Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Recent advances in fluorescent and colorimetric chemosensors for the detection of chemical warfare agents: a legacy of the 21st century
Vinod
Kumar
 *a,
Heejeong
Kim
*a,
Heejeong
Kim
 b,
Bipin
Pandey
b,
Bipin
Pandey
 d,
Tony D.
James
d,
Tony D.
James
 *c,
Juyoung
Yoon
*c,
Juyoung
Yoon
 *b and
Eric V.
Anslyn
*b and
Eric V.
Anslyn
 *d
*d
aProcess and Technology Development Division, Defence Research & Development Establishment, Jhansi Road, Gwalior 474002, India. E-mail: vkpal77@yahoo.co.in
bDepartment of Chemistry and Nanoscience, Ewha Womans University, Seoul 03760, Korea. E-mail: jyoon@ewha.ac.kr
cDepartment of Chemistry, University of Bath, Bath, BA2 7AY, UK. E-mail: t.d.james@bath.ac.uk
dDepartment of Chemistry, The University of Texas at Austin, Austin, Texas 78712-1224, USA. E-mail: anslyn@austin.utexas.edu
First published on 22nd December 2022
Abstract
Chemical warfare agents (CWAs) are among the most prominent threats to the human population, our peace, and social stability. Therefore, their detection and quantification are of utmost importance to ensure the security and protection of mankind. In recent years, significant developments have been made in supramolecular chemistry, analytical chemistry, and molecular sensors, which have improved our capability to detect CWAs. Fluorescent and colorimetric chemosensors are attractive tools that allow the selective, sensitive, cheap, portable, and real-time analysis of the potential presence of CWAs, where suitable combinations of selective recognition and transduction can be integrated. In this review, we provide a detailed discussion on recently reported molecular sensors with a specific focus on the sensing of each class of CWAs such as nerve agents, blister agents, blood agents, and other toxicants. We will also discuss the current technology used by military forces, and these discussions will include the type of instrumentation and established protocols. Finally, we will conclude this review with our outlook on the limitations and challenges in the area and summarize the potential of promising avenues for this field.
1. Introduction
The area of supramolecular chemistry emerged through inspiration from nature. Biological systems utilize a range of non-covalent interactions, such as hydrogen bonding, electrostatics, and π–π stacking, to facilitate essential processes, such as the functioning of proteins and nucleic acids. Over the last 50 years, supramolecular chemists have devoted their attention to mimicking these interactions and designing new chemical systems for a variety of different applications, such as catalysis1 and chemosensing,2 as well as the fabrication of molecular switches,3 smart materials,4 molecular machines,5 medicine,6 and nanomedicine.7 Notably, the design of new chemical sensors for analyte detection is crucial for chemical, biomedical, and defense applications. The detection of environmental pollutants, explosives, and chemical warfare agents (CWAs) is of particular interest to the scientific as well as the medical community. Next-generation molecular sensors and detection systems must focus more on prudent designs to control supramolecular interactions and enhance current properties. This review primarily focuses on the introduction of each type of lethal and non-lethal chemical weapon (conventional and novel CW agents) identified by the Chemical Weapon Convention (CWC). Examples of currently used detection methods/techniques/devices will also be discussed. We will discuss examples of currently reported chemical sensors with a particular focus on chromogenic and fluorogenic probes for CW detection. We hope that this review reveals how much this field has grown over just two decades to become a recognized and established branch of chemistry.A nuclear, chemical or biological weapon can cause widespread destruction and kill large numbers of people at one time. Chemical weapons are classified as weapons of mass destruction (WMD), like nuclear and biological weapons.8 Formerly, the term ‘NBC’ (i.e., nuclear, biological, and chemical) was coined to designate a WMD. At present, an accepted abbreviation is ‘CBRNE’ which describes chemical, biological, radiological, nuclear, and explosive weapons.9 Chemical, biological, and non-nuclear explosives have been demonstrated to cause extensive damage to the nervous system and/or have neurobehavioral effects in victims. Technically, chemical weapons are both the CW agents, and the used delivery systems such as bombs, rockets, artillery shells, and aerosols.9 CW agents are generally assumed to be gaseous (as depicted by movies), but they can also be in the liquid or solid-state. Therefore, to achieve the desired effect, CWAs are mostly delivered and disseminated in the form of aerosols. The release of aerosolized samples allows the particles to remain airborne for an indefinite period. The method of exposure of the CWAs depends on their physical and/or chemical properties. For example, if they have low vapor pressure, the likely route of exposure is via skin contact, and highly volatile CWAs are most likely exposed via respiratory, oronasal, and conjunctiva mucosal tissues. These exposures irritate the eyes, nose, and skin immediately.
1.1 Chemical weapons convention (CWC) and organization for the prohibition of chemical weapons (OPCW)
Production, stockpiling, transfer, and use of chemical weapons and their precursors are completely prohibited under the arms control treaty, as per the CWC established by the OPCW.10 The treaty entered into force on 29 April 1997 with the mandate to eliminate an entire category of weapons of WMD within a fixed time frame. At present, 193 States are committed to abiding by the regulation set by the OPCW. The production for research, medical, pharmaceutical, or protective purposes is still permitted but if produced in more than 100 grams, it must be reported to the OPCW.11 In 2013, the organization received the Noble Peace Prize for its legislative efforts in the non-proliferation of chemical weapons. The chemicals governed under the CWC are divided into three Schedules – Schedule 1, Schedule 2, and Schedule 3 (Table 1).12 Schedule 1 substances are chemicals that can be used as chemical weapons or used in the production of chemical weapons and have no or very limited additional use. Schedule 2 substances are chemicals that can be used as chemical weapons or used in the production of chemical weapons but have legitimate small-scale applications outside of CWs. Schedule 3 substances are chemicals that can be used as chemical weapons or used in the production/manufacture of chemical weapons, but also have legitimate large-scale industrial uses.| Schedule 1 substances | |
|---|---|
| 1 | Sarin: O-Isopropyl methylphosphonofluoridate |
| 2 | Soman: O-Pinacolyl methylphosphonofluoridate |
| 3 | Tabun: O-Ethyl N,N-dimethyl phosphoramidocyanidate |
| 4 | VX: O-Ethyl S-2-diisopropylaminoethyl methyl phosphonothiolate |
| 5 | Sulfur mustards |
| I. Mustard gas: Bis(2-chloroethyl)sulfide | |
| II. 2-Chloroethylchloromethylsulfide | |
| III. Bis(2-chloroethylthio)methane | |
| IV. Sesquimustards: | |
| V. Bis(2-chloroethylthiomethyl)ether | |
| VI. Bis(2-chloroethylthiomethyl)ether | |
| 6 | Lewisites: |
| I. Lewisite 1: 2-Chlorovinyldichloroarsine | |
| II. Lewisite 2: Bis(2-chlorovinyl)chloroarsine | |
| III. Lewisite 3: Tris(2-chlorovinyl)arsine | |
| 7 | Nitrogen mustards: |
| I. HN1: Bis(2-chloroethyl)ethylamine | |
| II. HN2: Bis(2-chloroethyl)methylamine | |
| III. HN3: Tris(2-chloroethyl)amine | |
| 8 | Saxitoxin |
| 9 | Ricin |
| 10 | Novichok nerve agents |
| 11 | Carbamates (quaternaries and bisquaternaries of dimethylcarbamoyloxypyridines) |
| Schedule 2 substances | |
|---|---|
| 1 | Amiton (VG): O,O-Diethyl S-[2-(diethylamino)ethyl]phosphorothiolate |
| 2 | PFIB: 1,1,3,3,3-Pentafluoro-2-(trifluoromethyl)-1-propene |
| 3 | BZ: 3-Quinuclidinyl benzilate |
| Schedule 3 substances | |
|---|---|
| 1 | Phosgene: carbonyl dichloride |
| 2 | Cyanogen chloride |
| 3 | Hydrogen cyanide |
| 4 | Chloropicrin: trichloronitromethane |
2. Chemical warfare agents
The large-scale use of toxic chemicals as weapons first became possible during the First World War (1914–1918).13 Initially, lachrymators (tear gases), sternutators (an agent used in chemical warfare that causes sneezing, irritation to the nose and eyes, pain in the chest, and nausea), and vomiting agents were used to harass but not kill the enemy.14 During the First and Second World Wars, poisonous chemicals were screened for their potential to be used as a weapon. Between these periods, several compounds were synthesized, or isolated from natural materials such as ricin, and examined to determine their applicability as a chemical weapon. Post-1945, a major focus was on the research and development of novel CW agents, with the extensive evaluation of their biochemistry, toxicology, and pharmacology. Despite these efforts, only a few candidates were identified that satisfied requirements, including acceptable production costs as well as appropriate physical, chemical, and toxicological properties. In total, 70 different CW agents were prepared, stockpiled, and weaponized in liquid, gas, or solid form.15 The stockpiling of CW agents is largely carried out in the form of unitary agents, and sometimes in binary forms.16 Unitary weapons are lethal chemical munitions that produce a toxic result in their existing state, such as GA, GB, SM, etc. Unitary agent chemicals are produced in a plant, loaded into a missile, and stored in a ready-to-use fashion. These munitions are highly toxic, and therefore, the storage, handling, and deployment of these chemicals need to be performed with extreme caution. Agents in the active form are also highly corrosive, thus posing the risk of leakage. Unlike unitary weapons, binary weapons involve nontoxic precursors that are mixed with the nerve agents and can be loaded into munitions just before deployment.Chemical agents are ordered into several categories according to their physiological mode of action, tactical purpose, or chemical structure.17 Based on lethality, they have also been classified as lethal CWAs and non-lethal CWAs. The categories are as follows:
i. Nerve agents
ii. Blister agents/vesicants
iii. Blood agents
iv. Choking agents or pulmonary agents
v. Riot-control agents or Harassing agents
(a) Tear agents
(b) Vomiting agents
(c) Malodorants
vi. Psychomimetic agents or incapacitating agents
vii. Toxins
Nerve agents, blister agents, and choking agents are generally electrophilic with varied reactivity and selectivity towards biological nucleophiles. They have been found to react with water to form toxic metabolites (e.g., HCl) and react with SH, OH, NH, and CO2 amino acid residues in proteins to form protein adducts and inhibit biological function. Similarly, the mustard agents, like sulfur mustard and nitrogen mustard, have been shown to react with NH and –P(O)O– residues in DNA.
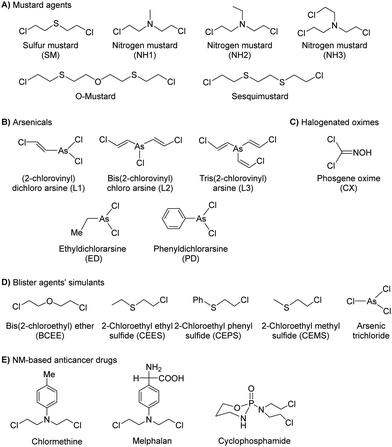
2.1 Nerve agents
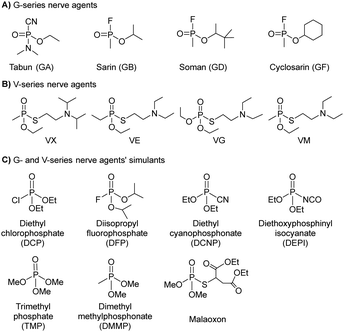
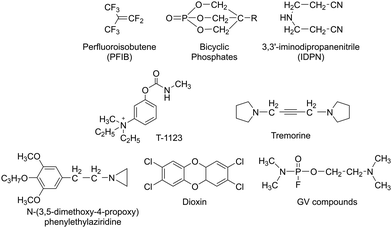
Nerve agents, also called nerve gases, are extremely toxic chemicals that act primarily by inhibiting the enzyme acetylcholinesterase (AChE) in both the peripheral and central nervous systems.18,19 This leads to an excess of the neurotransmitter acetylcholine (ACh) at synaptic junctions. Ultimately, death occurs due to asphyxiation or cardiac arrest. This can happen in minutes to hours depending on the route of exposure and the amount absorbed. The primary routes of exposure are ingestion, inhalation, and absorption through the skin. After exposure, the victim may experience involuntary salivation, lacrimation, urination, defecation, gastrointestinal pain, and vomiting. There are two main classes of nerve agents. The first is G-series agents: tabun (GA), sarin (GB), soman, (GD), and cyclosarin (GF) (Fig. 1(A)).20 G-series was named after the German scientist Gerhard Schrader, who was the first to synthesize such agents. He is now known as ‘the father of nerve agents’.21 In 1936 the first nerve agent GA (tabun) was developed. Subsequently, in 1939 Sarin was prepared, and then in 1944 GD (soman) emerged. Then in 1949 the somewhat obscure GF (cyclosarin) was developed, and in the 1950s VX came into existence.22 V-series agents are the second family of nerve agents, which include VE, VG (amiton), VM, VR, and VX (Fig. 1(B)). VX is the most studied nerve agent in this series. V stands for victory, venomous, or vicious, and VR stands for Russian VX. G-series agents are known as non-persistent, whereas the V-series are persistent because of their low vapor pressure. Sarin and VX are the chemical agents that were largely fielded in ammunition, rockets, artillery shells, airplane spray tanks, and landmines during war scenarios. Nerve agents were originally produced during the search for insecticides and because of the identified potent toxicity, they were subsequently evaluated for military use. These agents are generally identified by both, their chemical names and their two-letter NATO codes. The second letter is the specific identifier for each compound: GA (tabun), GB (sarin), GD (soman), and GF (cyclosarin).23 To mimic the physico-chemical characteristics of G-series and V-series nerve agents, various nerve agents’ simulants have also been identified which are presented in Fig. 1(C).All nerve agents are colorless and volatile liquids in their pure state at standard temperature and pressure. Their high volatility makes them a powerful weapon. G-agents produce a fruity odor and V-agents an amine odor. Sarin is soluble and soman is sparingly soluble in water, whereas VX and tabun exhibit intermediate solubility. Upon nerve agent exposure, generally, a combination of drugs having a complementary mode of action are administered. The combination treatment required pre-treatment with pyridostigmine bromide (which protects against irreversible inhibition of enzymes), and the use of atropine and oximes, such as pralidoxime chloride as post-exposure therapy. Derived from animal studies, human toxicity for nerve agents is estimated to range from 80 μg kg−1 (tabun) to 7 μg kg−1 (VX) for the LD50 by i.v. administration, percutaneous LD50 values were estimated to be 1000 mg for tabun, 1700 mg for sarin, 100 mg for soman, and 10 mg for VX, respectively, for a 70 kg person (Table 2).24 An additional feature that exists in all nerve agents is the presence of chirality (asymmetry) around the phosphorus atom.25 Studies show that the P(−)-stereoisomers of sarin and soman inhibit AChE several orders of magnitude better than the P(+)-stereoisomers. (−)-VX is only eight times more lethal than the (+)-stereoisomer. Hence, despite the modest selectivity of (−)-tabun towards the inhibition of AChE, this isomer is substantially more toxic in mice than (+)-tabun.26Fig. 2 portrays the normal enzymatic function of the AChE enzyme, as well as the mechanism of toxicity for the nerve agent, the aging process, and reactivation of the enzyme.
| Chemical agents | Toxicities in LD50 (mg kg−1) | Toxicities in LCt50 (mg min m−3) | IDLH (mg m−3) |
|---|---|---|---|
| Sulfur mustard (SM) | 100 | 900 | 0.7 |
| Nitrogen mustard (HN3) | 10 | 1500 | 0.003 |
| Lewisite (L) | 30 | 1400 | 0.1 |
| Tabun (GA) | 21.42 | 70 | 0.1 |
| Sarin (GB) | 24.28 | 35 | 0.1 |
| Soman (GD) | 0.71 | 35 | 0.05 |
| Cyclosarin (GF) | 0.14 | 35 | 0.05 |
| VX | 0.071 | 15 | 0.003 |
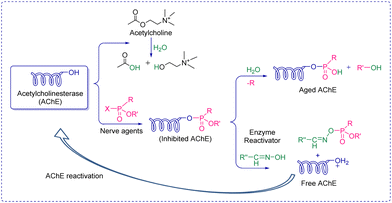 | ||
| Fig. 2 Schematic presentation of acetylcholine enzyme's function, inhibition, aging, and reactivation. | ||
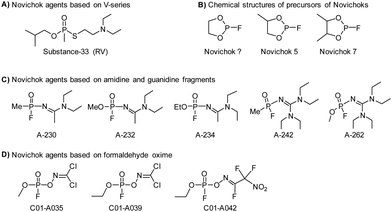
2.2 Novichok agents
The three recent assassination attempts, one on Sergei Skripal and his daughter, Yulia in the UK in the March 2018,27 then three months later on Charlie Rowley and Dawn Sturgess, two UK nationals,28 and very recently on Alexei Navalny of Russia in 2020,29 have sparked significant interest from the international community to further understand the relatively new class of nerve agents known as Novichoks (the Russian word for ‘newcomer’). Novichok agents (Fig. 3), also known as “N-series” agents, are organophosphorus compounds, similar to sarin and VX. They also inhibit the enzyme AChE.30 It is believed that these agents were secretly developed by the former U.S.S.R in 1970 during a Cold War-era weapons program (code-named FOLIANT). The program aimed to develop nerve agents that could not be stopped by chemical protective gear. They were also intended to be safer to handle, undetectable by conventional analytical tests, and safer to store and use than previous generations of CWAs. Novichok agents are considered more potent (10 times greater) than VX and can be applied in unitary and binary forms. Unitary agents were synthesized and used much like sarin, soman, and VX; i.e., the chemical structures were altered during production to have maximum potency. In general, the precursors are designed to be significantly less hazardous than the actual agents. Therefore, production, handling, and transportation are easier. A significant drawback of Novichok agents is their low stability in the environment.The OPCW recently concluded that there was insufficient information about the chemical structure and the toxicological property of Novichok agents. Until 2020, these agents were not formally listed by the CWC, primarily out of fear that their inclusion would reveal their exact chemical structures and this knowledge might be used by some countries or terrorist organizations to produce and use them. As of 2021, the OPCW has included them in their list of Schedule 1 chemicals.31 There is no mutual consensus on the nomenclature of these mysterious agents. However, their precursors are named Novichok? Novichok-5, and Novichok-7. Their reaction products are referred to by the symbol “A” with a three-digit number (hence the name “A-series compounds”). The “Novichok” designation refers to the binary form of the agent, with the final compound being referred to by its code number (e.g., A-232). The first developed agent was named Substance 33, which was very similar to VX (also known as VR; Russian VX), and it became a prototype for the series of Novichok agents. Subsequently, unitary chemical weapons were synthesized and the examples include A-230, A-232, and A-234.
From a chemistry perspective, they are organophosphorus compounds containing dihaloformamide and oxime groups (phosphorylated/phosphonylated oximes). These agents are highly toxic because the phosphorylated oximes are relatively unstable under normal conditions and the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–O– bond readily undergoes hydrolysis, resulting in the so-called aged form of AChE. Once aging has occurred, the enzyme is irreversibly inactivated (Fig. 2). A hypothesis somewhat supported by the aging half-time of A-230 which is similar to that observed in soman, i.e., 2–4 min. The treatment for poisoning by these mysterious nerve agents is similar to that of other nerve agents. The current treatment is symptomatic therapy (combination of an anticholinergic agent and anticonvulsant) or administration of so-called bioscavengers, such as butyrylcholinesterase. While drugs such as obidoxime, pralidoxime and HI-6 are designed to bind to free nerve agents in the patient's circulation and prevent the inhibition of AChE in tissue. This means detection needs to be accurate and rapid to facilitate administration of the required treatment before it is too late.
N–O– bond readily undergoes hydrolysis, resulting in the so-called aged form of AChE. Once aging has occurred, the enzyme is irreversibly inactivated (Fig. 2). A hypothesis somewhat supported by the aging half-time of A-230 which is similar to that observed in soman, i.e., 2–4 min. The treatment for poisoning by these mysterious nerve agents is similar to that of other nerve agents. The current treatment is symptomatic therapy (combination of an anticholinergic agent and anticonvulsant) or administration of so-called bioscavengers, such as butyrylcholinesterase. While drugs such as obidoxime, pralidoxime and HI-6 are designed to bind to free nerve agents in the patient's circulation and prevent the inhibition of AChE in tissue. This means detection needs to be accurate and rapid to facilitate administration of the required treatment before it is too late.
2.3 Carbamates
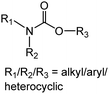
Carbamates (CBs) are another class of CW agents (fourth generation CW agents) that has recently been included in the list of Schedule 1 chemicals by the OPCW along with Novichok agents32 Carbamates are N-methyl carbamates derived from carbamic acid. Although, carbamic acid is unstable at room temperature, substitution using different alkyl/aryl, arylalkyl, and substituted alkyl/aryl groups at the amino as well as carboxylic groups of carbamic acid enhances the stability.33 CBs are used extensively as pesticides, as tranquilizers, and in the treatment of myasthenia gravis, glaucoma, anticholinergic poisoning, and paroxysmal atrial tachycardia.34Earlier, these agents were not covered by the CWC because they had never been used as chemical weapons, therefore they were initially omitted from the list of Schedule 1 chemicals. CBs are originally insecticides comparable to OP insecticides and exhibit similar toxicological effects to OP poisoning by causing carbamylation of acetylcholinesterase at neuronal synapses and neuromuscular junctions.35–38 The agents contain one or more quaternary amine centers that help them to penetrate neuromuscular junctions. They induce toxicity typically in less than 24 hours leading to miosis and rhinorrhea as primary indications of exposure at low levels. High concentrations of CBs result in vomiting, urination, or defecation, and finally loss of consciousness and convulsions in just 30 seconds, followed by cessation of breathing and flaccid paralysis. Chemically, CB nerve agents are mono- and bis-quaternaries of dimethyl carbamoyloxypyridines which are tabulated above (Table 3). Nerve agents based on CBs are stable in water but get rapidly hydrolyzed at high pH. Furthermore, basic peroxides also rapidly detoxify CBs.
