicating that it can be used as a highly sensitive and selective sensor capable of detecting heavy metal ions in water. Due to the selective interaction of sulfur and oxygen atoms with Hg2+ in LMOF-263, the removal rate of Hg2+ can reach 99.6% (Fig. 24b). However, when LMOF-263 is recycled, its structure shows signs of degradation, indicating the limited water stability of MOFs. Previously, only few MOFs have shown such high performance in detecting and capturing Hg2+ from aqueous solution. Wang and co-workers synthesized a scalable and cost-effective fluorescent MIL-101-NH2 using a simple one-step method (Fig. 24c).167 MIL-101-NH2 exhibited fluorescence detection limits of 0.0018 mM for Fe3+, 0.0016 mM for Cu2+ and 0.0052 mM for Pb2+ (Fig. 24d). The amino group of the MOFs chelates with metal ions to induce host–guest electron transfer, leading to fluorescence quenching, providing the high selectivity and sensitivity of MIL-101-NH2 for metal ion detection. MIL-101-NH2 possesses abundant amino groups on its surface, which serve as multiple chelating sites to interact with heavy metal ions, thereby facilitating the efficient adsorption of Fe3+, Cu2+ and Pb2+, the adsorption capacities for Fe3+, Cu2+ and Pb2+ were 3.5, 0.9 and 1.1 mM g−1 respectively. Moreover, Wang and co-workers developed an integrated electrode approach using sulfur-functionalized Zr-2,5-dimercaptobenzoic acid metal-organic frameworks (Zr-DMBD-MOFs) and coupled them with a three-dimensional microporous carbon (3D-KSC).168 This innovative architecture not only facilitated the efficient removal and detection of Hg2+ but also enhanced the detection sensitivity. The uniform dispersion of abundant Zr-DMBD-MOFs onto the interconnected framework of 3D-KSC, coupled with the presence of numerous thiol functional groups within the porous Zr-DMBD-MOFs, facilitated the selective enrichment of Hg2+ ions on the electrode surface. Significantly, this strategy eliminated potential interference from coexisting heavy metal ions, enabling accurate and precise detection of Hg2+ with a detection limit of 0.05 μM. This pioneering work highlights the potential of the Zr-DMBD-MOFs/3D-KSC integrated electrode as a promising platform for the sensitive and selective detection of Hg2+ ions, thus addressing a crucial need in environmental monitoring and remediation.
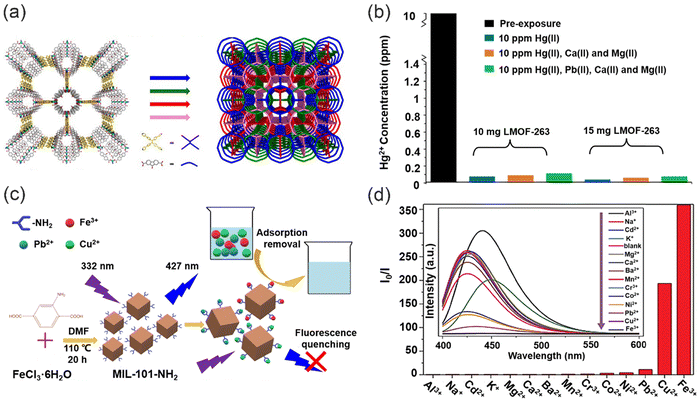 | ||
| Fig. 24 The structure and chelating sites of MOFs interact with heavy metal ions for the detection and adsorption of heavy metal ions. (a) Isoreticular luminescent metal–organic frameworks with Zn-based architectures. (b) The variation of Hg2+ concentrations for various amounts of LMOF-263 in mixed metal solutions. Reproduced from ref. 166 with permission from American Chemical Society. Copyright 2016. (c) The detection and removal of heavy metal ions by MIL-101-NH2. (d) Fluorescence response of MIL-101-NH2 towards various metal ions. Reproduced from ref. 167 with permission from Elsevier B.V. Copyright 2019. | ||
Core–shell MOF@MOF materials, synthesized by combining two MOFs, can integrate different characteristics and functionalities of individual MOFs, providing new functionalities to MOF@MOF structures. Wang and co-workers developed a novel NH2-MIL-101(Al)@ZIF-8 material with a unique core–shell nanoflower architecture by controlling the intramolecular nucleation and crystal growth processes through the regulation of polyvinylpyrrolidone (PVP) (Fig. 25a).169 The large specific surface area (1736.257 m2 g−1), substantial porosity (0.584 cm3 g−1), and uniformity in pore size (1.5 nm) of NH2-MIL-101(Al)@ZIF-8 contribute to its physical adsorption ability and enhance the adsorption performance. Significantly, the adsorption of Cu2+ on the material takes place via monolayer chemisorption between Cu2+ and the evenly distributed active sites on the material surface, while the adsorption capacity for Hg2+ can reach 459.52 mg g−1. Furthermore, the adsorption and detection abilities are both improved by the synergistic effect of NH2-MIL-101(Al)@ZIF-8, where the detection ability for Cu2+ was as low as 0.17 nM (Fig. 25b).
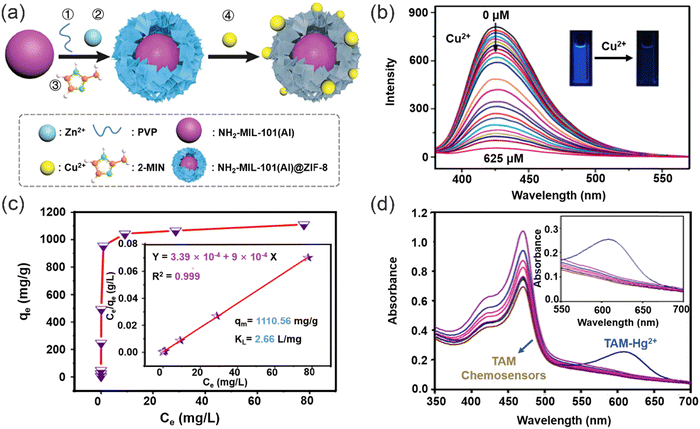 | ||
| Fig. 25 Fluorescence response and removal of heavy metal ions by MOFs with different structures. (a) The synthesis of core–shell NH2-MIL-101(Al)@ZIF-8 nanoflowers for the simultaneous detection and removal of Cu2+ ions. (b) Fluorescence spectra of the NH2-MIL-101(Al)@ZIF-8 suspension with the addition of various concentrations of Cu2+. Reproduced from ref. 169 with permission from Royal Society of Chemistry. Copyright 2018. (c) Langmuir isotherm and the linear relation inset for removal of Hg2+. (d) Effect of Hg2+ on absorbance spectra of TAM chemosensors. Reproduced from ref. 170 with permission from American Chemical Society. Copyright 2020. | ||
To further improve the sensitivity and adsorption capacity of MOFs for heavy metal ions. In 2020, Sewify and co-workers developed thioketone Al-MOFs monitors (TAM) via the direct immobilization of the chromophore on to the microporous surface of Al-MOFs.170 With the addition of Hg2+, a new absorption peak (λ = 600 nm) appeared (Fig. 25d) and the sensor exhibits a detection limit of 0.8 × 10−3 ppm for Hg2+ ions and can reach an adsorption capacity of 1110 mg g−1 for Hg2+ (Fig. 25c). Since organic receptors can be directly immobilized on to the microporous MOF backbone, real-time monitoring efficiency and sensitivity can be improved. Moreover, in the presence of Hg2+, the measured absorption spectra were significantly enhanced compared to other metal ions, confirming the superior selectivity of the Al-MOFs for Hg2+. In 2021, Safaei and co-workers developed a fluorescent MOF, [Zn2(DpTzTz)2 (BDC)2]·2DMF, which was composed of Zn2+, 2,5-di(4-pyridyl)thiazolo[4,5-D]thiazole (DpTzTz), and terephthalic acid. Due to the strong chelating sites of DpTzTz for mercury ions, the [Zn2(DpTzTz)2 (BDC)2]·2DMF exhibits exceptional removal capability with an adsorption capacity of up to 1428 mg g−1.171 Furthermore, coordination of Hg2+ with the sulfur and nitrogen atoms indicates that the incorporation of the rigid conjugated thiazolothiazole (TzTz) functional group, containing Lewis basic nitrogen and sulfur atoms, into the MOF is able to enhance host–guest interactions with Hg2+, enabling the MOF to detect and remove Hg2+.