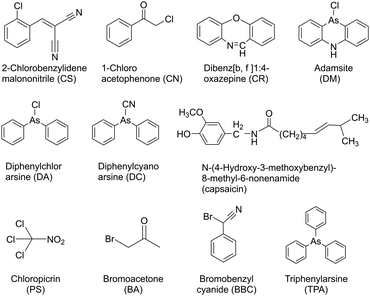
2.4 Blister agents
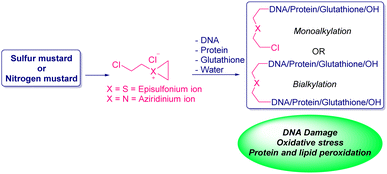
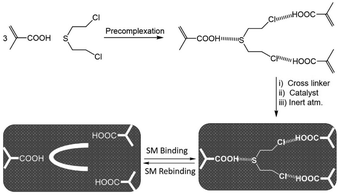
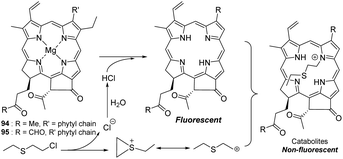
Blister agents or vesicants are substances that produce painful skin vesicles/blisters and the eyes, lungs, and mucous membranes are damaged. Symptoms can be instantaneous or can appear several hours following exposure. They have been placed in three major categories: mustard agents, e.g., sulfur mustard (H, HD, HT) and nitrogen mustard (HN-1, HN-2, HN-3); arsenicals, e.g., Lewisite (L); and halogenated oximes, e.g., phosphine oximes (CX) (Fig. 4(A)–(C)).SM has been the most extensively used blister/vesicant CWA over the past century.41 Upon exposure, it causes painful blisters and vesicles, hence its name. Symptoms occur within 2–24 h after exposure. Its toxicity lies in its ability to react with DNA, protein, and other nucleophilic biological fluids via the episulfonium cation, which is facilitated by the presence of water or other polarizing media.42 In DNA, it acts as a bifunctional alkylator and reacts at the N7 of guanine and the N3 of adenine at a ratio of 66% and 17%, respectively. The remaining 17% reacts as a cross-linked adduct involving two guanines on the same or complementary sugar-phosphate strands (Fig. 5).43 Due to the extreme toxicity, high persistence, and ease of manufacture, it was extensively used in both World Wars and has been regarded as ‘King of Warfare Gases’ with the highest military significance.44
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 90.52 As mentioned earlier, Agent HL was developed for use in the cold weather or at high-altitude and is a mixture of mustard gas (HD) and Lewisite (L), which British anti-Lewisite (BAL), regarded as dimercaprol, is a well-known antidote for Lewisite.53 Lewisite is prepared by the reaction between arsenic trichloride and acetylene in the presence of a suitable catalyst (e.g., anhydrous aluminum chloride). This reaction produces a mixture of 2-chlorovinylarsonous dichloride (Lewisite 1), bis(2-chloroethenyl)arsinous chloride (Lewisite 2), and tris(2-chlorovinyl)arsine (Lewisite 3).54 Lewisites are generally stable but exposure to moisture results in quick hydrolysis to 2-chlorovinyl-arsenious acid and a decrease in toxicity. Under mild basic conditions, hydrolysis of Lewisite results in the formation of Lewisite oxide. When exposed to a strong base, Lewisites decompose to yield acetylene and trisodium arsenate (Na3AsO4).55 Using the latter reaction, the concentration of Lewisite is quantified by measuring the amount of acetylene generated.56
90.52 As mentioned earlier, Agent HL was developed for use in the cold weather or at high-altitude and is a mixture of mustard gas (HD) and Lewisite (L), which British anti-Lewisite (BAL), regarded as dimercaprol, is a well-known antidote for Lewisite.53 Lewisite is prepared by the reaction between arsenic trichloride and acetylene in the presence of a suitable catalyst (e.g., anhydrous aluminum chloride). This reaction produces a mixture of 2-chlorovinylarsonous dichloride (Lewisite 1), bis(2-chloroethenyl)arsinous chloride (Lewisite 2), and tris(2-chlorovinyl)arsine (Lewisite 3).54 Lewisites are generally stable but exposure to moisture results in quick hydrolysis to 2-chlorovinyl-arsenious acid and a decrease in toxicity. Under mild basic conditions, hydrolysis of Lewisite results in the formation of Lewisite oxide. When exposed to a strong base, Lewisites decompose to yield acetylene and trisodium arsenate (Na3AsO4).55 Using the latter reaction, the concentration of Lewisite is quantified by measuring the amount of acetylene generated.56
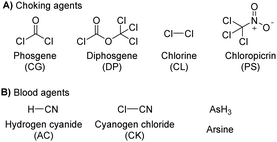
2.5 Blood agents
The blood agents in this category are mainly hydrogen cyanide (AC), cyanogen chloride (CK), and arsine (Fig. 6(A)). They are placed on the list of Schedule 3 toxic chemicals, per the CWC.59 These agents are named based on their effect on the oxygen-carrying capacity of RBCs. Hydrogen cyanide was used in World War I and II as a chemical weapon by the French, United States, and Italy. In addition to its use as a CWA, hydrogen cyanide is used in huge industrial processes for fumigation, electroplating, mining, and chemical synthesis.60 Among this class of CWAs, these are the least toxic agents, which mainly affect the retina and optic nerve leading to vision failure at higher doses. The toxicity of these agents is due to the affinity of the CN− ion for the heme a3 moiety of cytochrome c-oxidase found in mitochondria. This inhibits the electron transfer chain and leads to cellular hypoxia.61 Due to the acidity of gastric fluid (pH ∼ 1), substantial amounts of HCN can be released when cyanide salts (KCN and NaCN) are ingested.622.6 Choking agents
Choking agents are also known as lung-damaging agents or pulmonary agents that produce toxic inhalational injury as they attack lung tissue and primarily cause pulmonary edema.63 These agents pose a real threat to military and civilian personnel when used as chemical weapons. Choking agents include phosgene (CG), diphosgene (DP), chlorine (CL), and chloropicrin (PS) (Fig. 6(B)).64 Some of these chemicals (chlorine and phosgene) are produced in massive quantities for industrial purposes. The pulmonary agents, particularly phosgene and oxides of nitrogen, are relatively non-reactive and insoluble in aqueous solutions; hence, they readily penetrate respiratory bronchioles and alveoli. As expected, acylation reactions of biological proteins cause significant damage that ultimately leads to pulmonary edema.65 The symptom occurs in 20 minutes to 24 hours depending on the exposure dose and physicochemical properties of the agent.662.7 Toxins
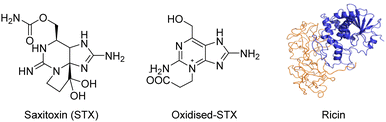
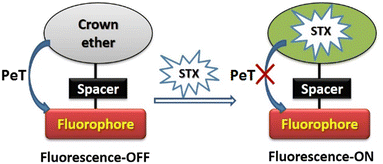
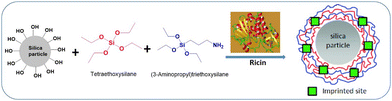
Toxins, such as ricin and saxitoxin (STX) (Fig. 7), are extremely toxic chemicals produced by living organisms, including animals, plants, and microbes. Therefore, these are categorized as both chemical and biological weapons.67,68 Their production, stockpiling, and use are prohibited under both the CWC and BWC (Biological Weapons Convention). Toxins are highly toxic natural products found in both proteinaceous and chemical forms. Among the list of various natural bioagents, ricin and STX have been weaponized and are included as Schedule 1 substances by the CWC.69 Due to their availability from natural sources, they can be used as domestic chemical weapons for terrorist activities. However, the relative ease of accessibility for ricin (from Caster beans) as compared to STX (either from natural sources or chemical synthesis) makes ricin a greater threat.2.8 Non-lethal chemical weapons
Non-lethal weapons (NLWs) or less-than-lethal weapons are designed to incapacitate personnel while minimizing fatalities, mitigating injury, and preventing any unwanted damage to structural property and the surrounding environment.77 For these reasons, they may be considered appropriate for riots and civilian peacekeeping operations, which are intended to produce temporary incapacitation of an individual. NLWs should have a rapid onset of incapacitating effects, easy dissemination and decontamination, long shelf-life, low cost, and no short- or long-term adverse effects on heterogeneous populations. These agents can be grouped as riot control agents, incapacitants, calmatives, and malodorants.3. Instrumental and chemical detection techniques: present status
The rapid identification of a CW agent is crucial to ensure the protection of first responders/emergency medical personnel, as well as for identifying the appropriate treatment for victims. In light of recent CW-related events (e.g., Salisbury, UK),90 there is increasing interest in developing new and effective instruments and chemical methods for both military and civilian purposes. This is underscored by the commonly seen spread of a CWA from the battlefield/target soldiers to civilian populations. Several types of commercially available military-based equipment exist. These require manual operation or can be fully automated.Current technologies rely on instrumental and chemical analyses for CWA detection, which can be applied to liquid, vapor, and contaminated solids. The former techniques generally exploit the physical characteristics of the chemical agents to develop the electronic equipment/devices. Considerable progress has been made with physical–electronic systems over the last 50 years. These systems are usually faster and more adaptable, allowing for the detection of other compounds/classes of compounds. The use of wet chemistry requires detection test kits that contain various dyes and enzymatic assays. Physical–electronic (instrumental) detection differs from chemical detection which is usually rather laborious to run. Furthermore, with detection kits, extensive use of (bio)chemicals can result in logistical difficulties. Due to the reaction design of each test, they may have a short shelf life. However, chemical detection is considered to be more reliable and cost-effective.
3.1 Instrumental techniques/methods
In general, current detection technologies consist of standoff and point detectors.91 Standoff detection refers to the ability of a detector to spot, evaluate, and identify an agent at a distance, while point detectors can be operated by on-site personnel who have more specific training and safety equipment. Point detectors detect chemical agents from a closer distance. The primary differences between standoff and point detectors are their size, weight, portability, and logistical support requirements. A wide variety of techniques based on standoff and point detection are commercially available and used by the armed forces. These are predominantly based on ion mobility spectrometry (IMS), flame photometry, gas chromatography (GC), and surface acoustic waves (SAW).92 In addition to this, electrochemical-based detectors93 and instruments based on techniques including molecularly imprinted polymer (MIP) sensors,94 biosensors,95 surface plasmon resonance (SPR),96 and conductive polymer sensors,97etc., are also in the advanced stages of development.Flame photometry exploits the emission of light from sulfur- and phosphorus-containing compounds in a hydrogen/air flame.98 These devices are therefore limited to these chemical species. Nevertheless, portable and automated detectors have been developed for on-site (in the field) detection of CW agents. These devices include AP2C, AP4C, and CHASE.99 IMS is a technique that separates ionized molecules that are then distinguished based on their mass, charge, and mobility in the gas phase.100 This affords a characteristic plot of the current generated over time for each type of CWA. The intensity of the peaks in the spectrum determines the relative concentration of the agent present. Chemical detectors based on this technology are GID-3, GID-2A, chemical agent monitors (CAMs), lightweight chemical detectors (LCDs), automatic chemical agent detection alarms (ACADAs or GID-4), rapid alarm identification device monitors (RAID series), individual/improved chemical agent monitors (ICAD), ChemPro 100, ICAM, M8A1 ACADA, RAID, and M-90.101 A classic SAW device consists of a piezoelectric crystal plate coated with a chemically selective polymer and two interdigital transducers.91,102 Light irradiation passes through a transparent medium, and the chemical species scatters the radiation beam. Differences between the incident beam and scattered radiation are measured. This difference in scattering is dependent upon the chemical structure of the molecule, which allows the identification of each type of CWA. The Joint Chemical Agent Detector (JCAD) and HAZMATCAD use SAW-based technology.103 SAW devices have advantages over other methods, including their relatively low cost and high detection sensitivity. More importantly, test samples can be analyzed directly in transparent glass vials or plastic bags, which significantly reduces the danger of exposure to first responders. At present Raman-based detectors available are FirstDefender, FirstDefender XL, and Joint Contaminated Surface Detector (JCSD).91,104 Infrared-based detectors have been developed and employed for point and standoff detection of CWAs. For these devices, IR radiation is passed through the test sample. Some radiations are absorbed whilst some are transmitted, resulting in the production of a spectrum with a unique molecular fingerprint for each CWA. The IR-based detectors most commonly used by the military are the M21 detector, Joint Service Lightweight Standoff Chemical Agent Detector (JSLSCAD), MIRAN SapphIRe, AN/KAS-1/1A, TravelIR HCI, HazMat ID, and IlluminatIR.91,101 IR-based detectors offer the advantage of high sensitivity and fast detection. Additionally, no sample preparation is required. An electrochemical sensor for CWA measures the change in the electrochemical signal caused by the interaction between the agents and an electrode. These sensors show high selectivity for a CWA but have poor sensitivity. An example of a currently available detector is SensorRAE.105 The CW Sentry Plus device is based on a combination of SAW arrays and electrochemical cells, which detect blood agents and choking agents along with nerve agents and blister agents.91 Another approach that is similar to electrochemical detection is the use of ion-selective electrodes (ISEs). In the case of sarin and soman, nerve agent hydrolysis generates fluoride ion which is then detected using a fluoride-selective electrode, whereas GA produces a cyanide ion detected using a cyanide-selective electrode.91 Gas chromatography (GC) coupled with a mass detector (GCMS) can be used to unambiguously detect most organic-based compounds, including CW agents, at very low concentrations (20–200 ng m−3).106 With the advent of more sophisticated analytical capabilities, GC combined with sensitive detectors, such as nitrogen phosphorus detection (NPD), flame photometric detection (FPD), and mass selective detection (MSD), can detect extremely low concentrations of CWAs (10–100 ppb). However, these instruments require a skilled operator and need routine maintenance. Therefore, the use of this on-site has little advantage over off-site analysis, when a chemical incident occurs at a distant site where the mobile lab cannot attend. Commercially available, portable GCMS-based instruments include HAPSITE (Inficon's), the EM series (Bruker's), and MM1 or MM2.91,101 In general, most of these devices/techniques have several limitations, such as low specificity and the inability to detect each type of CW agent. These detection methods are susceptible to interference and false-positive results. As a result, no single detection system can be relied upon to give an accurate result. Therefore, the recommended method is to simultaneously use two different types of detectors with different analytical techniques to obtain reliable and accurate data.
3.2 Chemical methods
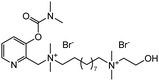
The chemical analysis involves measuring the change in color of test materials when they are exposed to the sample in question. This color change corresponds to the detection of a CWA and usually occurs via a chemical reaction between the CWA and reagent. This offers good selectivity, sensitivity, speed, ease of operation, and low cost. An important aspect of this strategy is that detection can be visualized by the naked eye. No specialized instruments need to be used, making this strategy less sophisticated and less expensive. The most common approaches use detector papers/tickets, detector tubes, and detection kits.91,101 These rapid detection kits have been employed by the military for several decades owing to being the fastest, cheapest, lightest, and simplest method for field use. Suspect liquid droplets or aerosol are typically evalauated using detector papers, whereas detector tubes and kits have been developed for evaluating gaseous CWAs and water samples, respectively. Detector papers/strips that are commercially available include M8 and M9, which produce different colors on exposure to liquid CW agents. Commercial examples of detection tubes, include Dräger tubes and residual vapor detection (RVD) kits. Where the detection reagents are impregnated into silica gel, which is supported on a glass tube. The test sample is passed through the dyes’ impregnated silica using a handheld suction pump. If a CWA is present, a color change is observed.107 Various types of M256 and Agentase chemical agent detection kits for military use are commercially available. The U.S. Army recognized Agentase sensors in 2004 as one of the greatest army inventions of 2003. In these kits, nerve agents impart a blue color by inhibiting a butyrylcholinesterase-mediated reaction cascade (Fig. 11(A)).108 For SM detection, the reaction between 4-(4-nitrobenzyl) pyridine, 3 and SM is exploited. Heating the sample at 80 °C followed by treatment with sodium hydroxide results in a change from colorless to blue, which enables the identification of SM (Fig. 11(B)).109 In the case of blood agents, the analyte reacts with mercury chloride to yield hydrogen chloride (HCl). This induces a color change for pH-sensitive dyes, such as methyl red or thymol blue.110 For the detection of cyanogen chloride, silica gel is impregnated with 4-benzylpyridine, 4, and barbituric acid (yellow). The presence of cyanogen chloride causes a cascade of reactions that affords a final pink-colored product (Fig. 11(C)).111 Choking agents, such as phosgene, are also detected using 4-(4-nitrobenzyl) pyridine to generate a brick red color from its original yellow color (Fig. 11(D)).112,113 Based on similar chemical reactions, a testing kit consisting of test bottles and test reagents was constructed to analyze the chemical agent present in aqueous media. A major limitation to chemical analysis is that the test is only as specific as the dyes used. Each dye can only react with one class of agent at one time.107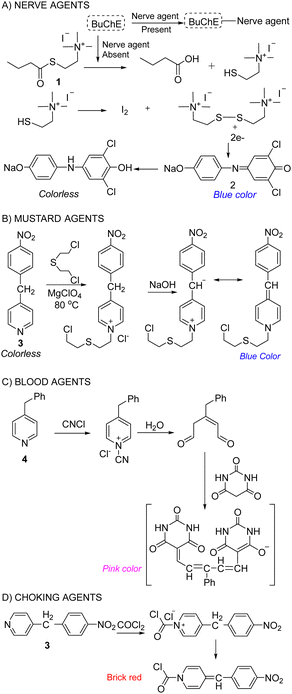 | ||
| Fig. 11 Classic chemical reactions used in the detection of (A) nerve agents, (B) mustard agents, (C) blood agents, and (D) choking agents. | ||
As mentioned briefly, currently used chemical technologies have several limitations, such as low specificity, poor sensitivity, and the inability to detect and differentiate all CW agents. Excellent selectivity is a key design criterion for CWA detection. However, achieving this feature is exceptionally challenging due to the electrophilic nature of most CWAs. Several non-toxic species have the potential to interfere with testing and afford false-positive results. Moreover, regulatory bodies are continuously imposing new stringent requirements for CWA analysis; while current detection systems may work, they may fall short of new selectivity and sensitivity requirements. Therefore, the continued advancement of chemical sensors is essential for ensuring vigilance against the threat of CWAs. As will be seen throughout this review, new methods are being developed and the potential of chemical probes is emerging as a promising option for use in CW detection in practical applications. The development of chemical sensors is attractive not only in terms of specificity and economic reasons but also for their significantly lower logistical and operational burden on military forces and civilians.
4. Fluorescent and colorimetric chemosensors: design and development
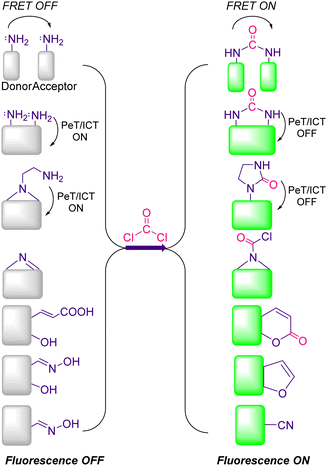
The term “chemical sensor” or “chemosensor” refers to a molecule of abiotic origin that signals the presence of matter or energy. Therefore, a chemosensor can be defined as a molecule that interacts reversibly with an analyte and transforms chemical information into analytically useful and measurable signals.114–116 The measurable signal is the result of two different processes occurring during host molecule/receptor and analyte/guest interaction i.e., molecular recognition and signal transduction.110 The molecular recognition is based on size, shape, geometry complementarity, and the presence of specific physical and chemical interactions. The signal transduction occurs with a concomitant change in one or more properties of the system such as absorption, fluorescence, and redox potentials. Sometimes, spacers are placed between the receptor and reporting units which can establish the geometry of the system and tune the electronic interaction between the two active moieties. When the binding interaction between the receptor and indicator is irreversible, the chemosensor is then termed a “chemodosimeter”.117Most chemosensors fit in either of three main approaches in supramolecular chemistry, namely (i) indicator–spacer–receptor (ISR)118 (ii) indicator displacement assay (IDA)119 (iii) a chemodosimeter approach or activity-based sensing.115,120 Typically, there are three approaches invariably used by supramolecular chemists while developing chemosensors for the detection of an analyte via various modes of sensing mechanism. Important sensing mechanisms include photoinduced electron transfer (PeT), intramolecular charge transfer (ICT), excimer formation, fluorescence resonance energy transfer (FRET), and excited-state intramolecular proton transfer (ESIPT). Two main types of optical chemosensors i.e., fluorescent and colorimetric are utilizing these sensing mechanisms. In the colorimetric sensors, there is a change in the electronic absorption spectra of the receptor, and the changes can be seen with the naked eye.121 Fluorescent chemosensors can change their fluorescence after the interaction with the analyte.122 Fluorescent and colorimetric chemosensors are widely used in organic, biological, and medicinal chemistry and environmental sciences for monitoring cations and anions. Fluorescent sensors are much more sensitive than the colorimetric ones and allow measurements in situ and in vivo.
4.1 Fluorescent and colorimetric chemosensors for the detection of nerve agents: an update
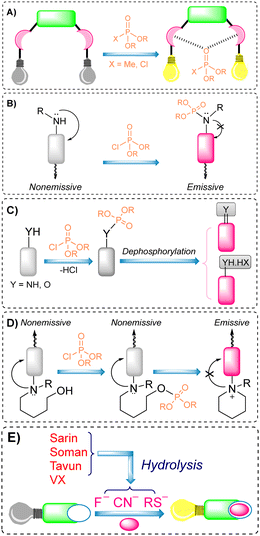
Over the preceeding several decades the importance of the design and development of colorimetric and fluorescence based chemosensors for CWA detection is becoming increasingly important. Nowadays, detection is achieved using two main tactics: (i) reaction-based or activity-based sensing and (ii) receptor-based sensing. The covalent approach exploits the reactivity of the analyte with a functional group on the chemical sensor. The reaction leads to a measurable optical response. This strategy is usually irreversible, meaning the developed sensor cannot be reused. On the other hand, CWAs can also be recognized via non-covalent reversible interactions (supramolecular approach), providing the ability to reuse chemical sensors. However, the supramolecular approach often displays poor selectivity and sensitivity, which can lead to frequent false-positive responses.The detection of nerve agents is accomplished by exploiting the reactions of the nucleophilic functionalities on a probe with the electrophilic phosphate ester. The resultant product leads to a detectable optical signal (colorimetric and/or fluorescence) (Fig. 12), either by suppression of photo-induced electron transfer (PeT), change in internal charge transfer (ICT), or an intramolecular cyclization reaction. A recent strategy includes the detection of hydrolyzed products from the nerve agents. These include fluoride ions, CN ions, and thiols (Fig. 12(E)). Various research groups around the world are continually devoting considerable attention to the design and development of new and alternative strategies. The development of chemical probes for nerve agents’ detection has been covered in the literature by Martínez-Máñez123 and Yoon.124 The structures of these chemical sensors (5–28) can be found in Fig. 13.125–148 Since they have previously been discussed, we have focused on reports that enable nerve agent detection from August 2017 to date.124
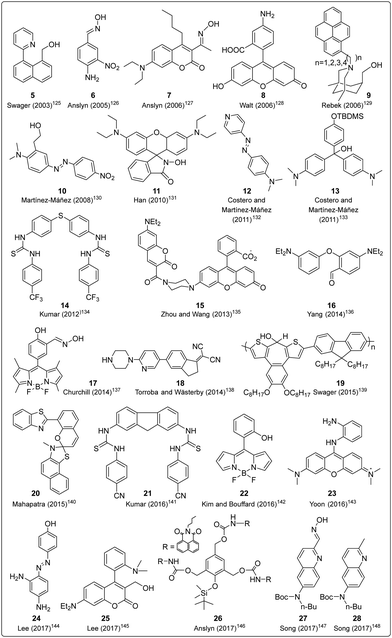 | ||
| Fig. 13 Chemical structures of the molecular probes designed for the detection of nerve agents that were previously discussed by Martínez-Máñez123 and Yoon.124 | ||
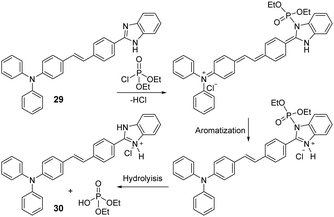
Mondal and co-workers constructed a triphenylamine-benzimidazole-based chemosensor (29) that provides a specific colorimetric and fluorescence change for the detection of diethyl chlorophosphate (DCP) in a THF/H2O (4/1, v/v) solution (Fig. 14).149 The triphenylamine moiety acts as the electron-donor group while the benzimidazole is an electron-acceptor. First, the phosphoryl unit adds to the benzimidazole moiety, which is then hydrolyzed to form protonated species (30). The overall process essentially increases the ICT efficiency of the network and generates dramatic changes in both the absorbance and emission profiles. The authors further used probe 29 by immobilizing it on a TLC plate for the vapor phase detection of DCP.
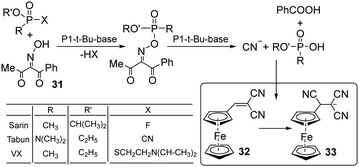
Following an unconventional strategy, Kumar et al. developed a system containing a single molecular probe that enables the simultaneous detection of three nerve agents: sarin, tabun, and VX (Fig. 15).150 In this approach, oxime (31) was used to react with each nerve agent and afford the corresponding nucleophilic hydrolyzed species (F−, CN−, and thiols). Each species reacted with electrophilic dye 32 to change its photophysical properties. As shown in Fig. 15, 32 interacts with cyanide and affords the new chemical species 33. This protocol showed no interference from close competitors, such as electrophilic agents and blister agents.