In conclusion, due to their remarkable properties, including high surface area, large pore size, and abundant adsorption sites, MOFs exhibit great potential for both detecting and removing heavy metal ions with high efficiency. However, future research should be directed towards improving the rate that MOFs can adsorb heavy metal ions from water. Additionally, previous research has shown that the introduction of carbon dots into COF and MOF matrices can enhance their luminescent properties.172,173 The introduction of carbon dots into MOF or COF matrices not only facilitates structural modifications but also serves as a surface modifier, reducing surface defects in the materials, thereby enhancing the optical stability of the composite.174–176 However, although the introduction of carbon dots can improve the luminescent properties, there is still scope for improvement in terms of detection sensitivity and adsorption capacity.
4.3. Porous silica materials
Porous silica is a silica material consisting of interconnected channels and pores with unique properties.177 The shape, size, and distribution of these channels and pores can be precisely controlled through various fabrication methods and processing conditions. Previous research has demonstrated that by immobilizing organic ligands onto mesoporous silica, composite materials can be formed, exhibiting unique properties.178,179 These organic ligands possess selective adsorption and high dispersion capabilities, which combined with the high specific surface area and excellent chemical stability of mesoporous silica,180 enable the composite material to interact with heavy metal ions and other metal ions (Fig. 26).181–183 Therefore, these composite materials exhibit tremendous potential for the detection and adsorption of heavy metal ions.Mesoporous silica is a porous silicon dioxide material with a highly ordered pore structure.184 The hydroxyl groups on its surface can be used to anchor functional organic ligands onto mesoporous silica through reversible covalent bonds and non-covalent interactions such as hydrogen bonding, enabling the detection and adsorption of target ions (Fig. 27a). For example, Awual synthesized a functional ligand, N,N-disalicylidene-4,5-dimethyl-phenylenedene (DDPD), and anchored it onto mesoporous silica for the detection and removal of Cu2+ from wastewater.185 Complexation of Cu2+ ions with functional groups from the organic ligand, facilitated the detection of Cu2+ ions through naked-eye observation or UV-vis absorption measurements (Fig. 27b), with a detection limit of 0.37 μg L−1. Moreover, the immobilization of the organic ligand within highly ordered mesoporous silica provides additional active sites for the interaction between the DDPD material and Cu2+ ions. The structure of mesoporous silica also enhances the adsorption capacity of the composite material for Cu2+, with a maximum adsorption capacity of 183.81 mg g−1. Similarly, in 2023, Awual and co-workers impregnated mesoporous silica with N,N-bis(salicylidene)1,2-bis(2-aminophenylthio) ethane (BSBAE), resulting in an optical composite material (OCM) for the detection and removal of Cd2+.186 Due to the non-covalent interaction between the hydroxyl groups of the mesoporous silica and the BSBAE ligand, as well as the presence of van der Waals forces and reversible covalent bonds, the OCM can interact with Cd2+ ions without disrupting the original structure of mesoporous silica. Furthermore, effective desorption of Cd2+ from the OCM was achieved using HCl, with a desorption efficiency of up to 99%. The OCM exhibited a high recyclability after being rinsed with water (Fig. 27c), highlighting one of the main advantages of mesoporous silica composite materials.
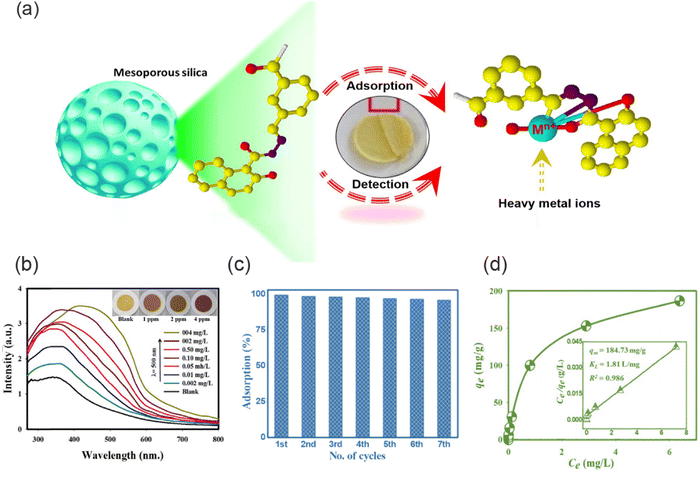 | ||
| Fig. 27 Mechanism of mesoporous silica materials for the detection and removal of heavy metal ions. (a) The mechanism of action of the organic ligand in mesoporous silica. Reproduced from ref. 187 with permission from Elsevier B.V. Copyright 2021. (b) The sensitivity of the composite material at different concentrations of Cu2+. Reproduced from ref. 185 with permission from Elsevier B.V. Copyright 2016. (c) Recycling efficiency of optical composite material. Reproduced from ref. 186 with permission from Elsevier B.V. Copyright 2022. (d) Langmuir isotherm and the linear relation inset for removal of Cu2+. Reproduced from ref. 188 with permission from Elsevier B.V. Copyright 2023. | ||
In addition to enabling effective detection and adsorption of heavy metal ions through the incorporation of specific functional groups, further enhancement of detection sensitivity and adsorption capacity of mesoporous silica materials can be achieved through the optimization of the fabrication methods and material processing.187 For example, Salman and co-workers immobilized the functional organic ligand, 4-tert-octyl-4-((phenyl)diazenyl)phenol, onto mesoporous silica to prepare a composite adsorbent.188 Then by adjusting the pH, a lower detection limit of 0.28 μg L−1 and an increased maximum adsorption capacity of 184.7 mg g−1 for Cu2+ by the material was achieved (Fig. 27d). The pore size, porosity, and surface charge of mesoporous silica may vary under different pH conditions, which can influence the interactions between the mesoporous silica materials and Cu2+. Therefore, optimizing the pH is crucial to achieve the best detection and adsorption performance.
In conclusion, mesoporous silica composite materials exhibit several advantages such as selective adsorption, high adsorption capacity, and recyclability, rendering them highly promising for applications in environmental monitoring and wastewater treatment. Furthermore, the stability of mesoporous silica can be further enhanced, and its adsorption performance can be improved by employing diverse functional group modification strategies and optimizing the structure.
5. Conclusions and perspectives
5.1. Conclusions
In summary, this review provides an overview of recent advancements in the field of advanced functional materials, specifically chromophore-based functional materials, for the detection and removal of heavy metal ions. Based on the synergistic interplay between the chromophores and substrate materials such as polymers and porous materials, the advanced functional materials developed exhibit superior performance for heavy metal ion detection and removal compared to conventional materials. Various types of chromophores endow the substrate materials with excellent heavy metal ion recognition capabilities, outstanding mechanical properties, and enhanced efficiency in metal removal. Meanwhile, the distinctive structure of the substrate materials contributes to the increased stability and environmental sustainability of the chromophores. Through an in-depth analysis of the reviewed work, it is evident that a wide range of sophisticated platforms have been developed to meet the increasing demand for efficient detection and removal of diverse heavy metal ion contaminants. The cutting-edge technologies and innovative strategies discussed in this review have led to remarkable progress in the design of functional materials that are not only convenient, stable, and rapid but also sensitive, environmentally friendly, and recyclable. These materials exhibit great potential for precise detection, even at ultra-low detection limits, and efficient removal of heavy metal ions. The advancements in selectivity and adsorption capacity have significantly enhanced the overall efficiency and practicability of heavy metal ion detection and removal processes. The integration of chromophores with polymers and porous materials, utilizing optical detection and chemisorption methods, has emerged as a promising approach for the treatment of various heavy metal ions pollutants. However, it is important to acknowledge that certain challenges still need to be overcome. Most functional materials can only detect and adsorb specific heavy metal ions. However, wastewater often contains a mixture of heavy metal ions, and the competitive adsorption among them can affect the removal efficiency of functional materials towards specific heavy metal ions. Furthermore, the concept of utilizing functional materials for the simultaneous detection and removal of heavy metal ions is still in its infancy. The preparation and use of these materials involves complex steps, making them unsuitable for real-world wastewater treatment. Although current functional materials exhibit the capability to accomplish these dual tasks, the detection efficiency of these bifunctional materials may not match that of dedicated sensor materials, and their adsorption capacity may be limited compared to conventional adsorbent materials. Moreover, actual wastewater is in a flowing state, and continuous flow can exert a level of “wear and tear” on the materials, leading to damage. Meanwhile, most exhausted functional materials are disposed of by being buried or incinerated, leading to a potential threat to the environment. As such, significant additional research should focus on improving the selectivity, stability of materials towards the detection and removal of heavy metal ions as well as maintaining their sustainability and cost-effectiveness.5.2. Perspectives
The integration of chromophore-based sensors with polymers and porous materials displays significant potential and while remarkable progress has been made for the treatment of heavy metal ion pollution, the sensing and extraction systems are still at an early stage and critical challenges remain, which restricts their further utility in real world applications. These novel heavy metal ion detection and removal systems based on functional materials still need to be improved to provide: (i) enhanced sensing and extraction ability: the systems should be capable of detecting heavy metal ions sensitively in a stable and constant manner, facilitating the detection in drinking water. In addition, among all the heavy metal ions, the coexistence of some cations such as Cd2+, Pb2+, Cu2+, Cr6+ and Hg2+ results in a decrease in the adsorption capacity of the functional material for the target heavy metal ions due to competing adsorption. Therefore, the design of highly selective materials is required which could be achieved by decorating the materials with additional multifunctional groups; (ii) Increasing the stability: although some systems based on non-covalent bonding have been developed for the detection and removal of heavy metal ions, the stability of systems based on non-covalent bonding is susceptible to environmental factors such as temperature and pH when compared to more stable covalent bonding systems. Therefore, the development of more stable systems for the detection and removal of heavy metal ions should be further investigated; (iii) multifunctional systems: most of the prepared systems can only detect or remove a single heavy metal ion, and when multiple heavy metal ions coexist, the detection and removal abilities are reduced, which limits their development for practical applications. Therefore, the rational design of systems capable of detecting and removing various heavy metal ions simultaneously is required to meet the needs of practical systems; (iv) enhanced mechanical performance: the treatment of wastewater necessitates the implementation of continuous flow processes, demanding materials with extraordinary mechanical properties that are well-suited for such applications. Achieving the requisite mechanical stability under dynamic flowing conditions is essential to withstand the high velocities inherent in wastewater treatment. (v) Appropriate dimensions: thicker materials excel in removal efficiency but have reduced sensing capability, while thinner materials exhibit exceptional sensing performance but lower adsorption capacity. As such, precise structural design is essential to achieve enhanced detection and removal of heavy metal ions. (vi) Hybrid design strategy: designs that couple a film detection layer with an adsorbent material layer to achieve high-efficiency detection and adsorption. (vii) Sustainability: eco-friendly materials are required to facilitate the development of green chemistry practices. Recyclable and degradable materials like cellulose, chitosan and lignin have attracted great interest due to their abundance in nature and they are cheap and readily available. Systems constructed based on these materials for the simultaneous detection and removal of heavy metal ions possessing outstanding stability and reusability are superior to other common materials; (viii) smart phone-based on-site analysis systems: traditional laboratory-based analyses, which involves complex sample preparation, specialized personnel and/or expensive instrumentation, are unsuitable for simple and comprehensive environmental monitoring. Therefore, reliable, cost-effective, and easy-to-use multifunctional and portable quantification systems need to be developed; (ix) safe reuse or disposal of used materials: currently, most of the materials are discarded and disposed of after the detection and removal of heavy metal ions. Therefore, to avoid secondary pollution, more advanced disposal or up-cycling methods need to be developed.189As a final thought, although the sensing platforms based on the materials discussed in this review still face many challenges, it is gratifying to note that current research provides hope for the construction of practical systems in the not-too-distant future. Systems with catalytic decontamination abilities are particularly interesting and deserve concerted development, since they have the ability to convert pollutants to harmless or low toxicity species. In summary we anticipate that with the continuous development of chemical materials and detection strategies, that advanced functional systems will be developed for the treatment of various heavy metal ions enabling improved environmental remediation and clean drinking water for everyone. Such progress is expected to continue in the coming years, enabling us to address the challenges of heavy metal ion contamination with ever increasing efficacy and efficiency.
Conflicts of interest
T. D. J. was a High-Level Foreign Expert of North China Electric Power University during the preparation of this review.Acknowledgements
This research is supported by the National Natural Science Foundation of China (No. 21607044, 51878273), and the Fundamental Research Funds for the Central Universities (No. 2023MS146). T. D. J. wishes to thank the Royal Society for a Wolfson Research Merit Award and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01).References
- S. Bolisetty, M. Peydayesh and R. Mezzenga, Chem. Soc. Rev., 2019, 48, 463–487 RSC.