Exploiting the well-known chemistry used in the RVD kit for the detection of nerve agents, Wang and co-workers used the combination of bovine serum protein and gold nanoclusters to afford 34, which enabled the detection of organophosphorus pesticides in water (Fig. 16).15134 was prepared using gold nanoclusters protected by bovine serum protein (BSA-AuNCs) and a fluorescent substrate, thereby making the complete ensemble fluorescent. This ensemble was found to undergo fluorescence quenching when thiocholine (TC) was released by AChE-mediated enzymatic hydrolysis of acetylthiocholine iodide (ATCI). As expected, the hydrolysis of ATCI is inhibited in the presence of nerve agents (parathion methyl), thus preventing the production of TC and any change in fluorescence at 630 nm. This method was also implemented for the detection of OP pesticides in food samples.
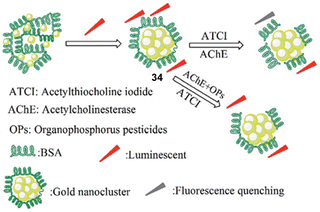 | ||
| Fig. 16 The sensing mechanism of 34 for parathion methyl in water. Reproduced with permission from ref. 151 Copyright (2017) Royal Society of Chemistry. | ||
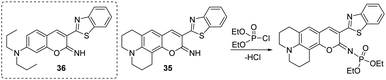
Peng and co-workers developed two on-off chemosensors that contained a nucleophilic imine moiety for the rapid detection of DCP in DMF (Fig. 17).152 The iminocoumarin-benzothiazole-based probes (35 and 36) demonstrated a notable decrease in their fluorescence emission at 526 nm and 531 nm, respectively, within 10 seconds of the addition of DCP. The fluorescence color of 35 changed from green-emissive to light-yellow-emissive, while for 36 a change from green to pink-yellow was observed. The mechanism of detection involves the nucleophilic addition of imine with DCP and the elimination of HCl.
There is no difference in terms of the reactivity of probes 35 and 36. However, 35 displayed a high fluorescent quantum yield, which is ascribed to the increased co-planarity and rigidity of the scaffold from the quinolizidine ring. The present method was also applied for the recognition of DCP in the gaseous state, with significant color changes that are easily observed by the naked eye.
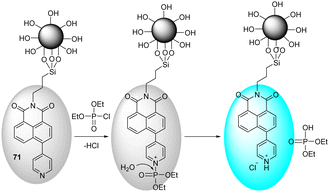
The immobilization of chemical sensors onto inorganic materials (e.g., silica) affords new smart materials with improved attributes. Silica-based hybrid materials demonstrate numerous benefits, such as high selectivity, sensitivity, recyclability, and robustness compared to discrete probes.153 The Ruracka group developed a silica-based material that contained the BODIPY chemical probe 37, which is designed to undergo intramolecular cyclization in the presence of nerve agents.15437 was immobilized on the walls of mesoporous silica materials (SBA-15) to prevent aggregation-caused quenching (ACQ) in water. On interaction with nerve agents (sarin, soman, and tabun), phosphorylation of the hydroxyl group occurred. This was followed by intramolecular cyclization with the pyrrolidine nitrogen to afford the cyclic product 38. This cyclization gave rise to changes in color and fluorescence (Fig. 18).
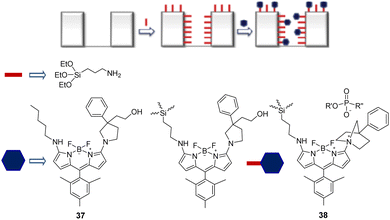 | ||
| Fig. 18 The sensing mechanism of SBA-15-bound 37 for sarin, soman, and tabun in water. Reproduced with permission from ref. 154. Copyright (2017) Elsevier B.V. | ||
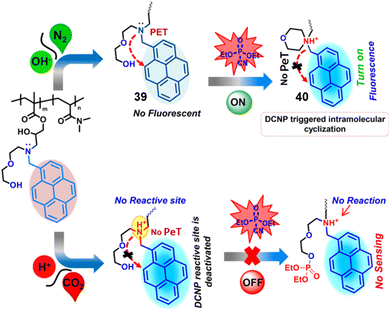
The direct incorporation of a chemical sensor into the polymer backbone is one of the most attractive ways to develop a reversible supramolecular sensor (Fig. 19).155,156 Lee et al. reported a pyrene-based polymeric probe (39) for the off-on sensing of DCNP in THF/H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) mixed solutions and in the vapor phase.157 The polymer sensor (39) contains 2-(2-((pyren-1-ylmethyl)-amino)-ethoxy)-ethanoline (38), in which pyrene is used as the fluorophore and the hydroxyl group is the nerve-agent-recognition unit. The reaction between the hydroxyl arm and DCNP resulted in phosphorylation, followed by intramolecular cyclization which led to the formation of the morpholino cation, 40. Initially, 39 was found to be nonfluorescent due to PeT. However, upon DCNP-mediated cyclization and formation of 40, PeT is inhibited and a significant increase in the fluorescence emission at 395 nm was observed. Probe 39 is tunable via CO2 and pH for the detection of DCNP, and the sensing system is reversible without any build-up of by-products. The tertiary amine groups of the polymer can be protonated to form quaternary ammonium salt upon exposure to CO2 gas, and the resulting protonated amine reverted to the original tertiary amine units in the presence of N2 or the absence of CO2.
1, v/v) mixed solutions and in the vapor phase.157 The polymer sensor (39) contains 2-(2-((pyren-1-ylmethyl)-amino)-ethoxy)-ethanoline (38), in which pyrene is used as the fluorophore and the hydroxyl group is the nerve-agent-recognition unit. The reaction between the hydroxyl arm and DCNP resulted in phosphorylation, followed by intramolecular cyclization which led to the formation of the morpholino cation, 40. Initially, 39 was found to be nonfluorescent due to PeT. However, upon DCNP-mediated cyclization and formation of 40, PeT is inhibited and a significant increase in the fluorescence emission at 395 nm was observed. Probe 39 is tunable via CO2 and pH for the detection of DCNP, and the sensing system is reversible without any build-up of by-products. The tertiary amine groups of the polymer can be protonated to form quaternary ammonium salt upon exposure to CO2 gas, and the resulting protonated amine reverted to the original tertiary amine units in the presence of N2 or the absence of CO2.
 | ||
| Fig. 19 The sensing mechanism of 39 for DCNP. Reproduced with permission from ref. 157. Copyright (2017) American Chemical Society. | ||
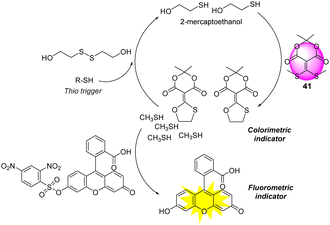
Auto inductive cascades can amplify the signal response and allow for the detection of a single analyte with ultra-high sensitivity.158,159 Anslyn and co-workers reported a unique auto inductive cascade strategy for the detection of V-type nerve agents (Fig. 20).160 This protocol was designed for the optical detection of thiols, which are the hydrolyzed products of V-type agents. Meldrum's acid-based conjugate acceptor (41) was used as an additional thiol source for signal amplification. The autoinduction is initiated by a thiol-disulfide exchange with the release of β-mercaptoethanol, which in turn reacts with 41 to release two equivalents of methyl sulfide. As shown in Fig. 20, this results in a self-propagating cycle that continues until all of 41 is consumed. As a result, this two-step integrated protocol produces a precise diagnostic assay for ultra-trace quantitation of V-type nerve agent detection.
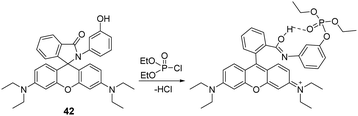
A rhodamine-based chemosensor (42) (Fig. 21) was developed by Sahoo and co-workers for the successful in vivo and in vitro imaging of DCP of DCP in catfish brains.16142 was initially colorless and non-fluorescent. However, the addition of DCP into the H2O/CH3CN (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) solution led to gas-phase-induced ring-opening of the spirolactam ring. This resulted in a 100-fold increase in the emission intensity and a color change from colorless to pink. 42 was used to image DCP in the A549 human cell line and study the distribution of DCP in vivo. This demonstration shows chemosensors can be used to study the effects of nerve agents in living systems, providing opportunities to identify therapeutic countermeasures.
1, v/v) solution led to gas-phase-induced ring-opening of the spirolactam ring. This resulted in a 100-fold increase in the emission intensity and a color change from colorless to pink. 42 was used to image DCP in the A549 human cell line and study the distribution of DCP in vivo. This demonstration shows chemosensors can be used to study the effects of nerve agents in living systems, providing opportunities to identify therapeutic countermeasures.
Che and co-workers reported a unique self-assembly approach for the selective and highly sensitive detection (15 ppb) of DCP in the vapor phase using a fluorescent nanofiber (Fig. 22).162 The fluorescent perylene diimide (PDI) derivative, functionalized with the 4-(hydroxymethyl)benzyl group and a dodecyl chain, was found to self-assemble through light-sensitive internanofiber hydrogen-bonding interactions. In this study, it was found that 4-(hydroxymethyl)benzyl was crucial to the formation of the hierarchical nanofiber, and this structure gave rise to a greater fluorescent quantum yield with respect to its monomeric unit, thereby amplifying the sensing signal. Light irradiation of the internanofiber hydrogen bonds weakened the interactions, resulting in a decrease in the fluorescence emission intensity. The exposure of DCP to the light-irradiated system resulted in the retightening of the nanofibers, enhancing the fluorescence emission intensity.
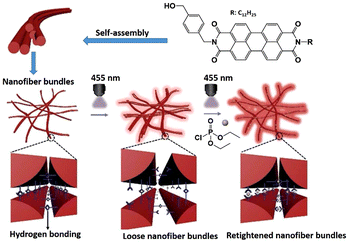 | ||
| Fig. 22 The sensing mechanism of perylene diimide (PDI) derivative-based nanofibers for DCP. Reproduced with permission from ref. 162. Copyright (2018) American Chemical Society. | ||
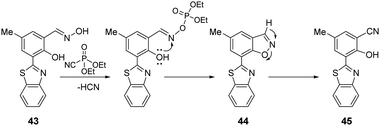
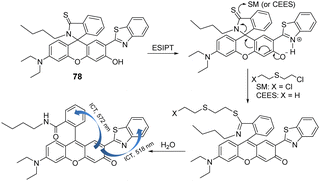
Yoon and co-workers reported the benzothiazole-based chemosensor (43), which is functionalized with an oxime unit, for the selective detection of the nerve agent surrogate DCNP (Fig. 23).163 The new ESIPT (excited-state intramolecular proton transfer) fluorescent probe 2-(2′-hydroxyphenyl)-benzothiazole (43) demonstrated that the hydroxyl group in the oxime moiety undergoes nucleophilic reaction with DCNP, followed by ring-closing and ring-opening to afford the highly fluorescent nitrile derivative 45via the benzisoxazole intermediate 44. An overall 60-fold enhancement in the fluorescence emission intensity at 480 nm was observed. The electron-withdrawing cyano group present in 43 results in strong fluorescence because it inhibits the PeT quenching process responsible for inefficient emission from the probe. Demonstrating its real-life application, the authors used an electrospinning technique to develop 43-containing nanofibers, which were used for vapor detection.
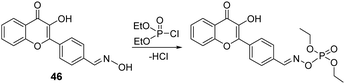
Wang and co-workers established a similar oxime-based chemosensor (46) for the detection of DCP in methanol (Fig. 24).164 In the presence of DCP, a notable fluorescence enhancement accompanied by an emission color change was observed within 90 s by the naked eye in the solution. This is attributed to the fact that the addition of the phosphate group suppresses the intramolecular rotation, thereby increasing the fluorescence of the probe. Dye-coated test strips were constructed for the detection of DCP vapor.
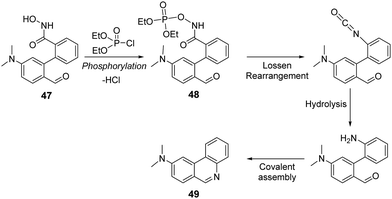
Yang and co-workers reported a “covalent-assembly-based” fluorescent probe (47) for the detection of DCP in acetonitrile (Fig. 25).165 This strategy exploits the Lossen rearrangement with DCP to form cyclic fluorescent product 49 (λem = 418 nm). As seen in Fig. 25, the hydroxamic acid group of 47 was shown to react with DCP to form a phosphoryl intermediate 48 that quickly undergoes Lossen rearrangement to produce an isocyanate intermediate. Since this reaction occurs in water, isocyanate transforms into an aniline intermediate that condenses to form a fluorescent phenanthridine system (49). This sensor featured excellent selectivity, fast response (within 100 s), and a low limit of detection (10.4 nM) toward DCP, even in the gas state.
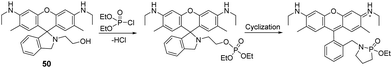
Similar to previous reports,118,122 Son and co-workers reported a rhodamine-deoxylactum-based colorimetric and fluorometric sensor (50) for the instantaneous detection of DCP in DMF (Fig. 26).166 The phosphorylation of hydroxy-arm and the subsequent intramolecular-cyclization-induced ring-opening of the deoxylactum afforded the observed fluorimetric (564 nm) and colorimetric response. 50 was applied to the development of a gas sensing device in the form of polyurethane-based test strips, which were able to detect DCP at a concentration of 9.66 × 10−9 M.
Using a smartphone and an easily assembled LEGO box, Anslyn, Marcotte, and co-workers developed a technique for the simultaneous detection and differentiation of G- and V-series nerve agent mimics for real-world applications (Fig. 27).167 Since fluoride and thiolate are the key constituents of these agents, the detection of these anions can serve as a way to indirectly detect the respective nerve agents. In this study, fluoride anions and thiols (Fig. 27) were used to initiate a self-propagating cascade reaction that amplifies fluorescence signals drastically in a ratiometric manner. It was shown that only one equivalent of fluoride or thiol was needed to activate the cascade reaction of 51 and produce further equivalents of fluoride or thiol. This caused the fluorescent signal to grow exponentially. In both self-propagating cascade reactions, 4-aminonaphthalimide (52) was used as the fluorophore. For signal amplification, benzoyl fluoride and Meldrum's acid-based conjugate acceptor were employed as the latent source of fluoride and thiol, respectively. Using the chromaticity and LEGO approach, concentrations of the analytes were accurately determined and differentiated. To accomplish this, the images were digitally processed using a watershed and morphological erosion algorithm. Watershed and morphological erosion algorithms are used in image processing to generate boundaries between objects, and to remove pixels on that object's boundaries, respectively.
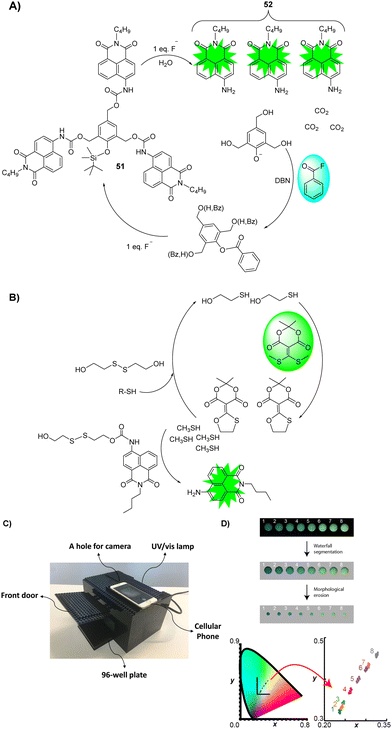 | ||
| Fig. 27 (A) Self-propagating cascade employing benzoyl fluoride as a latent source of fluoride for signal amplification. (B) Self-propagating protocol employs a Meldrum's acid-based conjugate acceptor as a latent source of thiol for signal amplification. (C) A homemade LEGO dark-box for imaging using a cell phone. (D) Fluorescent cascade reaction chromaticity corresponds to the analyte concentration quantitatively, and processing of the image using a watershed and morphological erosion algorithm. Reproduced with permission from ref. 167. Copyright (2018) American Chemical Society. | ||
Zhao and co-workers reported the ratiometric fluorescence detection of DCP vapors using a carbazole-based self-assembled nanofiber (53).168 In this super-fast and ultrasensitive detection method, the exposure of DCP to the nanofibers resulted in the phosphorylation of pyridine, leading to fluorescent quenching within the diffusion length of the nanofibers. This amplified the fluorescence quenching (Fig. 28). At the same time, nanofibers amplified the intramolecular charge transfer emission by harvesting excitons within the diffusion length. The most likely interferents, in this case, could be DCNP; however, these did not interfere because of the relatively weak electrophilic ability of DCNP to form a pyridine-phosphorylated complex. Unfortunately, acidic interference showed an equal response, as in the case of DCP, thereby making acids a major interferent.
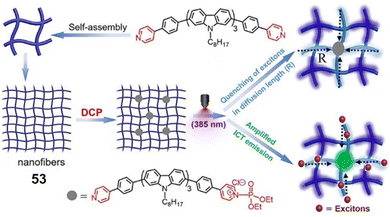 | ||
| Fig. 28 The sensing mechanism of self-assembled nanofibers (53) for DCP. Reproduced with permission from ref. 168. Copyright (2018) American Chemical Society. | ||
Reaction-based sensing methods are one of the best approaches for the development of colorimetric biosensors. As discussed in the introduction, commercial biosensors for nerve agent detection consist of the substrates acetyl(thio)choline or butyryl(thio)choline, which are hydrolyzed by AChE to afford choline and the corresponding acid. The hydrolyzed product is then detected via color change by using a pH indicator, redox indicator, or chromogenic reporter.169–171 In an attempt to improve upon existing biosensing technologies, Matěovský explored the combination of two triphenylmethane dyes, Guinea Green B and Fuchsin, for the visual detection of G and V agents via the indirect detection of thiocholine (Fig. 29) in water.172 Both dyes are sensitive to thiolate attack via Michael-type addition, which changes the optical properties in the form of a blue–red transition (i.e., from λmax 620 nm and 540 nm). The authors have also examined the stability of the sensing system by impregnating the reagents/substrates and dye into the glass nanofiber filter paper for NA detection to successfully demonstrate field applications.
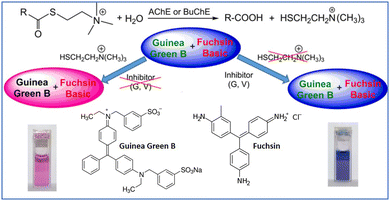 | ||
| Fig. 29 The combination of Guinea Green B and Fuchsin for G- and V-series nerve agents. Reproduced with permission from ref. 172. Copyright (2019) American Chemical Society. | ||
The Iyer group reported a portable sensor device for DCP detection using an amine-functionalized conjugated polymer (Fig. 30).173 Poly(3-(9,9-dioctyl-9H-fluoren-2-yl)benzene-1,2-diamine) (PFPDA) (54) was used as a sensory channel material for a two-terminal sensor, which provided the detection of the analyte with good selectivity and sensitivity (LOD: 5.88 ppb). The amplified current signal was obtained due to the redox interaction between the semiconducting polymer and DCP. Furthermore, the sensing response of the fabricated sensor was fast (3 s), reversible, and reproducible, demonstrating its enormous potential as a portable device for the on-site detection of nerve agents.
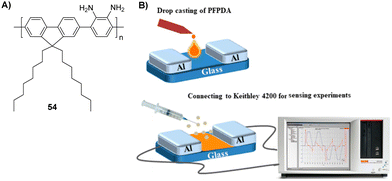 | ||
| Fig. 30 (A) Structure of PFPDA (54). (B) The sensing mechanism of an amine-functionalized conjugated polymer for G- and V-series nerve agents. Reproduced with permission from ref. 173. Copyright (2019) American Chemical Society. | ||
Koning and co-workers reported a chromophore-functionalized metal–organic framework (NU-1000) for the effective degradation and visual detection of VX (Fig. 31).174 The MOF was functionalized with Ellman's reagent (5,5′-dithiobis-(2-nitrobenzoic acid) or DTNB), which is a quantitative reporter for measuring thiol concentrations. The reaction between thiol and Ellman's reagent affords 5-mercapto-2-nitrobenzoic acid (TNB) with a bright yellow absorption (λabs = 412 nm).175 This functional MOF-based material (DTNB@ NU-1000) in neutral buffer degraded VX and afforded a visual response. This degradation showed a linear correlation with the concentration of VX, which allows for quantification.
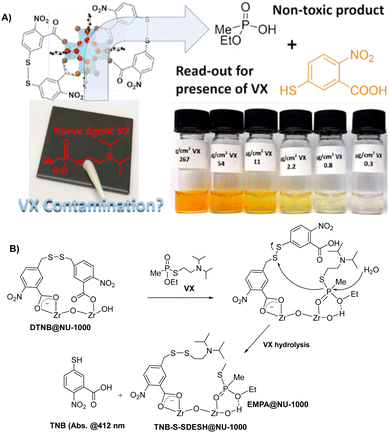 | ||
| Fig. 31 The sensing mechanism of DTNB@NU-1000 for VX. Reproduced with permission from ref. 174. Copyright (2019) American Chemical Society. | ||
Yoon and co-workers developed a fluorescent chemosensor (55), consisting of the anthracene carboxyimide fluorophore and a phosphorylative-reactive o-phenylenediamine unit (Fig. 32).176 The reaction between 55 and DCP in chloroform afforded a large emission enhancement of 55 at 588 nm with a fluorescence quantum yield of 3.2% within a minute. The calculated LOD was 88 nM. This turn-on response was attributed to the phosphorylation of o-phenylenediamine and the inhibition of PeT. Additionally, the 55-coated membrane was constructed for its potential for on-site analysis. Interestingly, 55 was also found to be selective and sensitive to choking agents, such as phosgene.
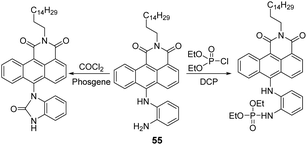 | ||
| Fig. 32 The sensing mechanism of 55 for DCP and phosgene in chloroform. Reproduced with permission from ref. 176. Copyright (2019) American Chemical Society. | ||
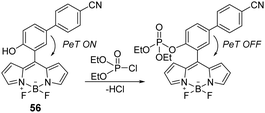
The boron dipyrromethene (BODIPY) scaffold has attracted significant interest in the realm of chemical biology for its use in fluorescent dyes177 and tags.178 Over the years, the BODIPY core has also been used to develop several chemical probes due to its ease of modification, good photostability, and environment-dependent fluorescence quantum yield.179 Attracted by these properties, Lu and co-workers developed a BODIPY-based sensor (56) for the detection of DCP in DMF.180 Probe 56 enabled the rapid (∼1 min) selective and sensitive detection of DCP (Fig. 33). This mechanism of detection was believed to be based on the inhibition of PeT upon phosphorylation of the hydroxyl unit found on 56. Encouraged by the performance of probe 56, the authors evaluated it for the detection of DCP using testing paper and investigated its practical applicability for on-site analysis.
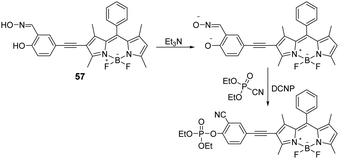
Another example of a BODIPY-based fluorescent sensor (57) for nerve agents’ detection was reported by Zhao and co-workers. In this design, the authors introduced the NA-reactive 2-salicylaldoxime unit onto the BODIPY scaffold via an ethynyl spacer (Fig. 34).18157 in acetonitrile solution was initially treated with the base triethylamine (NEt3), which led to a decrease in the fluorescence emission intensity at 520 nm. Subsequent phosphorylation of the phenol unit through treatment with DCNP resulted in a significant fluorescence enhancement at 560 nm. Probe 57 demonstrated an excellent LOD for DCNP (34 nM), fast response times (t1/2 = 175 s), and excellent selectivity (better than other OP compounds, such as DCP, DMMP, TPP, and TMP). They also investigated the detection of DCNP vapor using test strips with 57 immobilized.