- C. F. Carolin, P. S. Kumar, A. Saravanan, G. J. Joshiba and M. Naushad, J. Environ. Chem. Eng., 2017, 5, 2782–2799 CrossRef CAS.
- D. J. Sarkar, S. Das Sarkar, B. K. Das, B. K. Sahoo, A. Das, S. K. Nag, R. K. Manna, B. K. Behera and S. Samanta, Water Res., 2021, 192, 116853 CrossRef CAS PubMed.
- K. Vikrant and K.-H. Kim, Chem. Eng. J., 2019, 358, 264–282 CrossRef CAS.
- R. Li, H. Wu, J. Ding, W. Fu, L. Gan and Y. Li, Sci. Rep., 2017, 7, 46545 CrossRef CAS PubMed.
- A. B. Tabrizi, J. Hazard. Mater., 2007, 139, 260–264 CrossRef CAS PubMed.
- M. R. Awual, M. Ismael, T. Yaita, S. A. El-Safty, H. Shiwaku, Y. Okamoto and S. Suzuki, Chem. Eng. J., 2013, 222, 67–76 CrossRef CAS.
- C. Liu, R. Bai and Q. S. Ly, Water Res., 2008, 42, 1511–1522 CrossRef CAS PubMed.
- Y. Al-Degs, M. A. M. Khraisheh and M. F. Tutunji, Water Res., 2001, 35, 3724–3728 CrossRef CAS PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210–3244 RSC.
- C. Guo and J. Irudayaraj, Anal. Chem., 2011, 83, 2883–2889 CrossRef CAS PubMed.
- M. K. Nazeeruddin, D. Di Censo, R. Humphry-Baker and M. Gratzel, Adv. Funct. Mater., 2006, 16, 189–194 CrossRef CAS.
- G. H. Chen, Sep. Purif. Technol., 2004, 38, 11–41 CrossRef CAS.
- M. Li, N. Chen, H. Shang, C. Ling, K. Wei, S. Zhao, B. Zhou, F. Jia, Z. Ai and L. Zhang, Environ. Sci. Technol., 2022, 56, 10945–10953 CrossRef CAS PubMed.
- S. Su, B. Chen, M. He and B. Hu, Talanta, 2014, 123, 1–9 CrossRef CAS PubMed.
- F. A. Aydin and M. Soylak, J. Hazard. Mater., 2010, 173, 669–674 CrossRef CAS PubMed.
- T. G. Kazi, N. Jalbani, M. B. Arain, M. K. Jamali, H. I. Afridi, R. A. Sarfraz and A. Q. Shah, J. Hazard. Mater., 2009, 163, 302–307 CrossRef CAS PubMed.
- M. Tüzen, Food Chem., 2003, 80, 119–123 CrossRef.
- Y. Fang, Y. Zhang, L. Cao, J. Yang, M. Hu, Z. Pang and J. He, ACS Appl. Mater. Interfaces, 2020, 12, 11761–11768 CrossRef CAS PubMed.
- T. Kondo, W. J. Chen and G. S. Schlau-Cohen, Chem. Rev., 2017, 117, 860–898 CrossRef CAS PubMed.
- E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443–3480 CrossRef CAS PubMed.
- R. Freeman, T. Finder and I. Willner, Angew. Chem., Int. Ed., 2009, 48, 7818–7821 CrossRef CAS PubMed.
- Z.-H. Lin, G. Zhu, Y. S. Zhou, Y. Yang, P. Bai, J. Chen and Z. L. Wang, Angew. Chem., Int. Ed., 2013, 52, 5065–5069 CrossRef CAS PubMed.
- C. Zhao, G. Liu, Q. Tan, M. Gao, G. Chen, X. Huang, X. Xu, L. Li, J. Wang, Y. Zhang and D. Xu, J. Adv. Res., 2023, 44, 53–70 CrossRef CAS PubMed.
- E. S. Dragan, Chem. Eng. J., 2014, 243, 572–590 CrossRef CAS.
- E. A. Gendy, J. Ifthikar, J. Ali, D. T. Oyekunle, Z. Elkhlifia, I. I. Shahib, A. I. Khodair and Z. Chen, J. Environ. Chem. Eng., 2021, 9, 105687 CrossRef CAS.
- M. Hasan, M. A. Shenashen, M. N. Hasan, H. Znad, M. S. Salman and R. Awual, J. Mol. Liq., 2021, 323, 114587 CrossRef CAS.
- M. S. Salman, M. N. Hasan, K. T. Kubra and M. M. Hasan, Microchem. J., 2021, 162, 105868 CrossRef CAS.
- B. Ramalingam, T. Parandhaman, P. Choudhary and S. K. Das, ACS Sustainable Chem. Eng., 2018, 6, 6328–6341 CrossRef CAS.
- S. Biswas and R. Biswas, Chemosphere, 2023, 312, 137187 CrossRef CAS PubMed.
- L. Zhou, Y. Lin, Z. Huang, J. Ren and X. Qu, Chem. Commun., 2012, 48, 1147–1149 RSC.
- T. Liu, J. X. Dong, S. G. Liu, N. Li, S. M. Lin, Y. Z. Fan, J. L. Lei, H. Q. Luo and N. B. Li, J. Hazard. Mater., 2017, 322, 430–436 CrossRef CAS PubMed.
- L. Wang, Y. Wang, W. Li, W. Zhi, Y. Liu, L. Ni and Y. Wang, ACS Appl. Mater. Interfaces, 2019, 11, 40575–40584 CrossRef CAS PubMed.
- W. Wang, N.-K. Wong, M. Sun, C. Yan, S. Ma, Q. Yang and Y. Li, ACS Appl. Mater. Interfaces, 2015, 7, 8868–8875 CrossRef CAS PubMed.
- K. T. Kubra, M. S. Salman, H. Znad and M. N. Hasan, J. Mol. Liq., 2021, 329, 115541 CrossRef CAS.
- M. R. Awual, M. N. Hasan, M. M. Hasan, M. S. Salman, M. C. Sheikh, K. T. Kubra, M. S. Islam, H. M. Marwani, A. Islam, M. A. Khaleque, R. M. Waliullah, M. S. Hossain, A. I. Rasee, A. I. Rehan and M. E. Awual, Sep. Purif. Technol., 2023, 319, 124088 CrossRef CAS.
- M. S. Salman, M. C. Sheikh, M. M. Hasan, M. N. Hasan, K. T. Kubra, A. I. Rehan, M. E. Awual, A. I. Rasee, R. M. Waliullah, M. S. Hossain, M. A. Khaleque, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, Appl. Surf. Sci., 2023, 622, 157008 CrossRef CAS.
- A. Islam, S. H. Teo, Y. H. Taufiq-Yap, C. H. Ng, D.-V. N. Vo, M. L. Ibrahim, M. M. Hasan, M. A. R. Khan, A. S. M. Nur and M. R. Awual, Resour. Conserv. Recy., 2021, 175, 105849 CrossRef CAS.
- M. T. Chorsi, E. J. Curry, H. T. Chorsi, R. Das, J. Baroody, P. K. Purohit, H. Ilies and T. D. Nguyen, Adv. Mater., 2019, 31, 1802084 CrossRef PubMed.
- D. Zhang, Q. Chen, C. Shi, M. Chen, K. Ma, J. Wan and R. Liu, Adv. Funct. Mater., 2021, 31, 2007226 CrossRef CAS.
- B. K. Rani and S. A. John, J. Hazard. Mater., 2018, 343, 98–106 CrossRef CAS PubMed.
- S.-Y. Yu and S.-P. Wu, Sens. Actuators, B, 2014, 201, 25–30 CrossRef CAS.
- J. Luo, Z. Xie, J. W. Lam, L. Cheng, H. Chen, C. Qiu, H. S. Kwok, X. Zhan, Y. Liu and D. J. C. C. Zhu, Chem. Commun., 2001, 1740–1741 RSC.
- W.-M. Wang, D. Dai, J.-R. Wu, C.-Y. Wang, Y. Wang and Y.-W. Yang, Chem. – Eur. J., 2021, 27, 11879–11887 CrossRef CAS PubMed.
- H.-F. Xie, C.-J. Yu, Y.-L. Huang, H. Xu, Q.-L. Zhang, X.-H. Sun, X. Feng and C. Redshaw, Mater. Chem. Front., 2020, 4, 1500–1506 RSC.
- K. Rout, A. K. Manna, M. Sahu, J. Mondal, S. K. Singh and G. K. Patra, RSC Adv., 2019, 9, 25919–25931 RSC.
- B. Yuan, D.-X. Wang, L.-N. Zhu, Y.-L. Lan, M. Cheng, L.-M. Zhang, J.-Q. Chu, X.-Z. Li and D.-M. Kong, Chem. Sci., 2019, 10, 4220–4226 RSC.
- N. Sinha, L. Stegemann, T. T. Y. Tan, N. L. Doltsinis, C. A. Strassert and F. E. Hahn, Angew. Chem., Int. Ed., 2017, 56, 2785–2789 CrossRef CAS PubMed.
- D. Qi, J. Zhang, D. Zhang, M. Zhu, L. Gong, C. Su, W. Lu, Y. Bian and J. Jiang, Dyes Pigm., 2020, 173, 107941 CrossRef CAS.
- Y. Chen, W. Zhang, Y. Cai, R. T. K. Kwok, Y. Hu, J. W. Y. Lam, X. Gu, Z. He, Z. Zhao, X. Zheng, B. Chen, C. Gui and B. Z. Tang, Chem. Sci., 2017, 8, 2047–2055 RSC.
- K. Gupta, P. Joshi, R. Gusain and O. P. Khatri, Coord. Chem. Rev., 2021, 445, 214100 CrossRef CAS.
- R. Bhateria and R. Singh, J. Water Process Eng., 2019, 31, 100845 CrossRef.
- R. Baby, B. Saifullah and M. Z. Hussein, Nanoscale Res. Lett., 2019, 14, 1–17 CrossRef CAS PubMed.
- R. Gusain, N. Kumar and S. S. Ray, Coord. Chem. Rev., 2020, 405, 213111 CrossRef CAS.
- N. Bhardwaj and S. C. Kundu, Biotechnol. Adv., 2010, 28, 325–347 CrossRef CAS PubMed.
- D. Chen, K. Jiang, T. Huang and G. Shen, Adv. Mater., 2020, 32, 1901806 CrossRef CAS PubMed.
- G. Aragay, J. Pons and A. Merkoci, Chem. Rev., 2011, 111, 3433–3458 CrossRef CAS PubMed.
- E. Vunain, A. K. Mishra and B. B. Mamba, Int. J. Biol. Macromol., 2016, 86, 570–586 CrossRef CAS PubMed.
- J. Y. Lim, N. M. Mubarak, E. C. Abdullah, S. Nizamuddin, M. Khalid and Inamuddin, J. Ind. Eng. Chem., 2018, 66, 29–44 CrossRef CAS.
- X. Wang, Z.-J. Liu, E. H. Hill, Y. Zheng, G. Guo, Y. Wang, P. S. Weiss, J. Yu and Y.-W. Yang, Matter, 2019, 1, 848–861 CrossRef.
- X. Chen and S. S. Mao, Chem. Rev., 2007, 107, 2891–2959 CrossRef CAS PubMed.
- H. Li, D.-X. Chen, Y.-L. Sun, Y. B. Zheng, L.-L. Tan, P. S. Weiss and Y.-W. Yang, J. Am. Chem. Soc., 2013, 135, 1570–1576 CrossRef CAS PubMed.
- H. Li, L.-L. Tan, P. Jia, Q.-L. Li, Y.-L. Sun, J. Zhang, Y.-Q. Ning, J. Yu and Y.-W. Yang, Chem. Sci., 2014, 5, 2804–2808 RSC.
- R. Deng, N. Shen, Y. Yang, H. Yu, S. Xu, Y.-W. Yang, S. Liu, K. Meguellati and F. Yan, Biomaterials, 2018, 167, 80–90 CrossRef CAS PubMed.
- S. Chatterjee, X.-Y. Lou, F. Liang and Y.-W. Yang, Coord. Chem. Rev., 2022, 459, 214461 CrossRef CAS.
- Q. Niu, P. Gao, M. Yuan, G. Zhang, Y. Zhou, C. Dong, S. Shuang and Y. Zhang, Microchem. J., 2019, 146, 1140–1149 CrossRef CAS.
- Y. Liu, Y. Liu, L. Xu, J. Li, X. Liu, J. Liu and G. Li, Sens. Actuators, B, 2017, 249, 331–338 CrossRef CAS.
- G. Chen, J. Hai, H. Wang, W. Liu, F. Chen and B. Wang, Nanoscale, 2017, 9, 3315–3321 RSC.
- J. Zhou, H. Zhou, J. Tang, S. Deng, F. Yan, W. Li and M. Qu, Microchim. Acta, 2017, 184, 343–368 CrossRef CAS.
- P. Zuo, X. Lu, Z. Sun, Y. Guo and H. He, Microchim. Acta, 2016, 183, 519–542 CrossRef CAS.
- Z. Wang, C. Xu, Y. Lu, X. Chen, H. Yuan, G. Wei, G. Ye and J. Chen, Sens. Actuators, B, 2017, 241, 1324–1330 CrossRef CAS.
- S. Li, J. Luo, G. Yin, Z. Xu, Y. Le, X. Wu, N. Wu and Q. Zhang, Sens. Actuators, B, 2015, 206, 14–21 CrossRef CAS.
- E. F. C. Simoes, J. M. M. Leitao and J. C. G. Esteves da Silva, Microchim. Acta, 2016, 183, 1769–1777 CrossRef CAS.
- T. Hao, X. Wei, Y. Nie, Y. Xu, Y. Yan and Z. Zhou, Microchim. Acta, 2016, 183, 2197–2203 CrossRef CAS.