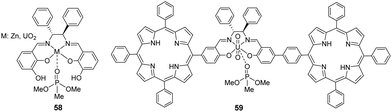
Sfrazzetto et al. demonstrated the ability to detect dimethyl methylphosphonate (DMMP) (a nerve agent simulant, Fig. 1(C)) by exploiting the metal coordination properties of phosphate esters with metal–salen complexes (Zn2+ and UO22+) (58) (Fig. 35).182 The interaction studies revealed that the coordination of DMMP with Zn–salen resulted in the complex adopting a square-based pyramidal geometry with molecular recognition occurring in a pseudo-axial position. In contrast, the UO2–salen complex adopted a bipyramidal geometry with DMMP coordinated to the fifth equatorial position.183 Additionally, the binding constant values of the coordination to the complex were further enhanced due to hydrogen bonding interactions between hydroxyl groups of 58 and DMMP. The recognition between Zn–salen and DMMP was performed in chloroform, which resulted in the rise of fluorescence emission at 430 nm. In an attempt to generate a colorimetric response, Pappalardo et al. developed the bis-porphyrin–salen–UO2 complex (59) for the detection of DMMP.184 The same coordination event led to significant color changes from light blue to purple in CHCl3. The presence of the porphyrin units was crucial for gaining these absorption properties owing to their extensive conjugation and photophysical properties. Since this coordination event is reversible, this sensor is particularly attractive for real-world applications as the device has the potential to be recycled.
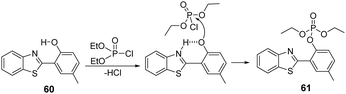
Bao and co-workers reported the ESIPT-based fluorescent chemosensor (60) for the detection of DCP in acetonitrile. Initially, 60 displayed fluorescence emission due to keto-/enol-form conversion. The phosphorylation of 60 by DCP resulted in the inhibition of the ESIPT mechanism, leading to a concomitant change in fluorescence emission intensity at 453 nm (Fig. 36).185 This could lead to turn-on fluorescence within six seconds, with high sensitivity and selectivity. The nucleophilicity of the phenol group is greatly enhanced by the proton transfer from the phenol group to the benzothiazole group to form the phosphorylated product (61), leading to a strong fluorescence response. 61 was immobilized in a polystyrene-based membrane for on the spot analysis of DCP vapor with the naked eye. This approach provides a practical, real-time detection kit for DCP environmental samples.
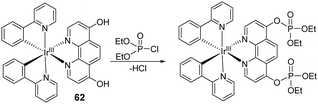
Khatua and co-workers synthesized the organometallic complex (62) for the detection of a nerve agent mimic in acetonitrile (Fig. 37).186 The reaction between 62 and DCP resulted in a ratiometric change in the fluorescence emission intensity from 518 nm to 565 nm in an acetonitrile solution at nanomolar concentrations. This change in luminescence was attributed to an increase in steric hindrance upon phosphorylation, which restricted intramolecular rotation. This led to the optical changes. 62-coated paper strips and PEO films which were prepared for visual detection of DCP vapor.
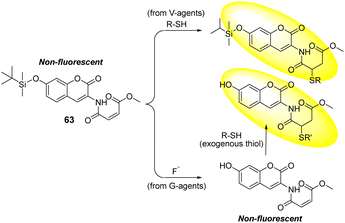
In an attempt to distinguish between G- and V-series nerve agents, Zhigang's group designed the coumarin-based fluorescent probe 63, which contained two different reactive units for fluoride anions (F−) and thiols, respectively. Fluoride is the hydrolyzed product of sarin and soman nerve agents and thiol is the hydrolyzed product of the VX nerve agent (Fig. 38).187 The two reactive groups are maleimide and silyl, which are specific for thiols. These react via conjugate addition and fluoride-mediated silyl deprotection, respectively. Distinct fluorescence responses were obtained in both cases. The reaction of 63 with thiols in an EtOH-HEPES buffer solution produces a clear peak in the fluorescence emission at 404 nm within 1 min. In the case of fluoride detection, the reaction of 63 with F− in the EtOH-HEPES solution initially led to no fluorescence change due to the formation of the desilylation product of 63. This desilylation product develops a response upon the addition of exogenous thiols, showing a new fluorescence emission peak at 460 nm.
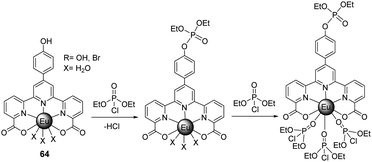
Ln(III)-based luminescent sensors are routinely developed for the detection of analytes due to their strong luminescence and reversible properties.188 The strong Lewis acidity and oxophillic nature of Ln(III) ions allow the selective coordination-mediated detection of phosphate-based nerve agents. The terpyridine-dicarboxylate-based Eu(III) probe, 64, was recently reported by Patra and co-workers as a turn-on luminescent sensor for the selective detection of nerve agent simulants, e.g., DCP, in acetonitrile (Fig. 39).189 Upon gradual addition of DCP, a subtle decrease was observed initially, followed by a rapid enhancement of the emission intensity of 64. This could be attributed to the fact that the nucleophilic attack at the electron-deficient phosphoryl–chloride bond of DCP by the peripheral phenolic –OH group of the antenna is known to be facile. This results in a minor decrease in the luminescence intensity, originating from f → f transitions. However, after the subsequent addition of DCP to 64, a notable increase in the luminescence intensity was observed, especially for the hypersensitive 5D0 → 7F2 transition band at 614 nm. This is due to the subsequent displacement of the labile water molecules from the coordination sphere.
Thiagarajan and co-workers evaluated the photophysical and aggregation-induced emissive (AIE) properties of the azine-based donor–π–acceptor probe (65) in different solvents with varying polarity (toluene, DCM, THF, MeOH, ACN, and DMF) (Fig. 40).19065 was shown to detect DCP in both its monomeric form and aggregated forms. In THF (Fig. 40(A)), the imine nitrogen of 65 was found to undergo phosphorylation, resulting in a red-shift in absorption (460 nm) and strong orange fluorescence with maximum emission at 513 nm. In a THF/water mixture (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) (Fig. 40(B)), DCP undergoes hydrolysis to form a diethyl phosphate and release hydrochloric acid. Subsequent protonation of the amine nitrogen of 65 resulted in changes in the absorption and emission spectra, with a absorption band shift from 417 nm to 350 nm and a emission shift from 570 nm to 406 nm.
70) (Fig. 40(B)), DCP undergoes hydrolysis to form a diethyl phosphate and release hydrochloric acid. Subsequent protonation of the amine nitrogen of 65 resulted in changes in the absorption and emission spectra, with a absorption band shift from 417 nm to 350 nm and a emission shift from 570 nm to 406 nm.
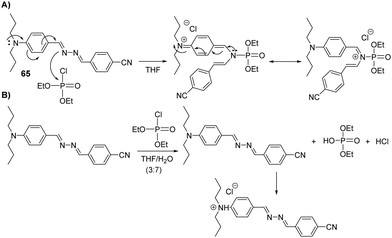 | ||
Fig. 40 (A) The sensing mechanism of 65 for DCP in THF. (B) The sensing mechanism of 65 for DCP in a THF/water mixture (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70). 70). | ||
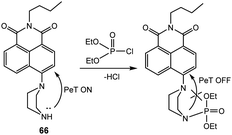
As shown in Fig. 41, naphthalimide-based fluorescent probe 66 was developed by Cheng's group for the detection of DCP in DMF.191 Initially, 66 was found to be weakly emissive, owing to PeT quenching from the piperazine nitrogen. The DCP-mediated phosphorylation of 66 resulted in the inhibition of PeT, which resulted in a significant fluorescence enhancement (150-fold) at 510 nm. In an attempt to explore practical applications for the vapor phase detection of DCP, probe 66 was also coated on filter paper; the desired response was observed, as expected.
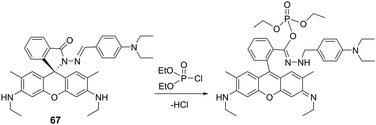
Bag and co-workers, reported the rhodamine-based chemical probe, 67, for the fluorimetric and colorimetric detection of DCP in ethanol (Fig. 42).192 The mechanism of detection was believed to be based on the phosphorylation of the carbonyl of the spirolactam unit, which induced ring-opening and afforded both an increase in absorption at 533 nm and a fluorescence increase at 553 nm (λex 480 nm).
In 2020, the functional polymer 68 containing (E)-2-(methyl(4-(pyridine-4-yldiazenyl)phenyl)amino)ethyl acrylate, N-(4-benzoylphenyl)acrylamide, and N,N-dimethyl acrylamide as polymer subunits was developed by Lee's group for the selective and visual detection of DCP in water (Fig. 43).193 An instant optical change from yellow to red was observed when 68 was treated with DCP in water. Quaternization of the pyridine groups of 68 was accompanied by a color change from yellow to pink due to strong interactions between electron-donating nitrogen atoms and electron-withdrawing quaternary ammonium (pyridinium) salts. Demonstrating the reusability of this polymeric system, a 2.0 M ammonia solution in ethanol was used to regenerate DCP-exposed films via deprotonation of the pyridine moiety. This was accompanied by a color change from pink back to yellow. This repeatability was shown in four successive cycles, which confirmed this system could provide an ideal solution for the detection of these analytes in a pure aqueous medium.
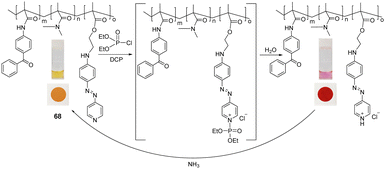 | ||
| Fig. 43 The sensing mechanism of colorimetric polymer 68 for DCP in water. Adapted with permission from ref. 193. Copyright (2019) American Chemical Society. | ||
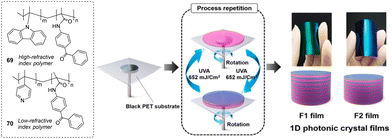
Lee and his group developed one-dimensional photonic crystal films for the colorimetric detection of a nerve agent mimic (DCP) (Fig. 44) in the vapor phase.194 The films were constructed via sequential, alternating layer deposition of polymers with high and low refractive indexes (69 and 70) containing poly(N-vinyl carbazole-co-benzophenone acrylate) and poly(4-vinyl pyridine-co-benzophenone acrylate) as photocrosslinkable units onto a black polyethylene terephthalate substrate. This was achieved via the spin-coating of each layer, followed by chemical immobilization onto the surface via UV-light irradiation. The polymers contained 7–15 mol% of photoactive benzophenone acrylate to promote film stability with high crosslinking density. The initial colors of the F1 and F2 films were green and cyan with maximum reflectance wavelengths of 540 and 490 nm, respectively. The color of the F1 film changed from green to yellow and finally to red when exposed to increasing DCP concentrations. F1 was chosen because it exhibited more diverse color transitions than F2 with increasing DCP concentrations. No response was observed when the films were treated with DMMP, TEP, TBP, or DCNP. The quaternary pyridinium salts generated by DCP detection can be dequaternized in the presence of an ammonium hydroxide solution. The sensitivity and visibility were enhanced with an increase in the relative humidity, and the detection process was reversible and repeatable over three cycles.
 | ||
| Fig. 44 Pictorial representation of the sensing ensemble based on one-dimensional photonic crystal films of high and low refractive index polymers (69 and 70) for DCP in the vapor phase. Reproduced with permission from ref. 194. Copyright (2020) Elsevier B.V. | ||
Takahashi and co-workers developed a gold nanoparticle (AuNPs) approach that could be used for the naked-eye detection of VX in a buffer solution (pH 9.0) (Fig. 45).195 The authors’ strategy focused on the detection of 2-(diisopropylamino)ethanethiol (DAET), i.e., the hydrolyzed product of VX. In this study, protonated DAET was found to interact with anionic-based AuNPs (capped with citric acid). As illustrated in Fig. 46, the electrostatic interaction between cationic DAET and anionic AuNPs promoted aggregation, leading to a color change from red to blue with a bathochromic shift from 520 nm to 680 nm (Fig. 46). This optical change is attributed to dipole coupling between the plasmons of neighboring aggregated particles.
 | ||
| Fig. 46 The sensing mechanism of AuNPs capped with citric acid for DAET in a buffer solution (pH 9.0). Reproduced with permission from ref. 195. Copyright (2021) Elsevier B.V. | ||
Zhang and co-workers exploited 1,8-naphthalimide-pyridine-functionalized hybrid SiO2 nanoparticles (71) for DCP detection in THF (Fig. 47).196 1,8-naphthalimide was used as the fluorescent reporter with the pyridine moieties used to react with DCP and DCNP. The addition of both DCP and DCNP resulted in phosphorylation of the pyridine moieties, followed by hydrolysis and their subsequent protonation. This mechanism resulted in a large enhancement in the fluorescence emission at 427 nm. Interference from mineral acids (e.g., HCl, HBr) is commonly observed for pyridine-based strategies. However, immobilizing 71 on the hydrophilic silica overcame this issue. This was attributed to mineral acids having a greater affinity for the surface of SiO2 NPs.
4.2 Fluorescent and colorimetric chemosensors for the detection of blister agents
The development of chemical sensors for the optical detection of blister agents has primarily focused on SM, rather than NM or Lewisite. This is believed to be due to the lack of appropriate molecular recognition units. In general, the design of chemosensors for the detection of SM targets the electrophilic alkyl-halides or the electrophilic episulfonium ions that form in a polar medium (Fig. 48).197 However, this nucleophilic strategy often leads to poor selectivity against other electrophilic CWA analytes (i.e., nerve agents). Therefore, fine-tuning of the chemosensor and experimental conditions are needed. Over the years, extensive efforts have been devoted by several research groups to develop SM-responsive probes with high selectivity and sensitivity. The reported approaches for SM detection must use the mimic CEES, which is far more reactive: SM (t1/2, 8.5 min at 25 °C in H2O) vs. CEES (t1/2, 44 s at 25 °C in H2O).198 The instant and accurate detection of SM is imperative for medical countermeasures. Current technologies require around a week to a month for analysis in dedicated laboratories.199![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, yielding luminescence at 382 nm which is about 130 times stronger than that of the free probe. Unfortunately, a major flaw with this approach was that the presence of any species with similar electrophilicity or acids (Lewis and Brønsted) are likely to cause interference and lead to a false-positive signal.
1, yielding luminescence at 382 nm which is about 130 times stronger than that of the free probe. Unfortunately, a major flaw with this approach was that the presence of any species with similar electrophilicity or acids (Lewis and Brønsted) are likely to cause interference and lead to a false-positive signal.
Using their expertise in indicator displacement assays (IDAs), Anslyn and Kumar reported the first-ever selective and sensitive fluorescent sensor for the detection of CEES201 and then again for SM (Fig. 50).202 In this design, a dithiol (73) was first allowed to react with CEES at 80 °C under pH 9 to form podand (74). 74 was then able to interact with the metal-indicator complex (75), resulting in the release of an indicator (coumarin fluorophore, ME) by forming metal-podant complex 76 with a concomitant turn-on fluorescence response at 460 nm. This strategy took advantage of the greater binding affinity of podand for Cd2+ ions. By taking advantage of the molecular design and reaction conditions, interference from nerve agents and other electrophilic agents was not observed. This is because nerve agents were rapidly hydrolyzed under these alkaline conditions (pH 9), and the less-reactive species, such as benzyl bromide and the bis-(2-chloroethyl)ether (BCEE), could not react under sensing conditions. The sensing ensemble was designed in such a way that it was directly implemented on bialkylating SM. Kumar et al. applied this same protocol to realize the selective and sensitive detection of SM.200 However, in the present case, it is necessary to use capping agents to tame the ligating properties of thiolate anions of 73, particularly when SM is absent. As a result, it is necessary to cap the thiol before performing experimental analysis. This process makes this method somewhat cumbersome.201
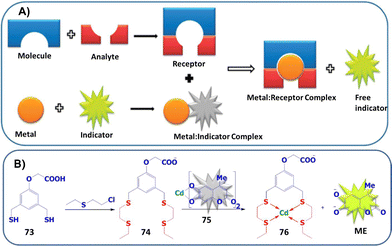 | ||
| Fig. 50 (A) Pictorial presentation of the IDA approach for SM detection. (B) Schematic illustration of chemical sensor components for CEES detection. Reproduced with permission from ref. 201. Copyright (2013) American Chemical Society. | ||
Ongoing efforts by Anslyn and Kumar resulted in the exploitation of squaraine (SQ) dyes for the colorimetric and fluorescent detection of CEES203 and SM in a basic methanolic solution.204 Squaraine dyes are a class of organic dyes that have an intense blue color with an absorption maxima between 630–670 nm and a fluorescence emission between 650–700 nm. The central, electron-deficient, four-membered ring is well-known to be susceptible to nucleophilic attack, which leads to its decolorization (Fig. 51(B)). In this approach, dithiol 73 was again used to react with CEES (and SM) and then exposed to SQ. If the blue color from SQ persists, this indicates the presence of SM. Alternatively, in the absence of CEES, 73 reacts with SQ, resulting in a color change from blue to colorless (Fig. 51). This sensing scheme was further extended by Kumar and co-workers for SM detection.202 Taking advantage of the visual detection of SM, the method was further explored for on-site detection by analyzing the sample in various matrices (soil and surface).
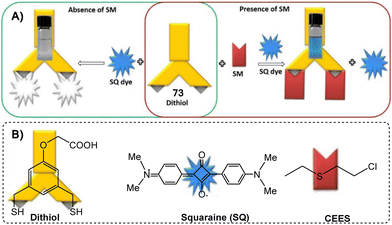 | ||
| Fig. 51 (A) Pictorial representation of a chemodosimeter approach for SM detection. (B) Chemical structure of the species involved in CEES sensing. Reproduced with permission from ref. 203. Copyright (2017) Royal Society of Chemistry. | ||
Pardasani and co-workers reported the rhodamine-6 G-based chemosensor, 77, for the detection of SM and NM in methanol/chloroform (4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). In this design, thioamide functionality was used for the selective recognition of SM and NM (Fig. 52).205 The S-alkylation of the thioamide functional group induced ring-opening of spirolactam, affording a simultaneous increase in absorption at 520 nm and fluorescence emission at 566 nm. This fluorescence response was observed between 15–60 min at room temperature and within 3 min at 60 °C. The selectivity of 77 was demonstrated against various alkyl halides, such as butyl iodide, butyl bromide, and BCEE.
1). In this design, thioamide functionality was used for the selective recognition of SM and NM (Fig. 52).205 The S-alkylation of the thioamide functional group induced ring-opening of spirolactam, affording a simultaneous increase in absorption at 520 nm and fluorescence emission at 566 nm. This fluorescence response was observed between 15–60 min at room temperature and within 3 min at 60 °C. The selectivity of 77 was demonstrated against various alkyl halides, such as butyl iodide, butyl bromide, and BCEE.
Using a similar design concept, Wang and co-workers reported a benzothiazole-functionalized rhodol with SM-reactive thioamide functionality (78) for the chromo-fluorogenic detection of SM in methanol (Fig. 53).206 Rhodol can be seen as a hybrid between rhodamine and fluorescein.207 Similar to rhodamine and fluorescein, rhodol (in the presence of appropriate external stimuli) can undergo spirocyclic ring-opening to form the highly fluorescent quinoid form. In the presence of SM, the solution of 78 changed from colorless to fuchsia. The reaction between SM and 78 was shown to induce ring-opening, which was found to be faster than the rhodamine-based sensor (20 min vs. 60 min). The authors attributed this rapid response to the benzothiazole-mediated ESIPT, where intramolecular proton transfer generated the photoinduced structural transformation of rhodol from the phenol form to the quinoid form. At room temperature and 60 °C, optical responses were observed within 25 min and 3 min, respectively. The selectivity was superior over alkyl halide, an oxygen analog of SM, and strong electrophiles (such as benzoyl chloride). Probe 78 was further used for the detection of SM in solution, soil, and air at ambient temperature, indicating its potential application for the on-site detection of SM.
Wolfbeis and co-workers developed enzyme-based test strips for the visual/photographic detection and quantification of SM vapor (Fig. 54).208 This strategy relies on the use of the enzyme haloalkane dehalogenase as a biorecognition element. This enzyme is known to degrade SM spontaneously into non-toxic thiodiglycol, chloride ions, and a proton. Using pH indicators present in the test strips, this change can be directly visualized from a color change of blue-green to yellow within 10 min. A quantitative readout was achieved using a conventional digital camera based on red-green-blue data acquisition. Selectivity was seen over 1,2-dichloroethane and ethyl bromoacetate, and excellent sensitivity was observed, especially at 0.01 ppm (for visual detection) and at 0.003 ppm (for red-green-blue read-out). These results make this approach extremely valuable as the strips are disposable, fairly rapid, and cost-effective for field use.
 | ||
| Fig. 54 Pictorial representation of photographic detection and quantitation of SM in the vapor phase. Reproduced with permission from ref. 208. Copyright (2016) American Chemical Society. | ||
In 2017, the mercaptomethylphenyl-modified tetraphenylethene (TPE) probe 79 was developed by Wang and co-workers for CEES detection in an aqueous solution (Fig. 55).209 The authors developed a mercaptomethylphenyl-modified tetraphenylethene probe that was allowed to react with CEES to form a sulfur-based podand with four open chains, i.e., 1,1,2,2-tetrakis(20-(ethylthio)propyl-[1,10-biphenyl]-4-yl)-ethene (80). These two staggered sulfur-based chains ligate with thiophilic metal ions, such as (Hg2+ and Cd2+ ions, form sandwiched structures). Such face-to-face molecular stacking eventually restricts the rotation of the phenyl rings of TPE. This process results in a fluorescence enhancement, leading to a selective fluorescence turn-on detection of SM simulants like CEES. The procedure shows high sensitivity (1.10 μM) and good selectivity over butyl iodide, butyl bromide, BCEE, and 2-chloroethyl ethyl ether.
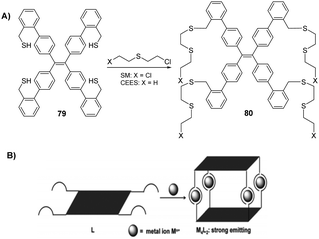 | ||
| Fig. 55 (A) The sensing mechanism of 79 for CESS and SM in an aqueous solution. (B) Its schematic presentation. Reproduced with permission from ref. 209. Copyright (2017) Royal Society of Chemistry. | ||
Kumar and co-workers developed probe 81 for the detection of SM in methanol. In this design, 81 incorporated the nucleophilic thioketone (thione) unit to interrupt the conjugation of the acridine orange (AO) dye scaffold (Fig. 56).21081 reacted with SM via the thione unit to afford the conjugated AO derivative with a change in turn-on response. The selectivity for SM over sarin, soman, tabun, VX, oxygen mustard, benzyl bromide, ethyl iodide, and HCl was achieved by fine-tuning the reaction conditions where methanolic potassium hydroxide acts as a base and was used to destroy any additional reactive interference. The detection is achieved within one minute, affording a color change from yellow to orange (443 nm to 502 nm) and a fluorescence change from green to yellow (518 nm to 555 nm) in less than a minute at 60 °C.210 To assess the feasibility of using 81 for real-time analysis of SM, soil samples, swab samples, and a kit for on spot the detection were used. 81-coated test strips displayed no response, even with organic solvents, acids, and bases. This method was highly sensitive when using chromogenic and fluorogenic techniques with calculated LODs of 0.02 mg (0.3 mM) and 0.005 mg (7.5 μM), respectively, which is the safe value (0.02 mg) to cause any blister on human skin.
A similar approach was developed by Tian and co-workers, in which the pyronin-based fluorescent probe 82 was capable of detecting an SM simulant with concentrations as low as 0.6 μM (in solution phase) and 0.25 ppm (in gaseous phase); these are below the AGEL-1 level of SM (Fig. 57).211 The S-alkylation of 82 with CEES was shown in DCM, which resulted in the formation of a highly fluorescent thiopyronin derivative (λem = 570 nm, 850–fold turn-on) with a marked shift in absorption from 445 nm to 567 nm. Excellent selectivity was seen for CEES over other interfering substances with similar reactivities, such as nerve agent's simulants (DCP and DCNP), acylating agents, an alkylating agent (ethyl iodide), and sulfur-containing compounds. An 82-treated TLC plate was developed and exposed to the vapor of CEES, which showed marked contrast in the color and fluorescence brightness (under illumination with 365 nm UV light) to those of the plate before the 2-CEES vapor treatment.