- W. Yao, N. Wu, Z. Lin, J. Chen, S. Li, S. Weng, L. Zhang, A. Liu and X. Lin, Microchim. Acta, 2017, 184, 907–914 CrossRef CAS.
- S. N. Baker and G. A. Baker, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- H. Li, Z. Kang, Y. Liu and S.-T. Lee, J. Mater. Chem., 2012, 22, 24230–24253 RSC.
- M. Zheng, Z. Xie, D. Qu, D. Li, P. Du, X. Jing and Z. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 13242–13247 CrossRef CAS PubMed.
- R. Zhang and W. Chen, Biosens. Bioelectron., 2014, 55, 83–90 CrossRef CAS PubMed.
- Y. Zhai, B. Zhang, R. Shi, S. Zhang, Y. Liu, B. Wang, K. Zhang, G. I. N. Waterhouse, T. Zhang and S. Lu, Adv. Energy Mater., 2022, 12, 2103426 CrossRef CAS.
- F. Limosani, E. M. Bauer, D. Cecchetti, S. Biagioni, V. Orlando, R. Pizzoferrato, P. Prosposito and M. Carbone, Nanomaterials, 2021, 11, 2249 CrossRef CAS PubMed.
- J. Zhao, C. Li, X. Du, Y. Zhu, S. Li, X. Liu, C. Liang, Q. Yu, L. Huang and K. Yang, Small, 2022, 18, 2200744 CrossRef CAS PubMed.
- A. Sekar, R. Yadav and N. Basavaraj, New J. Chem., 2021, 45, 2326–2360 RSC.
- W. Meng, X. Bai, B. Wang, Z. Liu, S. Lu and B. Yang, Energy Environ. Mater., 2019, 2, 172–192 CrossRef CAS.
- Y. Liu, Q. Zhou, J. Li, M. Lei and X. Yan, Sens. Actuators, B, 2016, 237, 597–604 CrossRef CAS.
- Q. Liu, N. Zhang, H. Shi, W. Ji, X. Guo, W. Yuan and Q. Hu, New J. Chem., 2018, 42, 3097–3101 RSC.
- Z. Ma, H. Ming, H. Huang, Y. Liu and Z. Kang, New J. Chem., 2012, 36, 861–864 RSC.
- Y. Xie, D. Cheng, X. Liu and A. Han, Sensors, 2019, 19, 3169 CrossRef CAS PubMed.
- Z. Y. Liu, W. Y. Jin, F. X. Wang, T. C. Li, J. F. Nie, W. C. Xiao, Q. Zhang and Y. Zhang, Sens. Actuators, B, 2019, 296, 126698 CrossRef CAS.
- S. Wen, T. Zeng, L. Liu, K. Zhao, Y. Zhao, X. Liu and H.-C. Wu, J. Am. Chem. Soc., 2011, 133, 18312–18317 CrossRef CAS PubMed.
- L. Tan, Z. Chen, C. Zhang, X. Wei, T. Lou and Y. Zhao, Small, 2017, 13, 1603370 CrossRef PubMed.
- Y. Li, H. Gao, Z. Qi, Z. Huang, L. Ma and J. Liu, Angew. Chem., Int. Ed., 2021, 60, 12985–12991 CrossRef CAS PubMed.
- W.-M. Wang, D. Dai, J.-R. Wu, C. Wang, Y. Wang and Y.-W. Yang, Dyes Pigm., 2022, 207, 110712 CrossRef CAS.
- D. P. Dubal, N. R. Chodankar, D.-H. Kim and P. Gomez-Romero, Chem. Soc. Rev., 2018, 47, 2065–2129 RSC.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- D. Dai, J. Yang, Y. Wang and Y. W. Yang, Adv. Funct. Mater., 2020, 31, 2006168 CrossRef.
- S. H. Byun, J. W. Chung and S. Y. Kwak, J. Water Process Eng., 2019, 29, 100757 CrossRef.
- K. N. Han, B. Y. Yu and S.-Y. Kwak, J. Membr. Sci., 2012, 396, 83–91 CrossRef CAS.
- W.-P. Zhu, J. Gao, S.-P. Sun, S. Zhang and T.-S. Chung, J. Membr. Sci., 2015, 487, 117–126 CrossRef CAS.
- E. Ferrari, E. Ranucci, U. Edlund and A.-C. Albertsson, J. Appl. Polym. Sci., 2014, 132, 41695 Search PubMed.
- A. Maleki, B. Hayati, F. Najafi, F. Gharibi and S. W. Joo, J. Mol. Liq., 2016, 224, 95–104 CrossRef CAS.
- M. Sajid, M. K. Nazal, Ihsanullah, N. Baig and A. M. Osman, Sep. Purif. Technol., 2018, 191, 400–423 CrossRef CAS.
- H. Yoo and S.-Y. Kwak, J. Membr. Sci., 2013, 448, 125–134 CrossRef CAS.
- J. Y. Sum, A. L. Ahmad and B. S. Ooi, J. Membr. Sci., 2014, 466, 183–191 CrossRef CAS.
- Y.-J. Tang, Z.-L. Xu, B.-Q. Huang, Y.-M. Wei and H. Yang, RSC Adv., 2016, 6, 45585–45594 RSC.
- M. Li, Z. Liu, S. Wang, D. G. Calatayud, W.-H. Zhu, T. D. James, L. Wang, B. Mao and H.-N. Xiao, Chem. Commun., 2018, 54, 184–187 RSC.
- H. Shao, D. Yin, D. Li, Q. Ma, W. Yu and X. Dong, ACS Appl. Mater. Interfaces, 2021, 13, 49288–49300 CrossRef CAS PubMed.
- M. Li, X. An, M. Jiang, S. Li, S. Liu, Z. Chen and H. Xiao, ACS Sustainable Chem. Eng., 2019, 7, 15182–15189 CrossRef CAS.
- M. Khan and I. M. C. Lo, J. Hazard. Mater., 2017, 322, 195–204 CrossRef CAS PubMed.
- W. L. Yan and R. B. Bai, Water Res., 2005, 39, 688–698 CrossRef CAS PubMed.
- M. Li, X. Li, M. Xu, B. Liu, M. Yang, Z. Chen, T. Gao, T. D. James, L. Wang and H. Xiao, Chem. Eng. J., 2021, 426, 131296 CrossRef CAS.
- A. Alam, Y. Zhang, H.-C. Kuan, S.-H. Lee and J. Ma, Prog. Polym. Sci., 2018, 77, 1–18 CrossRef CAS.
- J. Xu, X. Jie, F. Xie, H. Yang, W. Wei and Z. Xia, Nano Res., 2018, 11, 3648–3657 CrossRef CAS.
- X. Guo, D. Xu, H. Yuan, Q. Luo, S. Tang, L. Liu and Y. Wu, J. Mater. Chem. A, 2019, 7, 27081–27088 RSC.
- R. Liu, L. Dai, C. Si and Z. Zeng, Carbohydr. Polym., 2018, 195, 63–70 CrossRef CAS PubMed.
- Q. Yao, B. Fan, Y. Xiong, C. Jin, Q. Sun and C. Sheng, Sci. Rep., 2017, 7, 45914 CrossRef CAS PubMed.