Broome and co-workers reported a new strategy that uses a pyrene functionalized with a salicylic acid tether (83) to enable the selective detection of alkylating agents, such as methyl iodide (MeI), in a mixed PBS (phosphate-buffered saline) buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PBS/DMSO (v/v)) (Fig. 58).212 Initially, 83 was found to be weakly emissive. However, upon alkylation of the carboxylic acid with MeI, a 1000-fold increase in the fluorescence emission at 475 nm was observed. The mechanism of detection was proposed to be FRET-based, in which salicylic acid and pyrene act as FRET acceptors and FRET donors, respectively. The esterification resulting from alkylation by MeI turns on the fluorescence at 475 nm. The sensing is realized within 48 h (at 40 °C) or within 30 min at 90 °C using tetrabutylammonium iodide (Bu4NI) as a phase-transfer catalyst. Along with CW detection, the protocol was also implemented for the analysis of alkyl bromides and iodides, diazomethane, and their derivatives, as well as for pharmaceutical compounds like anticancer drugs, busulfan, and pipobroman (Fig. 59). Interestingly, 83 was the first probe of its kind to use oxygen as the nucleophile recognition unit; most sensors rely on either tertiary nitrogen or sulfur nucleophiles.
1 PBS/DMSO (v/v)) (Fig. 58).212 Initially, 83 was found to be weakly emissive. However, upon alkylation of the carboxylic acid with MeI, a 1000-fold increase in the fluorescence emission at 475 nm was observed. The mechanism of detection was proposed to be FRET-based, in which salicylic acid and pyrene act as FRET acceptors and FRET donors, respectively. The esterification resulting from alkylation by MeI turns on the fluorescence at 475 nm. The sensing is realized within 48 h (at 40 °C) or within 30 min at 90 °C using tetrabutylammonium iodide (Bu4NI) as a phase-transfer catalyst. Along with CW detection, the protocol was also implemented for the analysis of alkyl bromides and iodides, diazomethane, and their derivatives, as well as for pharmaceutical compounds like anticancer drugs, busulfan, and pipobroman (Fig. 59). Interestingly, 83 was the first probe of its kind to use oxygen as the nucleophile recognition unit; most sensors rely on either tertiary nitrogen or sulfur nucleophiles.
 | ||
Fig. 58 The sensing mechanism of 83 for alkylating agents, such as methyl iodide, in a mixed phosphate-buffered saline buffer (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 PBS/DMSO (v/v)). 1 PBS/DMSO (v/v)). | ||
Fluorescent probes with absorption and emission in the near-infrared region (650–900 nm) are more attractive for biological applications, as it permits deeper tissue penetration, low background interference, and low phototoxicity.213 One of the most notable reports on the NIR detection of SM was developed by Cao, Li, and Xiao. Among the series of reported fluorescent probes (84–87) (Fig. 60), 87 afforded the best response for SM combined with excellent selectivity.214 The reaction of 84–86 and 87 with SM produced fluorescence responses at 600 nm and 690 nm in 25–60 min and 10 min, respectively. The developed sensing systems were tested for SM selectivity against other interfering substances, such as nerve gas mimics, sulfur-containing compounds, inorganic compounds, alkylating agents, and hydrochloric acid, all of which did not lead to a change in fluorescence intensity. To provide new insights into SM-mediated injury, 87 was successfully applied to image SM distribution in live cells (HaCaT cells), and SM was found to accumulate in the mitochondria (Fig. 61). Examples like 87 can be used as tools for the study of SM in biological samples.
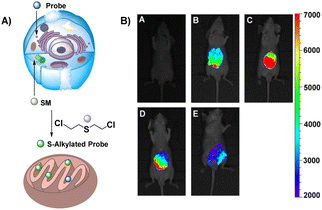 | ||
| Fig. 61 (A) Possible fluorescence mechanism of 87 concentrated in the mitochondria. (B) Representative fluorescent images for visualizing sulfur mustard levels in living SKH-1 mice using SiNIR-SM. Reproduced with permission from ref. 214. Copyright (2019) American Chemical Society. | ||
Che and co-workers reported the chemical sensor 88 for the simultaneous detection and differentiation of a sulfur mustard simulant (CEES) and DCP in the vapor phase (Fig. 62). This approach used nanofibers that were co-assembled with 88 and 89; through FRET, the photostability and fluorescence emission at 466 nm were enhanced.215 Upon exposure to DCP and CEES, fluorescence quenching occurred due to the formation of a complex between DCP or CEES and 88. This acts as an exciton trap to compete with FRET, thereby decreasing the emission. Additionally, combining 88 with 89 in nanofibers results in competitive CEES binding providing distinct fluorescence response, thereby facilitating the detection of DCP and CEES. The probes exhibited excellent selectivity for DCP and CEES against other test substrates, including DCNP, HCl, DMMP, common organic solvents, and water. The LOD for CEES was 0.3 ppm, which is among the lowest values ever reported.
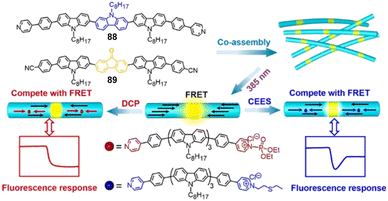 | ||
| Fig. 62 Schematic representation of the fluorescence detection and discrimination of DCP and CEES using the coassembled nanofibers with 88 and 89 in the vapor phase. Reproduced with permission from ref. 215. Copyright (2019) American Chemical Society. | ||
In an important development, rather than using a nucleophilic reaction for SM detection, Che and co-workers reported the ability to detect SM based on noncovalent interactions (sulfur–π and dipole–dipole interactions), thus affording desirable reversibility.216 The systems include hierarchical microspheres assembled from fluorene-based oligomer 90 or two fluorene-based oligomers (90 and 91) resulting in sensitive fluorescence detection of SM vapor (30 ppb) (Fig. 63). The responses of the systems were evaluated in terms of their fluorescence response with SM and interferences.
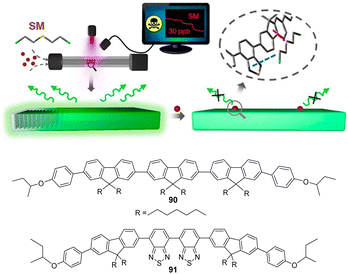 | ||
| Fig. 63 Schematic representation of the fluorescence detection of SM using hierarchical microspheres of fluorene-based oligomers (90) and (91) in the vapor phase. Reproduced with permission from ref. 216. Copyright (2019) American Chemical Society. | ||
Recently, Gall and co-workers explored a different approach using a FRET-based fluorescent probe (92) for the detection of reactive alkylating agents, such as CEES and alkyl halides, in DMSO/H2O (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (Fig. 64).217 The chemosensor contains a fluorophore and a quencher linked using a reactive spacer; upon reaction with CEES, the sample degrades to release the coumarin fluorophore, leading to an increase in the fluorescence signal at 477 nm. In probe 92, a nucleophilic tertiary amine reacts with electrophilic alkylating agents, to generate a tetraalkylammonium cation. In water elimination and separation of the quencher and fluorophore occurs (Fig. 65). In the probe, coumarin is employed as a fluorophore whose fluorescence is quenched by DABCYL (4-(dimethylaminoazo)benzene-4-carboxylic acid) (i.e., a quencher). The beauty of these techniques lies in their ability to detect not only SM simulants but also nitrogen mustard. Turn-on fluorescence was demonstrated with several alkyl halides over related interfering substances, like epoxides. The limit of detection for CEES was calculated to be 2.3 mM.
1) (Fig. 64).217 The chemosensor contains a fluorophore and a quencher linked using a reactive spacer; upon reaction with CEES, the sample degrades to release the coumarin fluorophore, leading to an increase in the fluorescence signal at 477 nm. In probe 92, a nucleophilic tertiary amine reacts with electrophilic alkylating agents, to generate a tetraalkylammonium cation. In water elimination and separation of the quencher and fluorophore occurs (Fig. 65). In the probe, coumarin is employed as a fluorophore whose fluorescence is quenched by DABCYL (4-(dimethylaminoazo)benzene-4-carboxylic acid) (i.e., a quencher). The beauty of these techniques lies in their ability to detect not only SM simulants but also nitrogen mustard. Turn-on fluorescence was demonstrated with several alkyl halides over related interfering substances, like epoxides. The limit of detection for CEES was calculated to be 2.3 mM.
In 2021, Kumar's group designed and developed an innovative protocol that employed luminol as a receptor and a fluorescence reporter for the detection of SM in a bicarbonate buffer (pH = 8.5) (Fig. 66).218 The identified reaction conditions needed to afford a response between 93 and SM used ionic liquid and water in an optimized concentration (0.31 M). The ionic liquid viz. 1-ethyl-3-methylimidazolium dicyanamide ([emim] [DCA]) not only enhanced the nucleophilicity of 93 by abstracting its enolic proton using a dicyanamide fragment but also stabilized the intermediate ion (episulfonium ion) of SM with the help of its positive counterpart, i.e., 1-ethyl-3-methylimidazolium. The combination of luminol and the ionic liquid in water exhibited a turn-on fluorescence response at 420 nm for SM within seconds with a sensitivity of 6 ppm. No interferences from reactive and non-reactive species were observed; these included nerve agents, reactive electrophilic agents, BCEE, benzyl bromide, and diethyl sulfide. In order to make the method field-deployable, a chromogenic response was attained by using a high concentration of 93. Significantly, the analysis of environmental samples, swab samples, and vapor phase detection can pave the way for the development of miniaturized, portable, and cost-effective chemosensor kits that can be used in the field and at strategic locations, such as airports and subways.
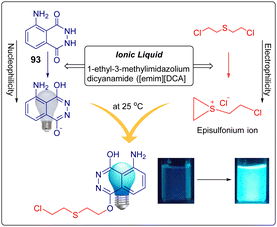 | ||
| Fig. 66 Schematic illustration of chemical sensing based on luminol (93) for SM. Reproduced with permission from ref. 218. Copyright (2021) American Chemical Society. | ||
Based on an IDA ‘proof of concept’ approach, Beer and co-workers developed and evaluated a turn-on fluorescent sensor for SM detection (Fig. 67). In this study, a dansyl fluorophore was ligated to gold nanoparticles (AuNPs) via imidazole and amine groups.219 The authors hypothesized that the displacement of the nitrogen of imidazole or amine, with the relatively soft sulfur donors present in SM/CEMS, release the dansyl fluorophore and affords a fluorescent response. The initial non-fluorescent conjugate is the result of an energy transfer process due to the proximity of functionalized fluorophores to a gold nanoparticle; this is attributed to through-space relaxation by nano surface energy transfer (NSET). Both AuNPs exhibited a weak fluorescence from the ligated dansyl group with emission maxima around 500 nm. The addition of the CEMS or SM produced a significant increase in the fluorescence intensity, which is due to the distance, dependent nature of the non-radiative processes. The response between both nanoparticles and analytes was almost instant and was complete within five minutes. Notably, analogous interfering agents, such as octanol and di-n-butyl ether, gave no significant fluorescence increase. Based on the SPECFIT analysis, the association constants between the host and guest were log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.65 ± 0.06 and log
K = 4.65 ± 0.06 and log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K = 4.19 ± 0.05 for CEMS and SM, respectively.
K = 4.19 ± 0.05 for CEMS and SM, respectively.
Dubey and co-workers exploited microfluidic paper-based analytical devices, i.e., μ-PADs, for the detection of blister agents along with nerve mimics by utilizing well-established chemistry (Fig. 68).220 μ-PADs are cellulose fiber networks possessing several advantages, such as very low cost, power-free application, portability and disposability, compatibility with a small volume of samples, and easy operation and construction. They are generally used for point-of-care diagnosis, biosensing, environmental monitoring, biomedical and pharmaceutical analysis, clinical diagnosis, and forensic investigations. In this study, a tree-shaped μ-PAD with one central channel and four test zones was designed. Out of the four test zones, two test zones were used for blister agents and nerve agents, respectively, and two were used for their respective controls. The detection of blister agents was based upon their reaction with para-nitrobenzyl pyridine in the presence of a base, which generates an intense purple/blue ionic product. The reaction of R6GH with nerve agents generated a fluorescent red/pink color. SM, NM, and nerve agents exhibited LOD values of 100 μM, 1 mM, and 2.5 mM, respectively.
 | ||
| Fig. 68 (A) Design of μ-PAD for the simultaneous detection of nerve agents and blister agents. (B) Colorimetric responses with sarin and SM (100 mM) on the μ-PAD. (C) Chemical structures of the dyes used. Reproduced with permission from ref. 220. Copyright (2012) Royal Society of Chemistry. | ||
Suryanarayana and co-workers developed molecularly imprinted polymers (MIPs) and evaluated them for the recognition of SM.221 Methacrylic acid, ethylene glycol dimethacrylate, and 2,2-azobisisobutyronitrile were polymerized in the presence of SM (Fig. 69). After the formation of SM-bonded polymers, SM was removed with the help of methanol, which formed an SM-imprinted site that is very specific to SM binding. A non-imprinted polymer (NIP) was similarly synthesized in the absence of SM. The SM-imprinted polymer exhibited a higher surface area than the control NIP. Enhanced SM binding to the MIP, when compared to the NIP, was observed with an imprinting efficiency (α) of 1.3. No interference was observed with SM MIP from BCEE.
Singh and co-workers reported an ultrasensitive fluorimetric biosensor for CEES using chlorophyll derivatives 94 and 95 (Fig. 70).222 In the presence of CEES, the chlorophyll fluorescence at 673 nm was quenched, which was attributed to the conversion of chlorophyll into its degredation products (as suggested by high-performance liquid chromatography profiles).222 This biosensor was found to be ultrasensitive with a LOD of 7.68 × 10−10 M.
Li and co-workers demonstrated the efficient encapsulation of SM and its simulants by per-ethylated pillar[5]arene (96) for the recognition of SM (Fig. 71).22396 exhibited strong binding affinity toward analytes in solution, as well as in the solid-state. The interaction between the host and guest was established using NMR spectroscopy. It was determined that the binding constant (Ka) between SM and the host was (6.2 ± 0.6) 3 × 103 M. In the complex, CH2–Cl protons of SM provide a very large upfield shift (δ: 0.42 ppm), while protons from 96 exhibit downfield shifts (Δδ = 0.26–0.30 ppm) due to de-shielding effects. XRD studies revealed the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 inclusion complex, which is driven by multiple C–H⋯πCl/S and S⋯π-interactions. The complex was found to be stable for more than six months in an exposed environment. This type of encapsulation of SM would be extremely beneficial in designing and developing materials for the detection and decontamination of CW agents and other toxicants.
1 inclusion complex, which is driven by multiple C–H⋯πCl/S and S⋯π-interactions. The complex was found to be stable for more than six months in an exposed environment. This type of encapsulation of SM would be extremely beneficial in designing and developing materials for the detection and decontamination of CW agents and other toxicants.
 | ||
| Fig. 71 Schematic illustration of (A) the per-ethylated pillar[5]arene host (96) and (B) single-crystal structures of host–SM complexes. Reproduced with permission from ref. 223. Copyright (2020) The Author(s). | ||
Bowman-James and co-workers studied a series of amide-based palladium(II) pincer complexes (97) coordinated with a solvent (CH3CN) for the recognition of CEES (Fig. 72).2241H NMR spectroscopy and X-ray crystallography confirmed the binding of CEES with palladium(II) pincer complexes, replacing the CH3CN with CEES. The soft nature of the palladium and sulfur atoms may account for the high affinity of the palladium complexes for CEES, as compared to the solvent. The crystal structure analysis revealed that the CEES molecule is shielded by aromatic walls of the aromatic ring, and data suggest that Pd–N1 distances are longer for the mustard structures by about 0.02 Å compared to the acetonitrile complexes. The effect of phenyl rings with various substitutions and with larger aromatic rings was systematically studied, revealing that substitution at the phenyl ring does not significantly affect the binding interaction. However, larger aromatic rings produced a significant increase in binding, possibly due to increased steric and electronic interactions. An NMR investigation revealed that the coexistence of cis- and trans-stereoisomers, corresponding with the two sides of the naphthalene rings oriented in the same (cis) or opposite (trans) direction. It is believed that the molecular wall effect due to the presence of aromatic rings appended perpendicular to the pincer plane observed with the mustard surrogate may lead to new applications, such as chemosensing, separation applications, and molecule/ion transport.
Astruc and co-corkers reported bifunctional 1,2,3-triazole derivatives with polyethylene glycol (PEG) (98) chain and a fluorescent dye (coumarin), which exhibit fluorescence in the presence of CEMS (2-chloroethyl methyl sulfide).225 When combined with AuNPs, this ensemble (containing 1,2,3-triazole derivatives, the polyethylene glycol (PEG) (98) chain, and a fluorescent dye) loses fluorescence due to the formation of triazole–AuNPs. Through a ligand-displacement process, CEMS was able to turn on the fluorescence (λmax = 472 nm) by displacing the AuNPs from a quenched complex of triazole–AuNPs, indicating the presence of the analyte (Fig. 73).
A pyridine-appended Mg–porphyrazine complex (99) was designed by Gupta and co-workers to perform molecular logic operations for the optical detection of CEES in CHCl3/CH3OH (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) (Fig. 74).226 The interaction of 99 with CEES leads to the perturbation/reduction in absorbance of the Q-band of 99. Treatment of the probe-CEES complex (100) with KMnO4 solution resulted in the reappearance of the Q-band (634 nm) of 99 and allowed the probe to be reused after a simple filtration step. This reversible sensing setup allowed for the creation of a platform that can generate different input-based combinatorial and sequential molecular logic gate(s).
1, v/v) (Fig. 74).226 The interaction of 99 with CEES leads to the perturbation/reduction in absorbance of the Q-band of 99. Treatment of the probe-CEES complex (100) with KMnO4 solution resulted in the reappearance of the Q-band (634 nm) of 99 and allowed the probe to be reused after a simple filtration step. This reversible sensing setup allowed for the creation of a platform that can generate different input-based combinatorial and sequential molecular logic gate(s).
Xi and co-workers developed the chemical probe N-(rhodamine-B)-thiolactam-2-n-butane (101), in which rhodamine and thiourea moieties played the roles of chromogenic and reactive groups, respectively (Fig. 75).227 The sensing ensemble with an SM simulant generated a chromo-fluorogenic response with the emergence of new absorption peaks at 560 nm and new emission peaks at 583 nm in the presence of the ionic liquid 1-butyl-3-methylimidazolium dicyandiamide ([BMIm]DCA). Owing to the better solubility of CEES in the ionic liquid (132.5%, w/w), the reaction between the probe and CEES was very rapid; it took place in 1 min at room temperature. Generally, this reaction takes place at an elevated temperature.
 | ||
| Fig. 75 The sensing mechanism of 101 for CEES in 1-butyl-3-methylimidazolium dicyandiamide as an ionic liquid. | ||
In the next development, Li and co-workers described the design, synthesis, and application of a selective and sensitive turn-on fluorescent probe (102) (Fig. 76) for SM detection in living cells (HT-22 cells) and whole animals (Aurelia coerulea polyps).228 A simple alkylation of the 102 by SM in the natural environment (H2O) leads to the formation of the fluorescent product with the emergence of an emission peak at 525 nm that indicates the presence of SM. The basic objective of the present study was to visualize the presence of intact SM in living cells and whole animals.
In 2021, Song et al. reported a similar fluorescent probe 103 that undergoes S-alkylation with CEES in ethanol. 4-Mercaptocoumarin-based chemical probes can detect blister agents (CEES, SM, and NH1) in both solution and vapor forms within five minutes (Fig. 77).229
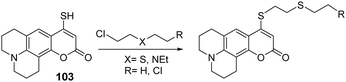 | ||
| Fig. 77 The sensing mechanism of 103 for CEES (X = S; R = H), SM (X = S; R = Cl), and NH1 (X = S; R = H). | ||
The next development was by Abuzalat and Kim's group who demonstrated the facile and trace-level detection of CEES using a fluorescein-encapsulated metal–organic framework (F@Zr-BTC) sensory material (Fig. 78).230 The fluorescein dye was encapsulated within the internal voids (with appreture diameters of 14 and 18 Å, respectively) of a Zr-based MOF using an in situ guest encapsulation method, resulting in enhanced fluorescence. When F@Zr-BTC was exposed to CEES, drastic fluorescence quenching at an emission wavelength of 534 nm occurred. This was attributed to the fact that the electron-deficient properties of the fluorescein affects the electronic interaction between CEES and F@Zr-BTC. Through an adsorptive phenomenon, there is a donor–acceptor electron transfer mechanism between CEES (electron donor) and F@Zr-BTC (electron acceptor), lowering the fluorescence intensity. The fluorescein present inside the Zr-BTC voids accepts the electrons from CEES. This sensing material also shows good selectivity to CEES, as compared to other analytes containing sulfur-like 2-mercapto ethanol, and H2S and DMMP.
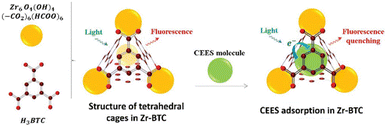 | ||
| Fig. 78 The sensing mechanism of the fluorescein-encapsulated metal–organic framework for CEES in ethanol. Reproduced with permission from ref. 230. Copyright (2021) Royal Society of Chemistry. | ||
In recent years, wearable electrochemical sensors have received great attention owing to their applicability, flexibility, portability, and biocompatibility. They also offer clinical monitoring of a patient at hospitals and personalized care at home.231 Arduini and co-workers realized the first wearable electrochemical biosensor for the on-site detection of SM and NM by exploiting paper-based, origami-like devices (Fig. 79).232 The detection was performed by monitoring the inhibitory effects of each agent on choline oxidase enzyme (ChOx), through the amperometric measurement of hydrogen peroxide (i.e., the enzymatic by-product). A nanocomposite of carbon black/Prussian blue (CB/PBNP) was used as a bulk modifier for conductive graphite ink, which constituted the working electrode. The sensing tool represents a critical point within the security field to provide early alarm systems.
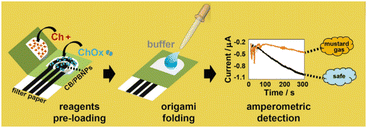 | ||
| Fig. 79 Pictorial representation of origami-like devices based on an electrochemical sensor for SM and NM. Reproduced with permission from ref. 232. Copyright (2019) Elsevier B.V. | ||
4.3 Fluorescent and colorimetric chemosensors for detection of blood agents
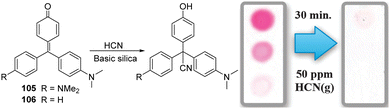 | ||
| Fig. 81 The sensing mechanism of 105 and 106 for HCN. Reproduced with permission from ref. 234. Copyright (2013) Royal Society of Chemistry. | ||
The detection of hydrogen cyanide gas along with the detection of cyanide in solution was realized by Song and co-workers using diethylaminoquinoline derivatives possessing dicyanovinyl substituents (Fig. 82).235 The chemodosimeter (107) was allowed to react with HCN via a Michael addition reaction. A polyethylene oxide-based test strip loaded with 107 was used to selectively detect HCN gas at 60 ppm.
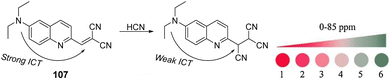 | ||
| Fig. 82 The sensing mechanism of 107 for HCN. Reproduced with permission from ref. 235. Copyright (2018) Elsevier B.V. | ||
A paper-based sensor was fabricated using a cobinamide-based indicator for the detection of HCN gas (Fig. 83).236 A piece of filter paper was impregnated with monocyanocobinamide [CN(H2O)Cbi]236 (108) facilitating the detection of HCN within 10 s of exposure at concentrations as low as 5.0 ppm. Cobinamide (Cbi), a cobalt-centered hydroxocobalamin, has the ability to bind up to two cyanide (CN−) ions. At neutral pH in water, it exists as the mixed hydroxy-aquo complex, known as aquohydroxocobinamide. Upon interaction with HCN, it forms monocyanocobinamide (CN(H2O)Cbi) and then diacyanocobinamide [(CN)2Cbi]. The change from CN(H2O)Cbi to dicyanocobinamide [(CN)2Cbi] produces a significant color change from orange (510 nm) to violet (583 nm).236 In the extension of the above studies, Greenawald et al. recently developed an RGB (red, green, blue) color sensor for HCN detection. A glass fiber filter paper was impregnated with CN(H2O)Cbi and placed above the RGB color sensor with on chip LED.237 Exposing the paper circle to HCN gas, resulted in a rapid color change. The device is controlled by a microcontroller, which transforms data into real-time RGB readouts, thereby permitting rapid color change analysis.
 | ||
| Fig. 83 (A) Chemical structure of probe 108 used for HCN detection. (B) RGB color sensor for HCN detection. Reproduced with permission from ref. 237. Copyright (2017) American Chemical Society. | ||
Gupta's group reported a chemodosimeter (109) that showed a notable chromo-fluorogenic response to HCN gas, as well as to cyanide in solution and on a solid surface (Fig. 84).238 The color response is generated due to the formation of an oxazole derivative from the reaction of 109 with HCN under slightly basic conditions (pH 8.5). The chemodosimeter detected HCN gas with a detection limit of 7 ppm, which is far below the dangerous limit.
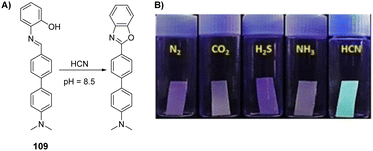 | ||
| Fig. 84 (A) The sensing mechanism of 109 for HCN. (B) Sensing of gases (i.e., N2, CO2, H2S, NH3, and HCN) on cellulose paper strips containing 109. Reproduced with permission from ref. 238. Copyright (2018) Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim. | ||
4.4 Fluorescent and colorimetric chemosensors for the detection of choking agents
4.5 Fluorescent and colorimetric chemosensors for the detection of toxins
Exploiting the fact that guanidiniums are known to bind with crown ethers, Gawley initially designed an anthracene-based crown ether (110) that was used for the fluorescence detection of STX with a binding constant of 3.88 × 103 M−1 in ethanol.241 This work was further advanced by evaluating a series of anthracene-based crown ethers (eleven derivatives) to analyze the effect of structural variations on binding and developing host derivatives that are suitable for incorporation into combinatorial libraries. The crown ethers exhibited good binding constant and higher selectivity over closely related interferents such as arginine, adenine, guanidinium ions, and o-bromophenol. In subsequent improvement, various sizes of crown ethers, e.g., 15-crown-5, 18-crown-6, 21-crown-7, 24-crown-8, and 27-crown-9 were evaluated.242 The study indicated that chemosensors with large crown ethers like 27-crown-9 are superior to previous probes for STX detection in terms of selectivity, sensitivity, and binding constant (2.29 × 105 M−1). Changing indicators from anthracenyl to a coumarin fluorophore leads to the selectivity of STX over Na+, K+, and Ca2+ in aza crown ether-based chemosensors (111).243 The main drive of the present research was to incorporate the spectral features of coumarin, such as large Stokes shifts (70–100 nm) and high quantum yields, into the STX chemosensor.
Tetrodotoxin (TTX), which is a guanidine-based neurotoxin, is another sodium channel blocker that binds with voltage-gated sodium channels and blocks the passage of sodium ions into the neuron.244 The mouse bioassay failed to distinguish between STX and TTX due to similarities in the clinical symptoms of the two toxins.245 The selectivity of STX over TTX was demonstrated by Gawley et al. using acridine-based crown ether chemosensors (112–113).246 However, the binding constant was similar to that of the anthracenyl derivative, suggesting a similar interaction between the host and guest. Two chemosensors with18-crown-6 (114) and 27-crown-9 rings (115) containing a boron azadipyrrin (a chromophore) were synthesized and evaluated by Gawley et al. for visual detection of STX.247 The use of boron azadipyrrin dye was justified by the authors due to the fact that the azadipyrrin dye with absorption λex = 650 nm is remote from any absorption bands due to STX (330 nm), thus reducing interference from the analyte. This is an improvement compared to previously developed probes based on coumarin (328 nm), acridine (350 nm), or anthracene (three bands from 360 to 390 nm). The visible sensor exhibits excellent sensitivity with LOD of 40 μM for STX and 100% fluorescence enhancement, displaying 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 toxin/crown stoichiometry and binding constant at 3–9 × 105 M−1.
1 toxin/crown stoichiometry and binding constant at 3–9 × 105 M−1.
Similarly, Leblanc's group placed two coumarin moieties in a single crown ether chemosensor (116) and observed a slight blue shift (7 nm) that was attributed to the destabilization of the excited state of the fluorophore by the analyte.248 The fluorescence spectroscopic results indicated two emission bands at 420 nm and 550 nm. The latter band is the result of the charge transfer (CT) process between two fluorophores, which are at a distance of about 8–10 Å. Hence, charge transfer occurs resulting in the red-shifted CT band (550 nm). In their next development, they further functionalized the surface of quartz with a coumaryl–aza-crown-6 derivative (117).249 The chemically modified quartz slide was placed under a bifurcated optical fiber and in the presence of STX a fluorescence enhancement (Ex = 332 nm, Em = 415 nm) was observed with a detection limit of 10−5 M for STX. As a substitute to the currently used mouse bioassay technique, the development of this functionalized material would be beneficial to fabricate an inexpensive and reusable nanosensor device.
In a fascinating approach, the authors integrated the major attributes of two approaches, i.e., MIPs and quantum dots (QDs). MIPs and QDs were utilized as the recognition elements and signal transducers, respectively, to design a new type of molecularly imprinted silica appended to quantum dots (MIP-QDs) for the detection of STX. The developed selective fluorescence nanosensor exhibited excellent fluorescence quenching in the presence of the analytes because of the complementary imprinted cavities on the surface of MIP-QDs (Fig. 88).250 The fluorescence on-off takes place in the presence/absence of STX. Using a surface-grafting technique, STX-imprinted sites were generated via the hydrolysis and condensation reaction of tetraethyl orthosilicate (TEOS) and 3-aminopropyl triethoxysilane (APTES) to provide –NH2 surface binding sites on the QD surface. The morphological data revealed that MIP-QDs have a larger external surface area and total pore volume than the non-imprinted polymer, thus providing more specific recognition sites to the STX than the NIPs. Structural analogs of STX, such as okadaic acid, gonyautoxin, anatoxin-a, and neosaxitoxin, were applied to assess the selectivity of MIP-QDs, the results confirmed that the imprinted cavities were only complementary for STX.
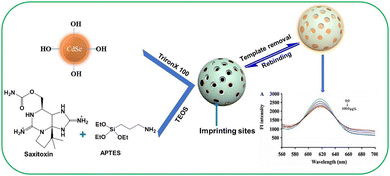 | ||
| Fig. 88 Pictorial representation of MIP-QD-based chemical sensing for the detection of saxitoxin. Reproduced with permission from ref. 250. Copyright (2017) Elsevier B.V. | ||
More recently, an ultrasensitive colorimetric biosensor based on gold nanoparticles (AuNPs) and an aptamer (AuNPs-aptamer biosensor) for specific detection of STX were reported by Zhang and Han (Fig. 89).251 The chromogenic response is the result of the aggregation of AuNPs (from AuNP functionalized with the aptamer) caused by the interaction between STX and the aptamer. The absorbance peak changes are attributed to the surface plasmon resonance absorption peak leading to a quantitative determination of the analyte. The present biosensor achieves the lowest STX detection limit of 10 fM within 30 min.
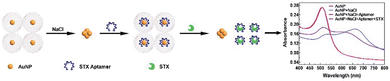 | ||
| Fig. 89 Schematic representation of an AuNP-based sensing ensemble for saxitoxin. Reproduced with permission from ref. 251. Copyright (2020) Royal Society of Chemistry. | ||
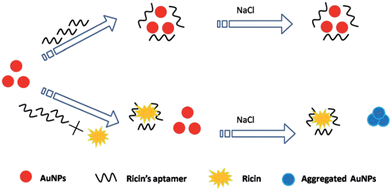 | ||
| Fig. 90 Schematic representation of an aptasensor based on modified AuNPs for ricin. Reproduced with permission from ref. 253 Copyright (2014) Royal Society of Chemistry. | ||
Employing the unique feature of gold nanoparticles possessing peroxidase-like activity, Li et al. demonstrated a simple and sensitive naked-eye detection technique for ricin (Fig. 91).254 3,3′,5,5′-Tetramethylbenzidine (TMB), a chromogenic substrate used in immunohistochemistry, exhibits strong affinity toward a negatively charged nanoparticle surface and gets oxidized in the presence of H2O2, leading to the enhancement of TMB absorbance (from light blue to deep blue). In this report, the authors established that the peroxidase-like activity of AuNPs can be enhanced by surface activation with a ricin-specific aptamer. In the prescence of ricin, the aptamer is desorbed from the AuNPs’ surface, resulting in a decrease in the catalytic abilities of the AuNPs and the absorbance of TMB. The protein-like thrombin (Th), glucose oxidase (GOx), and bovine albumin (BSA) did not interfere in the determination of ricin.
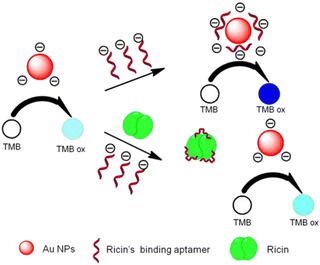 | ||
| Fig. 91 The sensing mechanism of the colorimetric aptasensor for ricin. Reproduced with permission from ref. 254. Copyright (2015) Royal Society of Chemistry. | ||
Russell's group presented a biosensing assay for the detection of a ricin mimic, Ricinus communis agglutinin 120 (RCA120) (Fig. 92).255 The approach is based on the aggregation of carbohydrate-stabilized AuNPs to generate a visual response, which was also monitored by UV-vis spectroscopy. To develop a bioassay, both long-chain 9-mercapto-3,6-dioxaoctyl-β-D-galactoside and short-chain 2-mercaptoethyl-β-D-galactoside derivatives were assembled onto gold nanoparticles, where RCA120 induced aggregation. The LOD for the toxin was 9 nM using the optimally presented galactose-stabilized nanoparticles.
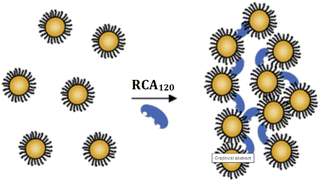 | ||
| Fig. 92 The sensing mechanism of the galactose-stabilized nanoparticles for the ricin mimic (RCA120). Reproduced with permission from ref. 255. Copyright (2008) Royal Society of Chemistry. | ||
Boopathi and co-workers developed a MIP for the recognition of ricin by adopting a two-step, procedure-based soft silane polymerization technique on a silica gel matrix using 3-aminopropyl triethoxysilane as a monomer and tetraethoxysilane as a crosslinker (Fig. 93).256 This technique does not require harsh reaction conditions and free radicals, which may affect the 3D structure of the protein during the imprinting process. The ricin-MIP exhibited nanopatterns, and it was found to be entirely different from the non-imprinted polymer (based on SEM imaging). The ricin-MIP exhibited an imprinting efficiency of 1.76, and it exhibited 10% interference from the structurally similar protein abrin.
Wang et al. reported the aptamer-modified micromotor-based turn-on fluorescence detection of ricin B toxin (Fig. 94).257 This concept is based on self-propelled chemically-powered micro motors consisting of reduced graphene oxide (rGO)/platinum (Pt) micromotors, which are modified with a specific ricin B aptamer tagged with fluorescein amidine dye. The aptamer displays a stable folding conformation under harsh environments, including wide pH (2–7) and temperature (4–63 °C) ranges. This approach exhibits rapid ‘on-the-fly’ binding of the toxin with a visual “off-on” fluorescent response that enables rapid detection of ricin at the ng mL−1 level and selective real-time measurements in diverse samples, which makes it attractive for defense applications.
 | ||
| Fig. 94 The sensing mechanism of the aptamer-modified micromotor-based fluorescence detection of ricin B. Reproduced with permission from ref. 257. Copyright (2016) American Chemical Society. | ||
5. Selectivity issues
Selectivity is the capability of a chemosensor to exclusively detect the target analyte in the presence of other species. This is one of the most challenging aspects during the design and development of the chemosensor as many molecular substances with similar structure, size, and charge can co-exist in complex media. Therefore, the incorportation of selectivity within the molecular receptor, requires elegant design of finely-tuned artificial receptors with optimized affinity for the analyte is of paramount importance. As such, the development of selective chemosensors is still one of the main frontiers in supramolecular chemistry. In order to impart selectivity into a chemosensor, a complete understanding of the target analyte and possible interferences is required.2Given the concern regarding the lethal nature of CW agents, selectivity become far more significant in the case of fluorescent and colorimetric chemosensors for the detection of CW Agents. Both blister agents and nerve agents are electrophilic in nature, and consequently all the developed molecular sensors are most likely to possess nitrogen, oxygen, and sulfur as a nucleophilic site in order to react with electrophilic agents, thus inducing the observed optical changes in the chemosensor. Obviously, this will lead to interference from other less toxic or non-toxic electrophilic agents such as sulfonyl chlorides, thionyl chloride, anhydrides and acid chlorides. Furthermore, in the case of chemosensors based on a nucleophilic center such as nitrogen, inferences from acids (organic/inorganic) is almost certain. In the presence of moisture, G-and V-series are susceptible to hydrolysis thus forming an organophosphoric acid and hydrofluoric/thio acid. Significantly, in many cases, the response of the sensing material to DCP and hydrochloric acid/hydrogen chloride is the same.258 Therefore, while detecting the real agents the presence of small amount of hydrolyzed product invariably occurs in the chemical agents and can interfere with the detection of CWAs. However, this situation can be avoided by removing the acid contaminants prior to measurement or by performing the detection studies in the presence of acid scavengers such as hexamethylenetetramine.
A literature survey indicates that in most cases, detection of CW agents were reported using DCP (in case of nerve agents) or CEES (in case of blister agents). In both the cases, the rate of reaction with chemosensors are different when compared to the respective real agents i.e. DCP or CEES react more quickly due to the weaker P–F or C–Cl bond.198,259 The specific detection of individual agents is highly desirable not only to reduce false positive signals but in addition from point of view of medical countermeasures. Since, in a war scenario, a specific antidote must be provided rapidly to the victim of a CWA attack. The different oximes (reactivators) are effective against different chemical agents, for example, pralidoxime chloride (PAM-Cl) is effective against GB and VX while HI-6 and HIo-7 provide better protection against GA and GD. Furthermore, in war-like situations, interference with acids could pose challenges for first responders or law enforcement agencies. Since these acidic gases may produce false positive signals that can cheat the detection device developed based on these chemosensors.
6. Conclusions and outlook
For more than a century, CW agents have constantly been posing serious threats to mankind, national security, and the environment. Broadly, the first half of the century was largely occupied with the development of various CW agents and their exploitation as weapons of mass destruction. In the second half of the century, efforts were made to improve detection, protection, and decontamination technologies against CW agents. In this review, we briefly introduced the long and unfortunate history of lethal and non-lethal chemical weapons in an attempt to provide an in-depth account of their chemistries and toxicities on a single platform. Here, we have pointed out the latest developments in CW detection technologies, and subsequently summarized currently used technologies in the military and peace-keeping operations. The past few decades have witnessed tremendous progress in the development of detection methods based on instrumental techniques and chemical detection principles. This has resulted in several technologies in the form of electronic devices and kits, which are currently being used by several nations.Ideally, to explore practical solutions to the existing problems related to selectivity, sensitivity, speed, portability, and cost, chemical sensors/detection are considered to be the more promising solution, particularly when exploring approaches based on supramolecular tools.260 Due to the diverse chemical nature of each class of CW agents (i.e., nerve agents, blister agents, blood agents, choking agents, and toxins), different chemical approaches have been exploited in each case.
Fluorescent and colorimetric chemosensors are among the most broadly discussed, researched, and applied synthetic chemical sensors. The structural diversity of these probes provides a range of exceptional electronic and optical properties. When combined with their robust chemistry and ease of manipulation, these probes have become attractive candidates for sensor applications. In most cases, optical detection systems rely on a suitable interaction between chemical probes and chemical agents.
The field of chemosensors for CW agents has seen tremendous progress in the past 20 years with the evolution of, probably, the first fluorescent chemosensor for CW agent mimics reported by Swager. This was followed by studies conducted by many groups,261 who have inspired countless researchers through their seminal contributions to the field of chemosensors. Most of these reports reveal different combinations of binding or reactive sites and signaling units coupled in different ways to develop selective and sensitive detection systems for diverse CWAs. Each segment was classified according to the signaling paradigm used. For example, (a) the binding site–signaling unit, (b) the displacement assay, and (c) chemodosimeters exhibiting ‘‘turn-off’’ or ‘‘turn-on’’ responses mediated by various sensing mechanisms, such as PeT, ITC, RET, and ESIPT. In most of the examples of chemosensors for the detection of nerve agents, blister agents, choking agents (phosgene), and blood agents (HCN), their electrophilic ability was utilized by incorporating a nucleophilic site in the chemical probe that eventually lead to the signal transduction. With the success of using a discrete molecular-like sensor, further attempts were undertaken to utilize the already developed science for the fabrication of technologies using polymers, hybrid materials, and nanomaterial—even for gas-phase detection—with much greater selectivity and sensitivity. In some cases, sensory materials have been integrated into devices that can be used to detect a diverse range of CW agents.
From the literature, it is evident that many innovative and sophisticated approaches have been explored for the detection of nerve agents and choking agents (phosgene). However, very few have been proposed for blister agents, blood agents (HCN), and toxins like saxitoxin and ricin. The last decade has seen a exponential growth in the development of chromo-fluorogenic probes for sulfur mustard after a report by the Anslyn group. Unfortunately, due to the high toxicity and unavailability of these chemical agents in academic environments, the majority of these approaches have been tested with simulants for nerve and blister agents, such as DCP and CEES, respectively. Both surrogates differ in chemical reactivity, which may lead to changes in the experimental settings while dealing with real agents in real-life scenarios. In addition, many of the chemical probes encounter challenges like interference, even with other non-toxic electrophilic species. These two major challenges were partially addressed by developing a strategy based on the detection of hydrolyzed products of nerve agents, which not only resolved these issues to some extent but also provided methods that can discriminate these compounds from one another.
Regardless of the notable progress, these emerging instrumental techniques are still confronting many difficulties owing to the inherent limitations of false-positive signals, operation complexity, and high cost. Chemical detection techniques have partly resolved the latter two problems, but selectivity is still a major concern. Given the challenges associated with CW detection—and because this is an opportunity for the scientific community to develop ‘ideal’ chemical sensor and detection systems—advanced, innovative, and more selective fluorescent and colorimetric chemosensors are of vital importance. Despite recent exciting reports, there are still only a small number of specific chemical probes, and this remains a largely unexplored area. Further efforts are needed to make advancements for the next-generation of responsive probes in terms of specificity. Furthermore, integrating different sensing approaches and designing and developing ‘single probes’ for the specific detection of various classes of CW agents will be a growing trend in the upcoming years. Additionally, superior performance can be achieved by developing efficient sensor materials that can be incorporated into conjugated/non-conjugated polymers, organic–inorganic hybrids, and nanomaterials. The merits of these smart and advanced materials include high sensitivity, simplicity, signal amplification, easy fabrication into devices, processability, stability, and reusability. The developed materials can further be transformed into responsive luminescent films, which in turn can be integrated with other technologies (e.g., electronics and imaging instruments) to fabricate prototype devices and kits.
In summary, we present a comprehensive review of various aspects of CW agents and the development of state-of-the-art detection techniques to protect the global community. Recent developments in the field of optical chemical sensors for CWAs are a new area that has been extensively discussed, covering each class of CW agents. Innovative advances in the chemistry of fluorescent and colorimetric probes continue to be carried out within the field of CW sensing, and we expect that chemosensor research will continue to expand and develop in the near future. We predict that new developments will address major challenges related to selectivity, sensitivity, and simplicity. These novel concepts and innovative ideas give us great hope that global security will be improved with CW chemosensors through the close collaboration between chemists, electronic engineers, and industrial partners. The national and international collaboration of these stakeholders should lead to exciting discoveries and commercial technologies in the field of chemical sensing for CWAs over the decades to come.
Abbreviations
| AC | Hydrogen cyanide |
| ACADA | Automatic chemical agent detection alarm |
| AChE | Acetylcholinesterase |
| AGEL | Acute exposure guideline level |
| AO | Acridine orange |
| APTES3 | Aminopropyl triethoxysilane |
| ATCI | Acetylthiocholine iodide |
| AuNP | Gold nanoparticle |
| BA | Blister agent |
| BCEE | Bis-2-chloroethylether |
| BMIDC | 1-Butyl-3-methylimidazolium dicyandiamide |
| BODIPY | Boron dipyrromethene |
| BSA | Bovine albumin |
| BSA | Bovine serum albumin |
| BWC | Biological weapon convention |
| BZ | 3-Quinuclidinyl benzilate atropine scopolamine |
| CAM | Chemical agent monitor |
| CB | Carbon black |
| Cbi | Cobinamide |
| C-CX | Chinese VX |
| CEES | 2 Chloroethyl ethyl sulfide |
| CG | Phosgene |
| ChO | Choline oxidase enzyme |
| CK | Cyanogen chloride |
| CL | Chlorine |
| CNS | Central nervous system |
| CT | Charge transfer |
| CWA | Chemical warfare agent |
| CWC | Chemical weapon convention |
| CX | Phosgene oxime |
| DA | Diphenylchlorarsine |
| DABCYL | 4-Dimethylaminoazobenzene-4-carboxylic acid |
| DAET | 2-Diisopropylaminoethanethiol |
| DC | Diphenylcyanoarsine |
| DCNP | Diethyl cyanophosphonate |
| DCP | Diethyl chlorophosphate |
| DEPI | Diethoxyphosphinyl isocyanate |
| DFP | Diisopropyl fluorophosphate |
| DMMP | Dimethyl methylphosphonate |
| DMMP | Dimethyl methylphosphonate |
| DMSO | Dimethyl sulfoxide |
| DP | Diphosgene |
| DTNB | 55-Dithio-bis-2-nitrobenzoic acid |
| [EMIM] [DCA] | 1-Ethyl-3-methylimidazolium dicyanamide |
| ESIPT | Excited-state intramolecular proton transfer |
| ET | Energy transfer |
| FPD | Flame photometric detection |
| FRET | Förster resonance energy transfer |
| GA | Tabun |
| GB | Sarin |
| GD | Soman |
| GF | Cyclosarin |
| GOx | Glucose oxidase |
| HD | Highly distilled |
| HPLC | High-performance liquid chromatography |
| ICAD | Individual/improved chemical agent detector |
| ICT | Internal charge transfer |
| IDA | Indicator displacement assay |
| ISE | Ion-selective electrodes |
| JCAD | Joint chemical agent detector |
| LCD | Lightweight chemical detector |
| LOD | Limit of detection |
| LSD | Lysergic acid diethylamide |
| ME | 4-Methylesculetin |
| MIP | Molecular imprinted polymer |
| MOF | Metal–organic framework |
| MSD | Mass selective detection |
| NA | Nerve agent |
| NATO | North atlantic treaty organization |
| NC | Nanocluster |
| NIP | Non-imprinted polymer |
| NIR | Near Infrared |
| NLW | Non-lethal weapon |
| NM | Nitrogen mustard |
| NMR | Nuclear magnetic resonance |
| NPD | Nitrogen phosphorus detection |
| NSET | Nano surface energy transfer |
| OP | Organophosphorus |
| OPCW | Organization for the prohibition of chemical weapon |
| PB | Prussian blue |
| PDI | Perylene diimide |
| PEG | Polyethylene glycol |
| PEO | Polyethyleene oxide |
| PeT | Photo-induced electron transfer |
| PS | Chloropicrin |
| QD | Quantaum dot |
| RAID | Rapid alarm identification device |
| RCA | Riot control agent |
| RCA-120 | Ricinuscommunis Agglutinin-120 |
| RGB | Red green blue |
| rGO | Reduced graphene oxide |
| RVD | Residual vapor detection |
| R-VX | Russian VX |
| SAW | Surface acoustic wave |
| SM | Nitrogen mustard |
| SQ | Squarine |
| STX | Saxitoxin |
| TC | Thiochloline |
| TEOS | Tetraethyl orthosilicate |
| Th | Thrombin |
| THC | Delta-9 tetrahydrocannibinol |
| THF | Tetrahydrofuran |
| TMB | 33′55′-Tetramethylbenzidine |
| TMP | Trimethyl phosphate |
| TPA | Triphenylarsine |
| TPE | Tetraphenylethene |
| TTX | Tetrodotoxin |
| WMD | Weapon of mass destruction |
| XRD | X-ray diffraction |
Conflicts of interest
There are no conflicts to declare.Acknowledgements
V. Kumar thanks the Director, DRDE Gwalior, for his keen interest and encouragement. J. Yoon thanks to the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1A2C3005420). TDJ wishes to thank the Royal Society for a Wolfson Research Merit Award and the Open Research Fund of the School of Chemistry and Chemical Engineering, Henan Normal University for support (2020ZD01). EVA thanks the Welch Regents Chair for continued support (F-0046).References
- M. Raynal, P. Ballester, A. Vidal-Ferran and P. W. N. M. van Leeuwen, Chem. Soc. Rev., 2014, 43, 1660–1733 ( Chem. Soc. Rev. , 2014 , 43 , 1734–1787 ) RSC.