- Q. Luo, X. Huang, Y. Luo, H. Yuan, T. Ren, X. Li, D. Xu, X. Guo and Y. Wu, Chem. Eng. J., 2021, 407, 127050 CrossRef CAS.
- F. Cheng, S. Zhang, L. Zhang, J. Sun and Y. Wu, Colloids Surf., A, 2022, 636, 128149 CrossRef CAS.
- X.-C. Yang, Y.-L. Yang, M.-M. Xu, S.-S. Liang, X.-L. Pu, J.-F. Hu, Q.-L. Li, J.-T. Zhao and Z.-J. Zhang, ACS Appl. Nano Mater., 2021, 4, 13986–13994 CrossRef CAS.
- J. Yang, Y. Li, Y. Zheng, Y. Xu, Z. Zheng, X. Chen and W. Liu, Small, 2019, 15, 1902826 CrossRef CAS PubMed.
- H. Maleki, Chem. Eng. J., 2016, 300, 98–118 CrossRef CAS.
- H. Maleki and N. Husing, Appl. Catal., B, 2018, 221, 530–555 CrossRef CAS.
- S. Zhao, W. J. Malfait, N. Guerrero-Alburquerque, M. M. Koebel and G. Nystroem, Angew. Chem., Int. Ed., 2018, 57, 7580–7608 CrossRef CAS PubMed.
- L. Zhi, W. Zuo, F. Chen and B. Wang, ACS Sustainable Chem. Eng., 2016, 4, 3398–3408 CrossRef CAS.
- H. Yuan, G. Yang, Q. Luo, T. Xiao, Y. Zuo, X. Guo, D. Xu and Y. Wu, Environ. Sci.: Nano, 2020, 7, 773–781 RSC.
- Z. Song, X. Chen, X. Gong, X. Gao, Q. Dai, T. T. Nguyen and M. Guo, Opt. Mater., 2020, 100, 109642 CrossRef CAS.
- L. Jing, S. Yang, X. Li, Y. Jiang, J. Lou, Z. Liu, Q. Ding and W. Han, Ind. Crop. Prod., 2022, 182, 114882 CrossRef CAS.
- R. Li, W. Liang, M. Li, S. Jiang, H. Huang, Z. Zhang, J. J. Wang and M. K. Awasthi, Int. J. Biol. Macromol., 2017, 104, 1072–1081 CrossRef CAS PubMed.
- X. Li, M. Li, Q. Shi, H. Guo, L. Wang, X. Guo, Z. Chen, J. L. Sessler, H. Xiao and T. D. James, Small, 2022, 18, 2201949 CrossRef CAS PubMed.
- J. Zhu, Q. Lu, C. Chen, J. Hu and J. Liu, J. Mater. Chem. C, 2017, 5, 6917–6922 RSC.
- L. Hu, B. Zhu, L. Zhang, H. Yuan, Q. Zhao and Z. Yan, Analyst, 2019, 144, 474–480 RSC.
- B. Kaur, N. Kaur and S. Kumar, Coord. Chem. Rev., 2018, 358, 13–69 CrossRef CAS.
- Y.-W. Lin, C.-C. Huang and H.-T. Chang, Analyst, 2011, 136, 863–871 RSC.
- S. Qin, X. Yu and L. Xu, J. Hazard. Mater., 2021, 405, 123978 CrossRef CAS PubMed.
- Z. Rahmani and M. Ghaemy, Opt. Mater., 2019, 97, 109356 CrossRef CAS.
- D. Xie, Y. Ma, Y. Gu, H. Zhou, H. Zhang, G. Wang, Y. Zhang and H. Zhao, J. Mater. Chem. A, 2017, 5, 23794–23804 RSC.
- M. Montalti, L. Prodi and N. Zaccheroni, J. Mater. Chem., 2005, 15, 2810–2814 RSC.
- R. Tian, Y. Qu and X. Zheng, Anal. Chem., 2014, 86, 9114–9121 CrossRef CAS PubMed.
- X. Huang, Y. Wang, X. Liao and B. Shi, J. Hazard. Mater., 2010, 183, 793–798 CrossRef CAS PubMed.
- S. Qin, L.-y Ma, X. Sun, X. Mao and L. Xu, J. Hazard. Mater., 2019, 366, 529–537 CrossRef CAS PubMed.
- R. Xie, Y. Qu, M. Tang, J. Zhao, S. Chua, T. Li, F. Zhang, A. E. H. Wheatley and F. Chai, Food Chem., 2021, 364, 130366 CrossRef CAS PubMed.
- H.-B. Cheng, Z. Li, Y.-D. Huang, L. Liu and H.-C. Wu, ACS Appl. Mater. Interfaces, 2017, 9, 11889–11894 CrossRef CAS PubMed.
- D. Dai, Z. Li, J. Yang, C. Wang, J.-R. Wu, Y. Wang, D. Zhang and Y.-W. Yang, J. Am. Chem. Soc., 2019, 141, 4756–4763 CrossRef CAS PubMed.
- I. Hisaki, C. Xin, K. Takahashi and T. Nakamura, Angew. Chem., Int. Ed., 2019, 58, 11160–11170 CrossRef CAS PubMed.
- M. A. Little and A. I. Cooper, Adv. Funct. Mater., 2020, 30, 1909842 CrossRef CAS.
- G. Singh, J. Lee, A. Karakoti, R. Bahadur, J. Yi, D. Zhao, K. AlBahily and A. Vinu, Chem. Soc. Rev., 2020, 49, 4360–4404 RSC.
- A. G. Slater and A. I. Cooper, Science, 2015, 348, aaa8075 CrossRef PubMed.
- Q. Guan, L.-L. Zhou and Y.-B. Dong, Chem. Soc. Rev., 2022, 51, 6307–6416 RSC.
- D. Rodriguez-San-Miguel, C. Montoro and F. Zamora, Chem. Soc. Rev., 2020, 49, 2291–2302 RSC.
- S. Yuan, X. Li, J. Zhu, G. Zhang, P. Van Puyvelde and B. Van der Bruggen, Chem. Soc. Rev., 2019, 48, 2665–2681 RSC.
- Y. Zhang, H. Li, J. Chang, X. Guan, L. Tang, Q. Fang, V. Valtchev, Y. Yan and S. Qiu, Small, 2021, 17, 2006112 CrossRef CAS PubMed.
- H.-L. Qian, F.-L. Meng, C.-X. Yang and X.-P. Yan, Angew. Chem., Int. Ed., 2020, 59, 17607–17613 CrossRef CAS PubMed.
- G. Li, J. Ye, Q. Fang and F. Liu, Chem. Eng. J., 2019, 370, 822–830 CrossRef CAS.
- S.-Y. Ding, M. Dong, Y.-W. Wang, Y.-T. Chen, H.-Z. Wang, C.-Y. Su and W. Wang, J. Am. Chem. Soc., 2016, 138, 3031–3037 CrossRef CAS PubMed.
- Y. X. Yu, G. L. Li, J. H. Liu and D. Q. Yuan, Chem. Eng. J., 2020, 401, 126139 CrossRef CAS.