- B. Wang and Eric V. Anslyn, Chemosensors: Principles, Strategies, and Applications, John Wiley & Sons, Inc., 2011, ISBN:9780470592069 DOI:10.1002/9781118019580.
- D. Dattler, G. Fuks, J. Heiser, E. Moulin, A. Perrot, X. Yao and N. Giuseppone, Chem. Rev., 2020, 120, 310–433 CrossRef CAS PubMed.
- D. B. Amabilino, D. K. Smith and J. W. Steed, Chem. Soc. Rev., 2017, 46, 2404–2420 RSC.
- S. Erbas-Cakmak, D. A. Leigh, C. T. McTernan and A. L. Nussbaumer, Chem. Rev., 2015, 115, 10081–10206 CrossRef CAS PubMed.
- J. Zhou, Y. Guocan and H. Feihe, Chem. Soc. Rev., 2017, 46, 7021–7053 RSC.
- B. S. Sekhon, Curr. Drug Targets, 2015, 16, 1407–1428 CrossRef CAS PubMed.
- Handbook of toxicology of chemical warfare agents, ed. R. C. Gupta, Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam, Boston, 3rd edn, 2020 Search PubMed.
- L. D. Prockop, J. Neurol. Sci., 2006, 249, 50–54 CrossRef CAS PubMed.
- https://www.opcw.org (Accessed November 07, 2022).
- https://www.opcw.org/chemical-weapons-convention/annexes/verification-annex/part-vi-regime-schedule-1-chemicals. (Accessed November 07, 2022).
- https://www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/annex-chemicals (Accessed November 07, 2022).
- L. Szinicz, Toxicology, 2005, 214, 167–181 CrossRef CAS PubMed.
- J. K. Smart, History of Chemical and Biological Warfare: An American Perspective, in Medical Aspects of Chemical and Biological Warfare, ed. F. R. Sidell, E. T. Takafuji, D. R. Franz, Office of the Surgeon General, Washington, DC, 1997. p. 15 Search PubMed.
- K. Ganesan, S. K. Raza and R. Vijayaraghavan, J. Pharm. BioAllied Sci., 2010, 2, 166–178 CrossRef CAS PubMed.
- https://web.archive.org/web/20130908034743/http://www.armscontrol.org/print/363 (Accessed November 07, 2022).
- R. Black, Development, historical use and properties of chemical warfare agents, In book, Chemical Warfare Toxicology, 2016, pp. 1–28 10.1039/9781782622413-00001.
- L. Szinicz, F. Worek, H. Thiermann, K. Kehe, S. Eckert and P. Eyer, Toxicology, 2007, 233, 23 CrossRef CAS PubMed.
- H. Rice, Toxicology of Organophosphorus Nerve Agents In book, Chemical Warfare Toxicology, 2016, pp. 81–116 10.1039/9781782622413-00081.
- S. M. Somani and J. A. Romano, Chemical Warfare Agents, CRC Press, Washington, DC, 2001, p. 447 Search PubMed.
- G. Schrader and H. Gebhardt, (Farbenfabriken Bayer AG): D. B. P. 767.830 (1939/1953).
- M. A. Hayoun, M. E. Smith, C. Ausman and H. D. Swoboda, Toxicology, V-Series Nerve Agent, StatPearls Publishing, Treasure Island (FL), Stat Pearls, 2020 Search PubMed.
- A. Watson, L. Hall, E. Raber, V. D. Hauschild, F. Dolislager, A. H. Love and M. L. H. Hum, Ecol Risk Assess., 2011, 17, 2–56 CrossRef CAS PubMed.
- P. Taylor, in Anticholinesterase agents, In The Pharmacological Basis of Therapeutics, ed. A. G. Gilman, L. S. Goodman, T. W. Rall, and F. Murad, Macmillan, New York, 6th edn, 1985, p. 110 Search PubMed.
- K. Mislow and J. J. Siegel, J. Am. Chem. Soc., 1984, 106, 3319–3328 CrossRef CAS.
- C. E. A. M. Degenhardt, G. R. Van Den Berg, L. P. A. De Jong and H. P. Benschop, J. Am. Chem. Soc., 1986, 108, 8290–8291 CrossRef CAS.
- S. Lewis, Salisbury, Novichok and International Law on the Use of Force, RUSI J., 2018, 163, 10–19 CrossRef.
- https://www.bbc.com/news/uk-44760875. (Accessed November 07, 2022).
- https://www.bbc.com/news/world-europe-43377698. (Accessed November 07, 2022).
- M. Kloske and Z. Witkiewic, Chemosphere, 2019, 221, 672–682 CrossRef CAS PubMed.
- https://cen.acs.org/policy/chemical-weapons/New-nerve-agents-added-Chemical-Weapons-Convention/97/web/2019/12. (Accessed November 07, 2022).
- https://www.opcw.org/chemical-weapons-convention/annexes/annex-chemicals/schedule-1 (Accessed November 07, 2022).
- P. Jäger, C. N. Rentzea and H. Kieczka, Carbamates and carbamoyl chlorides, Ullmann's encyclopedia of industrial chemistry, 2000 Search PubMed.
- A. K. Ghosh and M. Brindisi, J. Med. Chem., 2015, 58, 2895–2940 CrossRef CAS PubMed.
- A. Vale and M. Lotti, Organophosphorus and carbamate insecticide poisoning, Handbook of clinical neurology, 2015, 131, pp. 149–168 Search PubMed.
- A. McVicar, Carbamate Nerve Agents, Handbook of Chemical and Biological Warfare Agents, CRC Press, 2007, pp. 141–176 Search PubMed.
- A. M. King and K. A. Cynthia, Emerg. Med. Clin., 2015, 33, 133–151 CrossRef PubMed.
- J. Silberman and A. Taylor, Carbamate Toxicity, StatPearls Publishing LLC, USA, 2019, NCBI Number: NBK482183 Search PubMed.
- J. A. Romano, Jr., B. J. Lukey and H. Salem, Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics, CRC Press, London, 2007 Search PubMed.
- H. Q. Le and S. J. Knudsen, Emerg. Med. J., 2006, 23, 296–299 CrossRef CAS PubMed.
- M. Balali-Mood and M. Hefazi, Fundam. Clin. Pharmacol., 2005, 19, 297–315 CrossRef CAS PubMed.
- K. Kehe and L. Szinicz, Toxicology, 2005, 214, 198–209 CrossRef CAS PubMed.
- J. Jenner, Toxicology of Vesicants In book, Chemical Warfare Toxicology, 2016, pp. 29–80 10.1039/9781782622413-00029.
- M. Shohrati, M. Davoudi, M. Ghanei, M. Peyman and A. Peyman, Cutaneous Ocul. Toxicol., 2007, 26, 73–81 CrossRef CAS PubMed.
- A. Gilman, Am. J. Surg., 1963, 105, 574–578 CrossRef CAS PubMed.
- S. M. Somani, Toxicokinetics and toxicodynamics of mustard, in Chemical Warfare Agents, ed. S. M. Somani, Academic Press, New York, NY, 1992 Search PubMed.
- R. K. Singh, S. Kumar, D. N. Prasad and T. R. Bhardwaj, Eur. J. Med. Chem., 2018, 151, 401–433 CrossRef CAS PubMed.
- K. W. Kohn, J. A. Hartley and W. B. Mattes, Nucleic Acids Res., 1987, 15, 10531–10549 CrossRef CAS PubMed.
- A. Polavarapu, J. A. Stillabower, S. G. W. Stubblefield, W. M. Taylor and M. H. Baik, J. Org. Chem., 2012, 77, 5914–5921 CrossRef CAS PubMed.
- C. Descoteaux, K. Brasseur, V. Leblanc, S. Parent, E. Asselin and G. Berube, Steroids, 2012, 77, 403–412 CrossRef CAS PubMed.
- D. C. Keyes, J. L. Burstein, R. B. Schwartz and R. E. Swienton, Medical Response to Terrorism: Preparedness and Clinical Practice, Lippincott Williams & Wilkins, 2004, p. 16 Search PubMed.
- D. H. Rosenblatt, T. A. Miller and J. C. Dacre, Problem definition studies on potential environmental pollutants. II. Physical, chemical, toxicological, and biological properties of 16 substances. Technical Report 7509, U.S, Army Medical Bioengineering Research and Development Laboratory, Fort Detrick, Frederick, MD, 1975 Search PubMed.
- J. A. Vilensky and K. Redman, Ann. Emerg. Med., 2003, 41, 378–383 CrossRef PubMed.
- J. A. F. Compton, Military Chemical and Biological Agents: Chemical and Toxicological Properties, Telford Press, Caldwell, New Jersey, 1988, pp. 37–38 Search PubMed.
- C. M. Pechura and D. P. Rall, Institute of Medicine (US) Committee on the Survey of the Health Effects of Mustard Gas and Lewisite. Veterans at Risk: The Health Effects of Mustard Gas and Lewisite, National Academies Press, Washington (DC) (US), 1993 Search PubMed.
- G. Sandı, K. L. Brubaker, J. F. Schneider, H. J. O’Neill and P. L. Cannon Jr., Talanta, 2001, 54, 913–925 CrossRef PubMed.
- S. K. Singh, J. A. Klein, H. N. Wright and N. Tewari-Singh, Toxicol. Mech. Methods, 2021, 31, 288–292 CrossRef CAS PubMed.
- https://www.news9.com/story/40170458/fbi-investigationfinds-chemical-warfare-agent-inside-lawton-home. (Accessed November 07, 2022).
- T. T. Marrs, L. M. Robert and F. Sidell, Chemical warfare agents: Toxicology and treatment, John Wiley and Sons, Chichester, England, 2007 Search PubMed.
- L. Coentrao and D. Moura, Am. J. Emerg. Med., 2011, 29, 78–81 CrossRef PubMed.
- F. R. Sidell, E. T. Takafuji and D. R. Franz, Medical Aspects of Chemical and Biological Warfare, Office of the Surgeon General, Washington, DC, 1997 Search PubMed.
- D. Henschler, Hydrogen cyanide, potassium cyanide, and sodium cyanide [MAK Value Documentation, 2003], in The MAK-collection for occupational health and safety, ed. M. Tschickardt, Wiley Online Library, 2012, pp. 193–95 DOI:10.1002/3527600418.mb7490vere0019.
- H. Babad and A. G. Zeiler, Chem. Rev., 1973, 73, 75–91 CrossRef CAS.
- T. Zellner and F. Eyer, Toxicol. Lett., 2020, 320, 73–79 CrossRef CAS PubMed.
- J. Borak and W. F. Diller, J. Occup. Environ. Med., 2001, 43, 110–119 CrossRef CAS PubMed.
- C. Bast and B. Bress,Acute exposure guideline levels for selected airborne chemicals. Phosgene The National Research Council, Committee on Toxicology, The National Academies Press, Washington, DC, vol. 2, 2002 Search PubMed.
- P. D. Anderson, J. Pharm. Pract., 2012, 25, 121–129 CrossRef PubMed.
- M. A. Sierra and R. Martínez-Álvarez, J. Chem. Educ., 2020, 97, 1707–1714 CrossRef CAS.
- L. J. M. Madsen, Clin. Lab. Med., 2001, 21, 593–605 CrossRef PubMed.
- A. P. Thottumkara, W. H. Parsons and J. D. Bois, Angew. Chem., Int. Ed., 2014, 53, 5760–5784 CrossRef CAS PubMed.
- W. A. Catterall, Annu. Rev. Pharmacol. Toxicol., 1980, 20, 15–43 CrossRef CAS PubMed.
- Official Methods of Analysis, ed. K. Helrich, Association of Official Analytical Chemists (AOAC), Arlington, VA, 1990, pp. 881–882 Search PubMed.
- J. F. Lawrence, B. Niedzwiadek and C. Menard, J. AOAC Int., 2005, 88, 1714–1732 CrossRef CAS PubMed.
- L. Polito, M. Bortolotti, M. G. Battelli, G. Calafato and A. Bolognesi, Toxins, 2019, 11, 324–340 CrossRef CAS PubMed.
- S. Olsnes and A. Pihl, Biochemistry, 1973, 12, 3121–3126 CrossRef CAS PubMed.
- J. M. Lord, L. M. Roberts and J. D. Robertus, FASEB J., 1994, 8, 201–208 CrossRef CAS PubMed.
- A. Pearson, Nonproliferation Rev., 2006, 13, 152–188 Search PubMed.
- E. J. Olajos and H. Salem, J. Appl. Toxicol., 2001, 5, 355–391 CrossRef PubMed.
- L. J. Schep, R. J. Slaughter and D. I. McBride, J. R. Army Med. Corps, 2015, 161, 94–99 CrossRef PubMed.
- C. J. Hilmas, Riot Control Agents, in Handbook of Toxicology of Chemical Warfare Agents, ed. R. C. Gupta, Academic Press, 2nd edn, 2015, ch. 11, pp. 131–150 Search PubMed.
- Y. Dimitroglou, G. Rachiotis and C. Hadjichristodoulou, Int. J. Environ. Res. Public Health, 2015, 12, 1397–1411 CrossRef PubMed.
- R. Jabbour, H. Salem, B. Ballantyne and S. A. Katz, Arsenical Vomiting Agents, in Encyclopedia of Toxicology, ed. P. Wexler, Academic Press, 3rd edn, 2014, pp. 302–307 Search PubMed.
- J. Fusek, A. Dlabkova and J. Misik, in Handbook of toxicology of chemical warfare agents, ed. R. C. Gupta, Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam, Boston, 3rd edn, 2020, ch. 14, pp. 203–213 Search PubMed.
- B. Radke, L. Jewell, S. Piketh and J. Namiesnik, Arsenic Based Warfare Agents: Production, Use, and Destruction, Crit. Rev. Environ. Sci. Technol., 2014, 44, 1525–1576 CrossRef.
- J. M. Lakoski, W. B. Murray and J. M. Kenny, The Advantages and Limitations of Calmatives for Use as a Non-Lethal Technique (University Park, PA: Applied Research Laboratory, Pennsylvania State University, 2000), p. 48. https://erowid.org/psychoactives/war/war_article1.pdf (Accessed November 07, 2022).
- R. G. Sutherland, Chemical and Biochemical Non-lethal Weapons Political and Technical Aspects Publisher: SIPRI ISBN 978-91-85114-59-7, 2008, https://www.sipri.org/sites/default/files/files/PP/SIPRIPP23.pdf, accessed November 07, 2022.
- J. A. Romano Jr, B. J. Lukey and H. Salem, Chemical Warfare Agents: Chemistry, Pharmacology, Toxicology, and Therapeutics, CRC Press, London, 2007, pp. 367–372 Search PubMed.
- J. Bajgar, J. Kassa, J. Fusek, K. Kuca and D. Jun, in Handbook of toxicology of chemical warfare agents, ed. R. C. Gupta, 3rd edn, Elsevier/AP, Academic Press is an imprint of Elsevier, Amsterdam, Boston, 2020, ch. 27, pp. 403–412 Search PubMed.
- J. Matousek and I. Masek, On the new potential super-toxic lethal organophosphorus chemical warfare agents with intermediate volatility, ASA Newsletter, 1994, 1, 94–95 Search PubMed.
- https://www.opcw.org/sites/default/files/documents/2019/03/s-1731-2019(e).pdf. (Accessed November 07, 2022).
- Y. Sun and K. Y. Ong, Detection Technologies for Chemical Warfare Agents and Toxic Vapors, CRC Press, 2004 Search PubMed.
- Institute of Medicine (US) Committee on R&D Needs for Improving Civilian Medical Response to Chemical and Biological Terrorism Incidents. Chemical and Biological Terrorism: Research and Development to Improve Civilian Medical Response. Washington (DC), National Academies Press (US), 1999, 4, Detection and Measurement of Chemical Agents.
- A. A. Fatah, R. D. Arcilesi, J. C. Peterson, C. H. Lattin, C. Y. Wells and J. A. McClintock, in Guide for the Selection of Chemical Detection Equipment for Emergency First Responders, ed. U. D. O. H. Security, Washington, D.C., 3rd edn, 2007 Search PubMed.
- W. Lu, M. Xue, Z. Xu, X. Dong, F. Xue, F. Wang and Z. Meng, Curr. Org. Chem., 2015, 19, 62–71 CrossRef CAS.
- F. N. Diauudin, J. I. A. Rashid, V. F. Knight, W. M. Z. W. Yunus, K. K. Ong, N. A. M. Kasim, N. A. Halim and S. A. M. Noor, Sens. Bio-Sens. Res., 2019, 26, 100305 CrossRef.
- Y. Saylan, S. Akgonullu and A. Denizli, Biosensors, 2020, 10, 142 CrossRef CAS PubMed.
- H. Yoon, Nanomaterials, 2013, 3, 524–549 CrossRef CAS PubMed.
- Y. Sun and K. Y. Ong, Detection Technologies for Chemical Warfare Agents and Toxic Vapors, CRC Press, Boca Raton, Florida, 1st edn, 2004, p. 272 Search PubMed.
- Y. Seto, M. Kanamori-Kataoka, K. Tsuge, I. Ohsawa, H. Maruko, H. Sekiguchi, Y. Sano, S. Yamashiro, K. Matsushita, H. Sekiguchi, T. Itoi and K. Iura, Toxin Rev., 2007, 26, 299–312 CrossRef CAS.
- A. M. Marko, A. A. Osmo and E. T. S. Mika, Anal. Chem., 2010, 82, 9594–9600 CrossRef PubMed.
- R. Sferopoulos, A Review of Chemical Warfare Agent (CWA) Detector Technologies and Commercial-Off-The-Shelf Items, https://apps.dtic.mil/sti/pdfs/ADA502856.pdf, retrieved on October 26, 2021.
- Y. Pan, G. Zhang, T. Guo, X. Liu, C. Zhang, J. Yang, B. Cao, C. Zhang and W. Wang, Sens. Actuators, B, 2020, 315, 127986 CrossRef CAS.
- A. T. Nimal, U. Mittal, M. Singh, M. Khaneja, G. K. Kannan, J. C. Kapoor, V. Dubey, P. K. Gutch, G. Lal, K. D. Vyas and D. C. Gupta, Sens. Actuators, B, 2009, 135, 399–410 CrossRef CAS.
- R. R. Jones, D. C. Hooper, L. Zhang, D. Wolverson and V. K. Valev, Nanoscale Res. Lett., 2019, 14, 231–265 CrossRef PubMed.
- E. J. Pacsial-Ong and Z. P. Aguilar, Front. Biosci., 2013, 5, 516–543 CrossRef PubMed.
- Y. Sun and K. Y. Ong, Detection Technologies for Chemical Warfare Agents and Toxic Vapors, CRC Press, Boca Raton, Florida, 1st edn, 2004, pp. 190–194 Search PubMed.
- C. A. Valdez, R. N. Leif, S. Hok and B. R. Hart, Rev. Anal. Chem., 2018, 37, 20170007 CAS.
- G. M. Murray, detection and Screening of Chemicals Related to the Chemical Weapons Convention. Encyclopedia of Analytical Chemistry, 2006 DOI:10.1002/9780470027318.a0403.pub2.
- J. Epstein, R. W. Rosenthal and R. J. Ess, Anal. Chem., 1955, 27, 1435–1439 CrossRef CAS.
- A. Bussey, A. Clarke and J. Lambert, PCT/GB2004/001083 WO/2004/081561, March 11, 2004.
- B. Singh, P. P. Bhise, M. V. S. Suryanarayana, S. S. Yadav, V. K. Rao, B. G. Polke, D. Pandey, K. Ganesan and N. B. S. N. Rao, J. Sci. Ind. Res., 1999, 58, 25–30 CAS.
- V. Pitschmann, I. Tušarová, E. Halámek and Z. Kobliha, J. Serb. Chem. Soc., 2010, 75, 813–822 CrossRef CAS.
- N. Nakano, A. Yamamoto, Y. Kobayashi and K. Nagashima, Talanta, 1995, 42, 641–645 CrossRef CAS PubMed.
- T. W. Bell and N. M. Hext, Chem. Soc. Rev., 2004, 33, 589 CAS.
- A. W. Czarnik, Advances in Supramolecular Chemistry, JAI Press, Greenwich, Connecticut, 1993 Search PubMed.
- V. Kumar and A. Mazumder, Detection of Chemical Warfare Agents With Chemical Sensors, in Encyclopedia of Sensors and Biosensors, ed. R. Narayan, Elsevier, 2022, vol. 4, pp. 667–692 DOI:10.1016/B978-0-12-822548-6.00145-X.
- A. P. deSilva, H. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- S. Erbas-Cakmak, S. Kolemen, A. C. Sedgwick, T. Gunnlaugsson, T. D. James, J. Yoon and E. U. Akkaya, Chem. Soc. Rev., 2018, 47, 2228–2248 RSC.
- A. C. Sedgwick, J. T. Brewster, T. Wu, X. Feng, S. D. Bull, X. Qian, J. L. Sessler, T. D. James, E. V. Anslyn and X. Sun., Chem. Soc. Rev., 2021, 50, 9–38 RSC.
- W. Sun, M. Li, J. Fan and X. Peng, Acc. Chem. Res., 2019, 52, 2818–2831 CrossRef CAS PubMed.
- I. I. Ebralidze, N. O. Laschuk, J. Poisson and O. V. Zenkina, Colorimetric Sensors and Sensor Arrays, in Micro and Nano Technologies, Nanomaterials Design for Sensing Applications, Elsevier, 2019, ch. 1, pp. 1–39 Search PubMed.
- D. Wu, A. C. Sedgwick, T. Gunnlaugsson, E. U. Akkaya, J. Yoon and T. D. James, Chem. Soc. Rev., 2017, 46, 7105–7123 RSC.
- S. Royo, R. Martinez-Manez, F. Sancenon, A. M. Costero, M. Parra and S. Gil, Chem. Commun., 2007, 4839–4847 RSC.
- L. Chen, D. Wu and J. Yoon, ACS Sens., 2018, 3, 27–43 CrossRef CAS PubMed.
- S.-W. Zhang and T. M. Swager, J. Am. Chem. Soc., 2003, 125, 3420–3421 CrossRef CAS PubMed.
- K. J. Wallace, J. Morey, V. M. Lynch and E. V. Anslyn, New J. Chem., 2005, 29, 1469–1474 RSC.
- K. J. Wallace, R. I. Fagbemi, F. J. Folmer-Andersen, J. Morey, V. M. Lynth and E. V. Anslyn, Chem. Commun., 2006, 3886–3888 RSC.
- S. Bencic-Nagale, T. Sternfeld and D. R. Walt, J. Am. Chem. Soc., 2006, 128, 5041–5048 CrossRef CAS PubMed.
- T. J. Dale and J. Rebek, J. Am. Chem. Soc., 2006, 128, 4500–4501 ( Angew. Chem., Int Ed. , 2009 , 48 , 7850–7852 ) CrossRef CAS PubMed.
- A. M. Costero, S. Gil, M. Parra, P. M. E. Mancini, R. Martinez-Manez, F. Sancenon and S. Royo, Chem. Commun., 2008, 6002–6004 RSC.
- S. Han, Z. Xue, Z. Wang and T. B. Wen, Chem. Commun., 2010, 46, 8413–8415 RSC.
- S. Royo, A. M. Costero, M. Parra, S. Gil, R. Martinez-Manez and F. Sancenon, Chem. – Eur. J., 2011, 17, 6931–6934 CrossRef CAS PubMed.
- R. Gotor, A. M. Costero, S. Gil, M. Parra, R. Martinez-Manez and F. Sancenon, Chem. – Eur. J., 2011, 17, 11994–11997 CrossRef CAS PubMed.
- V. Kumar and M. P. Kaushik, Analyst, 2011, 136, 5151–5156 RSC.
- W. Xuan, Y. Cao, J. Zhou and W. Wang, Chem. Commun., 2013, 49, 10474–10476 RSC.
- Z. Lei and Y. Yang, J. Am. Chem. Soc., 2014, 136, 6594–6597 CrossRef CAS PubMed.
- Y. J. Yang, O. G. Tsay, D. P. Murale, J. A. Jeong, A. Segev and D. G. Churchill, Chem. Commun., 2014, 50, 7531–7534 RSC.
- G. B. Diaz de, D. Moreno, T. Torroba, A. Berg, J. Gunnars, T. Nilsson, R. Nyman, M. Persson, J. Pettersson, I. Eklind and P. Wasterby, J. Am. Chem. Soc., 2014, 136, 4125–4128 CrossRef PubMed.
- J. G. Weis and T. M. Swager, ACS Macro Lett., 2015, 4, 138–142 CrossRef CAS PubMed.
- A. K. Mahapatra, K. Maiti, S. K. Manna, R. Maji, S. Mondal, C. D. Mukhopadhyay, P. Sahoo and D. Mandal, Chem. Commun., 2015, 51, 9729–9732 RSC.
- V. Kumar, H. Rana, G. Raviraju, P. Garg, A. Baghel and A. K. Gupta, RSC Adv., 2016, 6, 59648–59656 RSC.
- T.-I. Kim, S. B. Maity, J. Bouffard and Y. Kim, Anal. Chem., 2016, 88, 9259–9263 CrossRef CAS PubMed.
- X. Zhou, Y. Zeng, C. Liyan, X. Wu and J. Yoon, Angew. Chem., Int. Ed., 2016, 55, 4729–4733 CrossRef CAS PubMed.
- M. Gupta and H.-I. Lee, Sens. Actuators, B, 2017, 242, 977–982 CrossRef CAS.
- Y. J. Jang, S. V. Mulay, Y. Kim, P. Jorayev and D. G. Churchill, New J. Chem., 2017, 41, 1653–1658 RSC.
- X. Sun, S. D. Dahlhauser and E. V. Anslyn, J. Am. Chem. Soc., 2017, 139, 4635–4638 CrossRef CAS PubMed.
- Y.-C. Cai, C. Li and Q.-H. Song, ACS Sens., 2017, 2, 834–841 CrossRef CAS PubMed.
- Y.-C. Cai, C. Li and Q.-H. Song, J. Mater. Chem. C, 2017, 5, 7337–7343 RSC.
- K. Aich, S. Das, S. Gharami, L. Patra and T. K. Mondal, New J. Chem., 2017, 41, 12562–12568 RSC.
- V. Kumar, G. Raviraju, H. Rana, V. K. Rao and A. K. Gupta, Chem. Commun., 2017, 53, 12954–12957 RSC.
- Q. J. Luo, Z. G. Li, J. H. Lai, F. Q. Li, P. Qiu and X. L. Wang, RSC Adv., 2017, 7, 55199–55205 RSC.
- M. S. J. Khan, Y.-W. Wang, M. O. Senge and Y. Peng, J. Hazard. Mater., 2018, 342, 10–19 CrossRef CAS PubMed.
- K. Rurack and R. Martinez-Manez, The Supramolecular Chemistry of Organic-Inorganic Hybrid Materials, Wiley, Hoboken, New Jersey, 2010, pp. 319–349 or 407–432 Search PubMed.
- E. Climent, M. Biyikal, K. Gawlitza, T. Dropa, M. Urban, A. M. Costero, R. Martinez-Manez and K. Rurack, Sens. Actuators, B, 2017, 246, 1056–1065 CrossRef CAS.
- J. M. Garcia, F. C. Garcia, F. Serna and J. L. de la Pena, Polym. Rev., 2011, 51, 341–390 CrossRef CAS.
- H. N. Kim, Z. Guo, W. Zhu, J. Yoon and H. Tian, Chem. Soc. Rev., 2011, 40, 79–93 RSC.
- M. Gupta and H.-l Lee, Macromolecules, 2017, 50, 6888–6895 CrossRef CAS.
- J. Yan, S. Lee, A. Zhang and J. Yoon, Chem. Soc. Rev., 2018, 47, 6900–6916 RSC.
- M. E. Roth, O. Green, S. Gnaim and D. Shabat, Chem. Rev., 2016, 116(3), 1309–1352 CrossRef CAS PubMed.
- X. Sun and E. V. Anslyn, Angew. Chem., Int. Ed., 2017, 56, 9522–9526 CrossRef CAS PubMed.
- H. S. Sarkar, A. Ghosh, S. Das, P. K. Maiti, S. Maitra, S. Mandal and P. Sahoo, Sci. Rep., 2018, 8, 1–7 CAS.
- X. Liu, Y. Gong, Y. Zheng, W. Xiong, C. Wang, T. Wang, Y. Che and J. Zhao, Anal. Chem., 2018, 90(3), 1498–1501 CrossRef CAS PubMed.
- L. Chen, H. Oh, D. Wu, M. H. Kim and J. Yoon, Chem. Commun., 2018, 54, 2276–2279 RSC.
- T. Qin, Y. Huang, K. Zhu, J. Wang, C. Pan, B. Liu and L. Wang, Anal. Chim. Acta, 2019, 1076, 125–130 CrossRef CAS PubMed.
- B. Huo, M. Du, A. Shen, M. Li, Y. Lai, X. Bai, A. Gong and Y. Yang, Anal. Chem., 2019, 91, 10979–10983 CrossRef CAS PubMed.
- G. Heo, R. Manivannan, H. Kim and Y.-A. Son, Dyes Pigm., 2019, 171, 107712 CrossRef CAS.
- X. Sun, A. A. Boulgakov, L. N. Smith, P. Metola, E. M. Marcotte and E. V. Anslyn, ACS Cent. Sci., 2018, 4, 854–861 CrossRef CAS PubMed.
- C. Sun, W. Xiong, W. Ye, Y. Zheng, R. Duan, Y. Che and J. Zhao, Anal. Chem., 2018, 90, 7131–7134 CrossRef CAS PubMed.
- K. Vymazalova, J. Kadlcak and E. Halamek, Def. Sci. J., 2012, 62, 399–403 CrossRef CAS.
- V. Pitschmann, L. Matejovsky, D. Vetchy and Z. Kobliha, Anal. Lett., 2016, 49, 2418–2426 CrossRef CAS.
- V. Pitschmann, L. Matejovsky, M. Lobotka, J. Dedic, M. Urban and M. Dymak, Biosensors, 2018, 8, 81–90 CrossRef CAS PubMed.
- L. Matejovsky and V. Pitschmann, ACS Omega, 2019, 4(25), 20978–20986 CrossRef CAS PubMed.
- N. Zehra, A. Kalita, A. H. Malik, U. Barman, M. A. Afroz and P. KrishnanIyer, ACS Sens., 2020, 5, 191–198 CrossRef CAS PubMed.
- M. C. de Koning, G. W. Peterson, M. V. Grol, I. Iordanov and M. McEntee, Chem. Mater., 2019, 31, 7417–7424 CrossRef CAS.
- G. L. Ellman, K. D. Courtney, V. Andres Jr. and R. M. Featherstone, Biochem. Pharmacol., 1961, 7, 88–95 CrossRef CAS PubMed.
- L. Zeng, H. Zeng, L. Jiang, S. Wang, J.-T. Hou and J. Yoon, Anal. Chem., 2019, 91, 12070–12076 CrossRef CAS PubMed.
- N. Boens, V. Leen and W. Dehaen, Chem. Soc. Rev., 2012, 41, 1130–1172 RSC.
- T. Kowada, H. Maeda and K. Kikuchi, Chem. Soc. Rev., 2015, 44, 4953–4972 RSC.
- A. C. Sedgwick, R. S. L. Chapman, J. E. Gardiner, L. R. Peacock, G. Kim, J. Yoon, S. D. Bull and T. D. James, Chem. Commun., 2017, 53, 10441–10443 RSC.
- Z. Lu, W. Fan, X. Shi, C. A. Black, C. Fan and F. Wang, Sens. Actuators, B, 2018, 255, 176–182 CrossRef CAS.
- S.-S. Li, Y.-C. Zheng, X.-M. Zhu, H.-B. Wang, L.-H. Liang, X.-Z. Wang, L. Yuan, F.-H. Zhang, H. Zheng and C.-L. Zhao, Sens. Actuators, B, 2021, 337, 129804 CrossRef CAS.
- J. G. Mao, Coord. Chem. Rev., 2007, 251, 1493–1520 CrossRef CAS.
- E. Butera, A. Zammataro, A. Pappalardo and G. T. Sfrazzetto, ChemPlusChem, 2021, 86, 681–695 CrossRef PubMed.
- C. M. A. Gangemi, U. Rimkaite, A. Pappalardo and G. T. Sfrazzetto, RSC Adv., 2021, 11, 13047–13050 RSC.
- X. Hua, H. Zeng, T. Chen, H. Q. Yuan, L. Zeng and G. M. Bao, Sens. Actuators, B, 2020, 319, 128282 CrossRef.
- S. K. Sheet, B. Sen and S. Khatua, Inorg. Chem., 2019, 58, 3635–3645 CrossRef CAS PubMed.
- W. Weihui, S. Shaohui, L. Jian, Z. Liang, L. Dan, X. Yanhua, W. Lianyuan, Z. Haiyan, S. Yonglin and J. Zhigang, Analyst, 2020, 145, 5425–5429 RSC.
- X. Wang, H. Chang, J. Xie, B. Zhao, B. Liu, S. Xu, W. Pei, N. Ren, L. Huang and W. Huang, Coord. Chem. Rev., 2014, 273–274, 201–212 CrossRef CAS.
- K. Gupta and A. K. Patra, ACS Sens., 2020, 5, 1268–1272 CrossRef CAS PubMed.
- M. Sathiyaraja and V. Thiagarajan, RSC Adv., 2020, 10, 25848–25855 RSC.
- H. Xu, H. Zhang, L. Zhao, C. Peng, G. Liu and T. Cheng, New J. Chem., 2020, 44, 10713–10718 RSC.
- K. C. Behera and B. Bag, Chem. Commun., 2020, 56, 9308–9311 RSC.
- T. N. Annisa, S.-H. Jung, M. Gupta, J. Y. Bae, J. M. Park and H.-L. Lee, ACS Appl. Mater. Interfaces, 2020, 12, 11055–11062 CrossRef CAS PubMed.
- S.-H. Jung, Y. J. Jung, B. C. Park, H. Kong, B. Lim, J. M. Park and H.-L. Lee, Sens. Actuators, B, 2020, 323, 128698 CrossRef CAS.
- F. Takahashi, Y. Kazui, H. Miyaguchi, T. Ohmori, R. Tanaka and J. Jin, Sens. Actuators, B, 2021, 327, 128902 CrossRef CAS.
- Y. Zhang, H. Mu, P. Zheng, Y. Zhao and M. Zhang, Sens. Actuators, B, 2021, 343, 130140 CrossRef CAS.
- Q. Q. Wang, R. A. Begum, V. W. Day and K. Bowman-James, Org. Biomol. Chem., 2012, 10, 8786–8793 RSC.
- Y. C. Yang, J. R. Ward and T. Luteran, J. Org. Chem., 1986, 51, 2756–2759 CrossRef CAS.
- https://www.science.org/news/2013/09/un-experts-find-convincing-evidence-large-scale-sarin-attack-syria. (Accessed November 07, 2022).
- S. Tal, H. Salman, Y. Abraham, M. Botoshansky and Y. Eichen, Chem. – Eur. J., 2006, 12, 4858–4864 CrossRef CAS PubMed.
- V. Kumar and E. V. Anslyn, J. Am. Chem. Soc., 2013, 135, 6338–6344 CrossRef CAS PubMed.
- H. Rana, G. Raviraju, A. Kumar, J. Acharya, A. K. Gupta and V. Kumar, Sens. Lett., 2019, 17, 352–357 CrossRef.
- V. Kumar and E. V. Anslyn, Chem. Sci., 2013, 4, 4292–4297 RSC.
- V. Kumar and H. Rana, RSC Adv., 2015, 5, 91946–91950 RSC.
- D. R. Goud, A. K. Purohit, V. Tak, D. K. Dubey, P. Kumar and D. Pardasani, Chem. Commun., 2014, 50, 12363–12366 RSC.
- H. Wang, J. Guan, X. Han, S. W. Chen, T. Li, Y. Zhang, M. S. Yuan and J. Wang, Talanta, 2018, 189, 39–44 CrossRef CAS PubMed.
- Y. M. Poronik, K. V. Vygranenko, D. Gryko and D. T. Gryko, Chem. Soc. Rev., 2019, 48, 5242–5265 RSC.
- S. Bidmanova, M. S. Steiner, M. Stepan, K. Vymazalova, M. A. Gruber, A. Duerkop, J. Damborsky, Z. Prokop and O. S. Wolfbeis, Anal. Chem., 2016, 88(11), 6044–6049 CrossRef CAS PubMed.
- H. Wang, D.-E. Wang, J. Guan, X. Han, P. Xue, W. Liu, M.-S. Yuan and J. Wang, J. Mater. Chem. C, 2017, 5, 11565–11572 RSC.
- V. Kumar, H. Rana, G. Raviraju and A. K. Gupta, Anal. Chem., 2018, 90, 1417–1422 CrossRef CAS PubMed.
- Y. Zhang, Y. Lv, X. Wang, A. Peng, K. Zhang, X. Jie, J. Huang and Z. Tian, Anal. Chem., 2018, 90, 5481–5488 CrossRef CAS PubMed.
- Y. L. Jiang and A.-M. Broome, ACS Sens., 2019, 4, 1791–1797 CrossRef CAS PubMed.
- Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16–29 RSC.
- W. Meng, M. Sun, Q. Xu, J. Cen, Y. Cao, Z. Li and K. Xiao, ACS Sens., 2019, 4, 2794–2801 CrossRef CAS PubMed.
- W. Xiong, Y. Gong, Y. Che and J. Zhao, Anal. Chem., 2019, 91, 1711–1714 CrossRef CAS PubMed.
- C. Qiu, X. Liu, C. Cheng, Y. Gong, W. Xiong, Y. Guo, C. Wang, J. Zhao and Y. Che, Anal. Chem., 2019, 91, 6408–6412 CrossRef CAS PubMed.
- W. Tuo, J. Bouquet, F. Taran and T. L. Gall, Chem. Commun., 2019, 55, 8655–8658 RSC.
- V. V. Singh, V. Kumar, U. Biswas, M. Boopathi, K. Ganesan and A. K. Gupta, Anal. Chem., 2021, 93, 1193–1199 CrossRef CAS PubMed.
- R. C. Knighton, M. R. Sambrook, J. C. Vincent, S. A. Smith, C. J. Serpell, J. Cookson, M. S. Vickers and P. D. Beer, Chem. Commun., 2013, 49, 2293–2295 RSC.
- D. Pardasani, V. Tak, A. K. Purohit and D. K. Dubey, Analyst, 2012, 137, 5648–5653 RSC.
- M. Boopathi, M. V. S. Suryanarayana, A. K. Nigam, P. Pandey, K. Ganesan, B. Singh and K. Sekhar, Biosens. Bioelectron., 2006, 21, 2339–2344 CrossRef CAS PubMed.
- S. Kaur, M. Singh and S. S. Flora, Appl. Biochem. Biotechnol., 2013, 171, 1405–1415 CrossRef CAS PubMed.
- B. Li, S. Li, B. Wang, Z. Meng, Y. Wang, Q. Meng and C. Li, iScience, 2020, 23, 101443 CrossRef CAS PubMed.
- Q.-Q. Wang, R. A. Begum, V. W. Day and K. Bowman-James, J. Am. Chem. Soc., 2013, 135, 17193–17199 CrossRef CAS PubMed.
- N. Li, P. Zhao, N. Liu, M. Echeverria, S. Moya, L. Salmon, J. Ruiz and D. Astruc, Chem. – Eur. J., 2014, 20, 8363–8369 CrossRef CAS PubMed.
- N. V. Singha, B. Shankar, R. Shanmugam, S. K. Awasthi and R. D. Gupta, Sens. Actuators, B, 2016, 227, 85–91 CrossRef.
- D. Li, H. Xi, S. Han and S. Zhao, Anal. Methods, 2021, 13, 484–490 RSC.
- W. Menga, H. Zhang, L. Xiao, X. Chend, M. Sunb, Q. Xub, Y. Cao, K. Xiao and Z. Li, Sens. Actuators, B, 2019, 296, 126678 CrossRef.
- M.-J. Xue, X.-Z. Wei, W. Feng, Z.-F. Xing, S.-L. Liu and Q.-H. Song, J. Hazard. Mater., 2021, 416, 125789 CrossRef CAS PubMed.
- O. Abuzalat, S. Homayoonnia, D. Wong, H. R. Tantawy and S. Kim, Dalton Trans., 2021, 50, 3261–3268 RSC.
- S. Shrivastava, T. Q. Trung and N.-E. Lee, Chem. Soc. Rev., 2020, 49, 1812–1866 RSC.
- N. Colozza, K. Kehe, G. Dionisi, T. Popp, A. Tsoutsoulopoulos, D. Steinritz, D. Moscone and F. Arduini, Biosens. Bioelectron., 2019, 129, 15–23 CrossRef CAS PubMed.
- D.-H. Lee, D.-N. Lee and J.-I. Hong, New J. Chem., 2016, 40, 9021–9024 RSC.
- R. Gotor, A. M. Costero, S. Gil, M. Parra, R. Martinez-Manez, F. Sancenon and P. Gavina, Chem. Commun., 2013, 49, 5669–5671 RSC.
- L. Zhong, H. Li, S.-L. Wang and Q.-H. Song, Sens. Actuators, B, 2018, 266, 703–709 CrossRef CAS.
- L. A. Greenawald, J. L. Snyder, N. L. Fry, M. J. Sailor, G. R. Boss, H. O. Finklea and S. Bell, Sens. Actuators, B, 2015, 221, 379–385 CrossRef CAS PubMed.
- L. A. Greenawald, G. R. Boss, J. L. Snyder, A. Reeder and S. Bell, ACS Sens., 2017, 2(10), 1458–1466 CrossRef CAS PubMed.
- R. C. Gupta, S. K. Dwivedi, R. Ali and A. Misra, ChemistrySelect, 2018, 3, 2025–2031 CrossRef CAS.
- X. Liu, N. Li, M. Li, H. Chen, N. Zhang, Y. Wang and K. Zheng, Coord. Chem. Rev., 2020, 404, 213109 CrossRef CAS.
- C. Guo, A. C. Sedgwick, T. Hirao and J. L. Sessler, Coord. Chem. Rev., 2021, 427, 213560 CrossRef CAS PubMed.
- R. E. Gawley, S. Pinet, C. M. Cardona, P. K. Datta, T. Ren, W. C. Guida, J. Nydick and R. M. Leblanc, J. Am. Chem. Soc., 2002, 124, 13448–13453 CrossRef CAS PubMed.
- H. Mao, J. B. Thorne, J. S. Pharr and R. E. Gawley, Can. J. Chem., 2006, 84, 1273–1279 CrossRef CAS PubMed.
- P. Kele, J. Orbulescu, T. L. Calhoun, R. E. Gawley and R. M. Leblanc, Tetrahedron Lett., 2002, 43, 4413–4416 CrossRef CAS.
- J. Lago, L. P. Rodríguez, L. Blanco, J. M. Vieites and A. G. Cabado, Mar. Drugs, 2015, 13, 6384–6406 CrossRef CAS PubMed.
- S. Burrell, S. Crum, B. Foley and A. D. Turner, TrAC, Trends Anal. Chem., 2016, 75, 10–23 CrossRef CAS.
- R. E. Gawley, M. Shanmugasundaram, J. B. Thorne and R. M. Tarkka, Toxicon, 2005, 45, 783–787 CrossRef CAS PubMed ; Corrigendum 46, 477.
- R. E. Gawley, H. Mao, M. M. Haque, J. B. Thorne and J. S. Pharr, J. Org. Chem., 2007, 72, 2187–2191 CrossRef CAS PubMed.
- J. Orbulescu, P. Kele, A. Kotschy and R. M. Leblanc, J. Mater. Chem., 2005, 15, 3084–3088 RSC.
- P. Kele, J. Orbulescu, R. E. Gawley and R. M. Leblanc, Chem. Commun., 2006, 1494–1496 RSC.
- A. Sun, J. Chai, T. Xiao, X. Shi, X. Li, Q. Zhao, D. Li and J. Chen, Sens. Actuators, B, 2018, 258, 408–414 CrossRef CAS.
- L. Qiang, Y. Zhang, X. Guo, Y. Gao, Y. Han, J. Sun and L. Han, RSC Adv., 2020, 10, 15293–15298 RSC.
- X. He, Current technologies for detection of ricin in different matrices, in Ricin Toxin, ed. J. W. Cherwonogrodky, Bentham Science, Oak Park, Illinois, 2014, ch. 2, pp. 38–56 Search PubMed.
- J. Hu, H. Dai, Y. Sun, P. Ni, Y. Wang, S. Jiang and Z. Li, RSC Adv., 2014, 4, 43998–44003 RSC.
- J. Hu, P. Ni, H. Dai, Y. Sun, Y. Wang, S. Jiang and Z. Li, RSC Adv., 2015, 5, 16036–16041 RSC.
- C. L. Schofield, B. Mukhopadhyay, S. M. Hardy, M. B. McDonnell, R. A. Field and D. A. Russell, Analyst, 2008, 133, 626–634 RSC.
- S. Pradhan, M. Boopathi, O. Kumar, A. Baghel, P. Pandey, T. H. Mahato, B. Singh and R. Vijayaraghavan, Biosens. Bioelectron., 2009, 25, 592–598 CrossRef CAS PubMed.
- B. Esteban-Fernández de Ávila, M. A. Lopez-Ramirez, D. F. Báez, A. Jodra, V. V. Singh, K. Kaufmann and J. Wang, ACS Sens., 2016, 1, 217–221 CrossRef.
- S. Fan, G. H. Dennison, N. FitzGerald, P. L. Burn, I. R. Gentle and P. E. Shaw, Commun. Chem., 2021, 4, 1–11 CrossRef.
- T. M. Alam, M. K. Kinnan, B. W. Wilson and D. R. Wheeler, ChemistrySelect, 2016, 1, 2698–2705 CrossRef CAS.
- W. Q. Meng, A. C. Sedgwick, N. Kwon, M. Sun, K. Xiao, X. P. He, E. V. Anslyn, T. D. James and J. Yoon, Chem. Soc. Rev., 2022 10.1039/D2CS00650B.
- L. Zeng, T. Chen, B. Zhu, S. Koo, Y. Tang, W. Lin, T. D. James and J. S. Kim, Chem. Sci., 2022, 13, 4523–4532 RSC.
| This journal is © The Royal Society of Chemistry 2023 |