- W.-R. Cui, W. Jiang, C.-R. Zhang, R.-P. Liang, J. Liu and J.-D. Qiu, ACS Sustainable Chem. Eng., 2020, 8, 445–451 CrossRef CAS.
- Y. Lu, Y. Liang, Y. Zhao, M. Xia, X. Liu, T. Shen, L. Feng, N. Yuan and Q. Chen, ACS Appl. Mater. Interfaces, 2021, 13, 1644–1650 CrossRef CAS PubMed.
- X. H. Xiong, Z. W. Yu, L. L. Gong, Y. Tao, Z. Gao, L. Wang, W. H. Yin, L. X. Yang and F. Luo, Adv. Sci., 2019, 6, 1900547 CrossRef PubMed.
- W.-R. Cui, C.-R. Zhang, W. Jiang, F.-F. Li, R.-P. Liang, J. Liu and J.-D. Qiu, Nat. Commun., 2020, 11, 436 CrossRef CAS PubMed.
- X. Li, Y. Liu, J. Wang, J. Gascon, J. Li and B. Van der Bruggen, Chem. Soc. Rev., 2017, 46, 7124–7144 RSC.
- M. Mon, R. Bruno, E. Tiburcio, M. Viciano-Chumillas, L. H. G. Kalinke, J. Ferrando-Soria, D. Armentano and E. Pardo, J. Am. Chem. Soc., 2019, 141, 13601–13609 CrossRef CAS PubMed.
- S. Wu, H. Min, W. Shi and P. Cheng, Adv. Mater., 2020, 32, 1805871 CrossRef CAS PubMed.
- M. Woellner, S. Hausdorf, N. Klein, P. Mueller, M. W. Smith and S. Kaskel, Adv. Mater., 2018, 30, 1704679 CrossRef PubMed.
- X. Jia, M. Peydayesh, Q. Huang and R. Mezzenga, Small, 2022, 18, 2105502 CrossRef CAS PubMed.
- Y. Peng, H. Huang, Y. Zhang, C. Kang, S. Chen, L. Song, D. Liu and C. Zhong, Nat. Commun., 2018, 9, 187 CrossRef PubMed.
- N. D. Rudd, H. Wang, E. M. A. Fuentes-Fernandez, S. J. Teat, F. Chen, G. Hall, Y. J. Chabal and J. Li, ACS Appl. Mater. Interfaces, 2016, 8, 30294–30303 CrossRef CAS PubMed.
- S.-W. Lv, J.-M. Liu, C.-Y. Li, N. Zhao, Z.-H. Wang and S. Wang, Chem. Eng. J., 2019, 375, 122111 CrossRef CAS.
- H. Yang, C. Peng, J. Han, Y. Song and L. Wang, Sens. Actuators, B, 2020, 320, 128447 CrossRef CAS.
- L. Zhang, J. Wang, X. Ren, W. Zhang, T. Zhang, X. Liu, T. Du, T. Li and J. Wang, J. Mater. Chem. A, 2018, 6, 21029–21038 RSC.
- A. Radwan, I. M. El-Sewify, A. Shahat, H. M. E. Azzazy, M. M. H. Khalil and M. F. El-Shahat, ACS Sustainable Chem. Eng., 2020, 8, 15097–15107 CrossRef CAS.
- S. Safaei, H. Kazemian and P. C. Junk, J. Solid State Chem., 2021, 300, 122267 CrossRef CAS.
- S. Qin, X. He, F. Jin, Y. Wang, H. Chu, S. Han, Y. Sun and L. Gao, RSC Adv., 2022, 12, 18784–18793 RSC.
- H. Guo, X. Wang, N. Wu, M. Xu, M. Wang, L. Zhang and W. Yang, Anal. Methods, 2020, 12, 4058–4063 RSC.
- L. Guo, Y. Song, K. Cai and L. Wang, Spectrochim. Acta, Part A, 2020, 227, 117703 CrossRef CAS PubMed.
- Y. Ma, G. Xu, F. Wei, Y. Cen, Y. Ma, Y. Song, X. Xu, M. Shi, S. Muhammad and Q. Hu, J. Mater. Chem. C, 2017, 5, 8566–8571 RSC.
- L. Xu, G. Fang, J. Liu, M. Pan, R. Wang and S. Wang, J. Mater. Chem. A, 2016, 4, 15880–15887 RSC.
- M. R. Awual, Chem. Eng. J., 2015, 266, 368–375 CrossRef CAS.
- K. T. Kubra, M. S. Salman, M. N. Hasan, A. Islam, M. M. Hasan and M. R. Awual, J. Mol. Liq., 2021, 336, 116325 CrossRef CAS.
- M. R. Awual, J. Environ. Chem. Eng., 2019, 7, 103378 CrossRef CAS.
- M. R. Awual, Mater. Sci. Eng., C, 2019, 101, 686–695 CrossRef CAS PubMed.
- M. R. Awual, M. M. Hasan, A. Shahat, M. Naushad, H. Shiwaku and T. Yaita, Chem. Eng. J., 2015, 265, 210–218 CrossRef CAS.
- K. T. Kubra, M. M. Hasan, M. N. Hasan, M. S. Salman, M. A. Khaleque, M. C. Sheikh, A. I. Rehan, A. I. Rasee, R. M. Waliullah, M. E. Awual, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, Colloids Surf., A, 2023, 667, 131415 CrossRef CAS.
- M. N. Hasan, M. S. Salman, M. M. Hasan, K. T. Kubra, M. C. Sheikh, A. I. Rehan, A. I. Rasee, M. E. Awual, R. M. Waliullah, M. S. Hossain, A. Islam, S. Khandaker, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, J. Mol. Struct., 2023, 1276, 134795 CrossRef CAS.
- M. R. Awual, J. Environ. Chem. Eng., 2019, 7, 103087 CrossRef CAS.
- M. R. Awual, Chem. Eng. J., 2017, 307, 85–94 CrossRef CAS.
- M. M. Hasan, K. T. Kubra, M. N. Hasan, M. E. Awual, M. S. Salman, M. C. Sheikh, A. I. Rehan, A. I. Rasee, R. M. Waliullah, M. S. Islam, S. Khandaker, A. Islam, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, J. Mol. Liq., 2023, 371, 121125 CrossRef CAS.
- K. T. Kubra, M. S. Salman, M. N. Hasan, A. Islam, S. H. Teo, M. M. Hasan, M. C. Sheikh and M. R. Awual, J. Mol. Liq., 2021, 338, 116667 CrossRef CAS.
- M. S. Salman, M. N. Hasan, M. M. Hasan, K. T. Kubra, M. C. Sheikh, A. I. Rehan, R. M. Waliullah, A. I. Rasee, M. E. Awual, M. S. Hossain, A. K. D. Alsukaibi, H. M. Alshammari and M. R. Awual, J. Mol. Struct., 2023, 1282, 135259 CrossRef CAS.
- M. Xiao, Z. Liu, N. Xu, L. Jiang, M. Yang and C. Yi, ACS Sens., 2020, 5, 870–878 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |