Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Quantitative 67Zn, 27Al and 1H MAS NMR spectroscopy for the characterization of Zn species in ZSM-5 catalysts†
Marija
Avramovska
a,
Dieter
Freude
*a,
Jürgen
Haase
a,
Alexander V.
Toktarev
b,
Sergei S.
Arzumanov
 b,
Anton A.
Gabrienko
b,
Anton A.
Gabrienko
 b and
Alexander G.
Stepanov
b and
Alexander G.
Stepanov
 *b
*b
aFaculty of Physics and Earth Sciences, Leipzig University, Linnéstraße 5, 04103 Leipzig, Germany. E-mail: freude@uni-leipzig.de
bBoreskov Institute of Catalysis, Siberian Branch of the Russian Academy of Sciences, Prospekt Akademika Lavrentieva 5, Novosibirsk 630090, Russia. E-mail: stepanov@catalysis.ru
First published on 10th October 2023
Abstract
67Zn MAS NMR spectroscopy was used to characterize the state of Zn in Zn-modified zeolites ZSM-5. Two 67Zn enriched zeolite samples were prepared: by solid-state exchange with metal 67Zn (Zn2+/ZSM-5 sample) and by ion exchange with zinc formate solution (ZnO/H-ZSM-5 sample), both containing ca. 3.8 wt% Zn. The elemental analysis, TEM, and quantitative BAS and aluminum analyses with 1H and 27Al MAS NMR have shown that Zn2+/ZSM-5 contains zinc in the form of Zn2+ cations, while both ZnO species and Zn2+ cations are present in ZnO/H-ZSM-5 besides BAS. 67Zn MAS NMR has detected the signal of Zn in a tetrahedral environment from ZnO species for both the activated and hydrated ZnO/H-ZSM-5 zeolite. The signal of Zn in an octahedral environment was detected for the hydrated Zn2+/ZSM-5 and ZnO/H-ZSM-5 zeolites. This signal may belong to zinc cation [HOZn]+ or Zn(OH)2 species surrounded by water molecules. Quantitative 67Zn MAS NMR analysis has shown that only 27 and 38% of zinc loaded in the zeolite is visible for the activated and hydrated ZnO/H-ZSM-5 zeolite, and 24% of Zn is visible for the hydrated Zn2+/ZSM-5. Zinc in the form of ZnO species is entirely visible in both the activated and hydrated ZnO/H-ZSM-5 zeolite, while Zn2+ cations are not detected at all for the activated sample and only 29% of Zn2+ cations is visible for the hydrated zeolite. Detection of only a part of Zn2+ cations in the form of [HOZn]+ or Zn(OH)2 species in octahedral environment presumes only partial hydrolysis of the bond of Zn2+ cation with framework oxygen and further solvation of the Zn species formed at hydrolysis by the adsorbed water.
Introduction
The conversion of light alkanes to highly valuable aromatic hydrocarbons can be effectively performed by Zn-modified zeolite catalysts.1–4 Various approaches have been used2,5 for zinc loading in the zeolite and, therefore, different types, dimensions, and localizations of zinc species, inside the zeolite pores and on the outer surface of the crystals, have been considered for the mechanism of catalysis.6–8 In this regard, it is crucial to characterize properly the state of zinc species loaded into a zeolite. In our recent work, we have used the following experimental techniques to study Zn species in zeolites:8 extended X-ray absorption fine structure (EXAFS), X-ray photoelectron spectroscopy (XPS) and diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), the latter for molecule complexes attached to the Zn species. An obvious idea is to use Zn NMR spectroscopy as well. However, there are certain difficulties regarding the application of the method. First, the only stable NMR-active isotope is 67Zn, which has a natural abundance of 4.10%.9 Second, the low gyromagnetic ratio, γ, amounting to γ/2π = 2.668532 MHz T−1,10 downgrades the molar receptivity of 67Zn nuclei with respect to 1H nuclei by a factor of 2.87 × 10−3. Third, the nuclear spin of 67Zn is I = 5/2 and the nuclear electric quadrupole moment of is 15.0 fm2,10 which limits the NMR observation of powders to the central transition (magnetic quantum numbers −1/2 ↔ +1/2) and causes a strong second-order quadrupole broadening of the NMR spectra. Fourth, the amount of Zn species usually loaded in a zeolite is relatively low, e.g., it is about 3.8 wt% our studies.8,11–13 A maximum value of 6 wt% can be found in the literature.14The combination of the four factors lowers drastically the sensitivity of Zn NMR compared to the mostly used NMR isotopes. Only the first problem can be overcome by using 67Zn enriched materials for Zn loading in zeolites. A corresponding study was performed by Qi et al.14 They used 89.6% 67Zn enriched precursors for loading of 2 and 6 wt% of Zn in ZSM-5 zeolite and presented 67Zn NMR spectra and 1H spectra with dipolar 67Zn recoupling by irradiation of the 67Zn NMR frequency. The latter spectra were measured as a function of the irradiation time (up to 14.4 ms) and used for a quantitative determination of the synergic acid sites with the obtained amount being about 4 μmol g−1 for both, the 2 and 6 wt%, Zn-modified ZSM-5 samples.14 It turns out that the concentration of synergic acid sites, provided in the study of Qi et al.,14 is three orders of magnitude smaller than the concentration of Zn atoms.
NMR spectroscopy can be applied as a quantitative technique, if a one-pulse excitation (observation of the Bloch decay) and a reference sample with a known concentration of the nucleus under study are used. For echo measurements, the echo intensity loss by the transverse relaxation must be taken into account. The sensitivity-enhancement technique quadrupolar Carr–Purcell–Meiboom–Gill (QCPMG NMR)15 can be hardly used for concentration determinations because the obtained time-domain signal increases with the number of observed echoes. The present study applies 67Zn, 27Al, and 1H MAS NMR spectroscopy on zeolites ZSM-5 zeolites modified with 67Zn enriched zinc, in order to find out which information for the characterization of Zn-containing catalysts can be obtained by quantitative NMR spectroscopy. 27Al recoupled 1H MAS NMR spectroscopy (TRAPDOR)16,17 was also applied to get additional information.
Methods
Synthesis of Zn2+/ZSM-5 zeolite and sample preparation
The parent zeolite is a template-free synthesized NH4-ZSM-5 (Si/Al = 13) zeolite provided by the Tricat GmbH. The ammonium form was transformed into the hydrogen form by calcination at 500 °C for 5 h. The characteristics of this H-ZSM-5 zeolite were previously described.18 A solid-state exchange reaction between BAS of the acid H-ZSM-5 zeolite and zinc powder was used to prepare Zn2+ – exchanged zeolite.19,20 The zeolite was activated under vacuum at 673 K for 20 h (residual pressure < 10−3 Pa), and the reaction with zinc vapor was performed at 773 K for 7 h in a vacuumed glass ampule loaded with the activated zeolite and metallic zinc (Zn/Al = 5). We used a 90% 67Zn enriched Zn metal of US Services Inc. (John Kilby), whereas a Zn metal with a 4.10% natural abundance of 67Zn was used for previous studies.19 It follows an evacuation at 773 K for 15 h. 1H MAS NMR spectroscopy has shown19 that such a procedure leads to the removal of bridged SiOHAl groups. The concentration of Zn in the product in the resulting sample is 3.8 wt%, as determined by elemental analysis (ICP-OES). We denote this sample as activated Zn2+/ZSM-5. A portion of the Zn2+/ZSM-5 zeolite was exposed to atmospheric water for 24 h and then again activated at 673 K for 20 h under vacuum and further sealed in a 3-mm-glass ampoule for the NMR measurement. This sample is denoted as reactivated Zn2+/ZSM-5. Another sample, sealed in a glass tube, was opened and kept for 24 h under air before 67Zn NMR measurements meanwhile rehydration took place. We denote the sample as hydrated Zn2+/ZSM-5.Synthesis of ZnO/H-ZSM-5 zeolite and sample preparation
Zn-modified ZSM-5 zeolite, containing both Zn2+ cations and highly dispersed ZnO species or small clusters of ZnO located in the channels of the zeolite, was synthesized by the ion exchange with zinc formate solution, 67Zn(HCOO)2. Details of the synthesis procedure is described further. The elemental analysis has shown that the content of Zn in the sample is 3.86 wt%.ZnO/H-ZSM-5 zeolite was activated under vacuum (673 K for 20 h) and a portion of it was loaded with a small amount of adsorbed benzene and then sealed in a 3 mm-glass ampoule for the NMR measurement. We denote the sample as activated ZnO/H-ZSM-5. Another portion of the activated sample was kept in air for 24 h and further filled into the MAS rotor for the measurement. We denote the sample as hydrated ZnO/H-ZSM-5.
Transmission electron microscopy
The Themis-Z 3.1 (TFS, USA) microscope, equipped with a field emission cathode with a monochromator and two aberration correctors, was used to study Zn-modified zeolite samples at an accelerating voltage of 200 kV. Energy dispersive X-ray spectroscopy (EDX) was performed with a four-segment Super-X detector (an energy resolution of 120 eV) in the scanning dark-field mode (HAADF-STEM) with elemental mapping along the characteristic lines of the spectrum from each point of the analyzing region. For the electron microscopy studies, Zn2+/ZSM-5 and ZnO/H-ZSM-5 zeolites were dispersed in ethanol using ultrasound and deposited on a perforated carbon-film-coated copper grid (3 mm diameter).NMR methods
67Zn and 27Al MAS NMR experiments were performed at room temperature on a Bruker AVANCE 750 spectrometer at 17.6 T with high-power Bruker probes and a MAS frequency of 10 kHz. Larmor frequencies were about 195 and 47 MHz for 27Al and 67Zn, respectively. A narrow-bore X probe was used for 67Zn MAS NMR spectroscopy. 67Zn MAS NMR measurements were done using a Hahn echo with a 16-phase cycle and a delay of one rotation period, 100 μs, between π/2- and π-pulses. For each zeolite sample, the number of scans was 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with a delay of 2 s between the scans, a dwell time of 20 μs, a size of 16 k, and a unique receiver gain. The estimated longitudinal 67Zn relaxation time for Zn-modified zeolite samples is smaller than 200 μs. We have T1 ≈ 1 s for ZnO powder and used a repetition delay of 5 s for the ZnO reference sample.
000 with a delay of 2 s between the scans, a dwell time of 20 μs, a size of 16 k, and a unique receiver gain. The estimated longitudinal 67Zn relaxation time for Zn-modified zeolite samples is smaller than 200 μs. We have T1 ≈ 1 s for ZnO powder and used a repetition delay of 5 s for the ZnO reference sample.
Additional 67Zn measurements with a Hahn-echo pulse delay of 200 μs did not show a significant signal decay compared to the 100 μs pulse distance for all samples. Therefore, transverse relaxation can be neglected for the 100 μs pulse distance. This is important for NMR concentration determinations by using the Hahn-echo intensity.
For 27Al MAS NMR, the number of scans were 500 with a delay of 0.5 s between the scans, a dwell time of 20 μs, a size of 4 k and a unique receiver gain. A selective π/4 (non-selective π/12) pulse was used to get spectral intensities independent of different quadrupole coupling constants CQ.
To determine the quantity of BAS in the zeolite samples with 1H MAS NMR, the experiments were performed at 9.4 T on a Bruker Avance-400 spectrometer equipped with a broad-band double-resonance-MAS probe. Zirconia rotors (4 mm outer diameter) with the inserted sealed 3 mm glass tube were spun at 5–10 kHz by dried compressed air at room temperature (300 K). 1H MAS NMR spectra were recorded by the Hahn-echo pulse sequence (π/2–τ–π–τ-acquisition), where τ equals one rotor period (100–200 μs). The excitation pulse length was 4.5 μs (π/2), and typically 32 scans were accumulated with a 4–60 s delay. In double-resonance, 1H{27Al} TRAPDOR experiments16,17 Hahn-echo sequence was applied to the 1H channel with irradiation of aluminum during both τ periods. The 27Al nutation frequency of the irradiation field was about 100 kHz.
All chemical shift references correspond to the IUPAC convention.21
Reference samples
NMR concentration determination requires a reference (internal or external) with a known concentration of the corresponding nucleus. For the determination of the concentration of zeolite OH groups (Brønsted acid sites, BAS) with 1H MAS NMR, we have used a certain quantity of benzene adsorbed on activated (dehydrated) zeolite sample as an internal reference.18,22For determination of the quantity of Zn species by 67Zn MAS NMR, ZnO powder with a molar mass of 81.41 g mol−1 and a 67Zn natural abundance of 4.10% was used as an external 67Zn reference. We have 0.041 NA/81.41 of 67Zn nuclei per gram of ZnO (NA is the Avogadro constant). Thus, the specific concentration (67Zn number per 1g ZnO powder) is cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) ZnO = 5.036 × 10-4 × NA g−1. 0.131 gram of ZnO powder was filled into the MAS rotor. It corresponds to NA × 6.6 × 10−5 of 67Zn nuclei in the rotor.
ZnO = 5.036 × 10-4 × NA g−1. 0.131 gram of ZnO powder was filled into the MAS rotor. It corresponds to NA × 6.6 × 10−5 of 67Zn nuclei in the rotor.
Aluminum concentration was determined by 27Al MAS NMR measurements of the hydrated samples in comparison with the zeolite Na-ZSM-5 (Si/Al = 15) of the Tricat GmbH, which is the sodium form for the parent zeolite of the Zn-modified samples.
Results
Sample characterization by electron microscopy
Before studying by MAS NMR, ZnO/H-ZSM-5 and Zn2+/ZSM-5 zeolites were characterized by transmission electron microscopy. Fig. 1 shows the obtained TEM images. Both samples, originating from the same parent H-ZSM-5, display a crystal size of 0.5–5 μm and a rod-shaped morphology, which is typical of MFI-type zeolites.23 The Zn2+/ZSM-5 sample represents the pure zeolite phase (Fig. 1a and b). For ZnO/H-ZSM-5, the particles notably different in size and mass density, compared to the zeolite crystals, can be seen (Fig. 1c and d). This evidences the presence of another phase deposited on the surface of the zeolite crystals. | ||
| Fig. 1 HAADF-STEM images of ZnO/H-ZSM-5 (a) and (b) and Zn2+/ZSM-5 (c) and (d) zeolites. EDX elemental mapping images of Si (blue), Al (green), and Zn (red) in the crystals of ZnO/H-ZSM-5 (e) and (f) and Zn2+/ZSM-5 (g) and (h) zeolites. See also Table 1 for relative atomic content of the elements as obtained for whole studied area (e)–(h) or selected ones as depicted in (f). | ||
For Zn2+/ZSM-5 (Fig. 1e and f), EDX elemental mapping shows Zn, Al, and Si are uniformly distributed in the zeolite crystals, with Al/Zn atomic ratio being around 2 (Table 1). This confirms the presence of only Zn2+ species in the sample and almost full substitution of BAS by zinc cations. For ZnO/H-ZSM-5 (Fig. 1g and h), there is a uniform distribution of Si and Al within the zeolite crystals, whereas the smaller particles, observed in HAADF-STEM images, are enriched with Zn. Therefore, it is reasonable to conclude that these particles are ZnO-like agglomerates on the external surface of ZSM-5 crystals. Elemental composition determined for different areas in Fig. 1h and presented in Table 1 demonstrates that the crystals of ZnO/H-ZSM-5 zeolite also contain Zn, and its quantity is similar to that for the Zn2+/ZSM-5 sample. This may indicate the presence of Zn2+ cations and small ZnO-like clusters inside ZnO/H-ZSM-5 pores8 together with larger ZnO-agglomerates on the external surface.
| Fig. 1 image | Studied area | Atomic content/% | ||
|---|---|---|---|---|
| Al | Si | Zn | ||
| e | Whole area | 7.66 | 87.09 | 5.26 |
| f | Whole area | 7.24 | 81.14 | 11.62 |
| Area 1 | 0.73 | 8.39 | 90.88 | |
| Area 2 | 7.98 | 88.16 | 3.86 | |
| Area 3 | 9.62 | 83.32 | 7.06 | |
| Area 4 | 4.60 | 54.43 | 40.98 | |
| g | Whole area | 7.43 | 87.94 | 4.62 |
| h | Whole area | 8.07 | 88.31 | 3.62 |
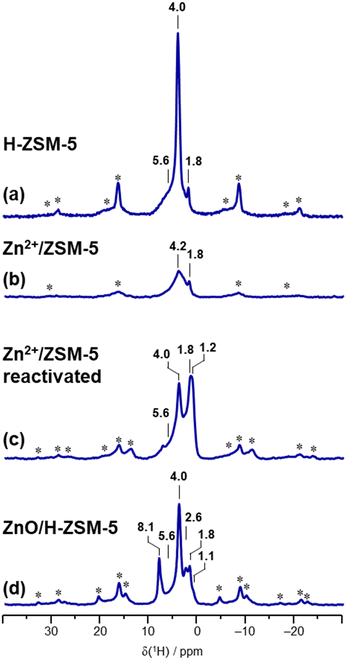
Sample characterization by 1H MAS NMR and evaluation of BAS quantity and zinc state
| Zeolite sample | OH concentration/μmol·g−1 | ||||
|---|---|---|---|---|---|
| Si–OH | Zn–OH | Al–OH | Si–OH–Al | Zeolite unit cell | |
| H-ZSM-5 | 40 ± 8 (terminal) | 96 ± 5 | 1290 ± 90 | |Aloct0.47 H7.38| [Altetr7.38 Si88.62 O192] | |
| Zn2+/ZSM-5 (3.80 wt% 67Zn) | 400 ± 70 (silanol nests) | |67Zn2+3.93| [Altetr7.85 Si88.15 O192] | |||
| ZnO/H-ZSM-5 (3.86 wt% 67Zn) | 70 ± 50 | 17 ± 16 | 140 ± 40 | 530 ± 170 | |Aloct0.47 (67ZnO)1.25 (67Zn2+)2.16 H3.06| [Altetr7.38 Si88.62 O192] |
Concentrations of aluminum species determined by 27Al MAS NMR spectroscopy
Fig. 4 presents the 27Al MAS NMR spectra of the reference sample, a hydrated zeolite Na-ZSM-5 (A), and the samples under study, the hydrated Zn2+/ZSM-5 (B) and the hydrated ZnO/H-ZSM-5 (C). The hydrated samples represent the activated samples that were opened and kept under air for 24 h. The weight analyses have shown that they contain 7 wt% water. Such value corresponds to the degree of hydration of about 25 water molecules per unit cell for Zn2+/ZSM-5 and ZnO/H-ZSM-5, respectively. Na-ZSM-5 reference sample contained 12 molecules of water per zeolite unit cell.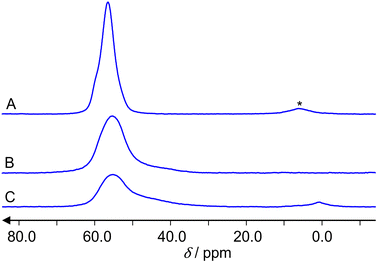 | ||
| Fig. 4 27Al MAS NMR spectra of the hydrated zeolites: Na-ZSM-5 (A), Zn2+/ZSM-5 (B) and ZnO/H-/ZSM-5 (C). The asterisk (*) denotes a spinning sideband. | ||
Spectra (A) and (B) show signals of tetrahedrally coordinated aluminum atoms AlO4 at about 60 ppm. A spinning sideband is observed only in the spectrum (A). Spectrum (C) shows in addition a signal of octahedrally coordinated aluminum AlO6 at about 3 ppm. No signal of five-fold coordinated aluminum AlO5, which would be expected at about 35 ppm, is observed. For the determination of the aluminum concentration, we consider the integral intensities of the spectra, the weight of the samples and the different Si/Al ratios (those are 12 for the parent H-ZSM-5 and 15 for the reference Na-ZSM-5). The accuracy of this approach for concentration determinations by 27Al MAS NMR is 15%. Within this limit, there is no significant deviation of the Si/Al ratio from the value 12 in the hydrated Zn2+/ZSM-5 and ZnO/H-ZSM-5 samples. Both samples show mainly (100 and 94% integral intensity, respectively) AlO4 coordination of framework aluminum atoms. Only the hydrated ZnO/H-ZSM-5 shows a weak (6% integral intensity) signal of extra-framework AlO6 aluminum. Spectra (B) and (C) have a shoulder of the AlO4 peak. Consequently, the spectra were deconvoluted by dmfit25 as presented for the ZnO/H-ZSM-5 sample in Fig. 5. The result is that the spectrum of ZnO/H-ZSM-5 sample consists of three signals with the following values of the chemical shift δ, quadrupole coupling constant CQ and relative integral intensities I: δ = 60.5 ppm with CQ = 5.2 MHz and I = 59%, δ = 60.5 ppm with CQ = 8.2 MHz and I = 35%, δ = 3.5 ppm with CQ = 4.0 MHz and I = 6%. The deconvolution of the spectrum of Zn2+/ZSM-5 gives two signals: δ = 60.5 ppm with CQ = 5.2 MHz and I = 79%, δ = 60.5 ppm with CQ = 8.2 MHz and I = 21%.
 | ||
| Fig. 5 Deconvolution of the 27Al MAS NMR spectra of the hydrated ZnO/H-ZSM-5 zeolite. (A) is the experimental spectrum, (B) the sum of the deconvoluted signals, (C) is the main AlO4 signal with δ = 60.5 ppm, CQ = 5.2 MHz, asymmetry parameter η = 0.5, (D) is a stronger quadrupolar broadened signal with the same chemical shift δ = 60.5 ppm, but CQ = 8.2 MHz, η = 0.5, (E) is the AlO6 signal with δ = 4.0 ppm, CQ = 4.0 MHz, η = 1. The deconvolution was performed by dmfit25 using a broadening of Em = 600. The signals at 60.5 ppm with CQ = 5.2 MHz, 60.5 ppm with CQ = 8.2 MHz, and 3.5 ppm with CQ = 4.0 MHz contain 59%, 35% and 6% of the total integral intensity, respectively. | ||
67Zn MAS NMR spectra of the zeolite samples
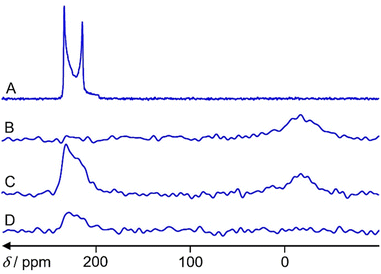
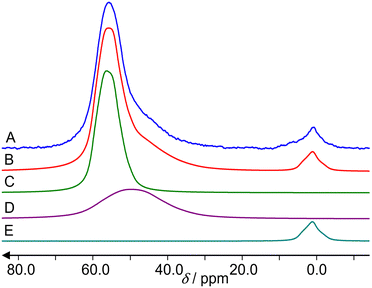
Fig. 6 shows 67Zn MAS NMR spectra of ZnO reference and the three samples under study. A fit of the spectrum in Fig. 6A by the program dmfit25 yields the following parameters for the ZnO signal: chemical shift δ = 240 ppm, quadrupole coupling constant CQ = 2.4 MHz, asymmetry parameter η = 0. This is in accordance with the literature data.26 Spinning sidebands cannot be observed.The hydrated Zn2+/ZSM-5 zeolite exhibits a signal in the region of 0 ppm (Fig. 6B), which belongs to octahedrally coordinated (ZnO6) Zn.27 A fit is less accurate due to the lower signal-to-noise ratio and a broadening of Em = 500 is used for the spectrum. It gives δ = 7 ppm, CQ = 3 MHz and η = 0.
The activated ZnO/H-ZSM-5 zeolite shows only the signal in the region of tetrahedrally coordinated (ZnO4) Zn species (Fig. 6D).27 A fit gives δ = 240 ppm, CQ = 2.5 MHz and η = 0.
The hydrated ZnO/H-ZSM-5 exhibits the signals of both tetrahedrally (ZnO4) and octahedrally (ZnO6) coordinated Zn species (Fig. 6C). A fit gives δ = 240 ppm, CQ = 2.5 MHz and η = 0 with 2/3 integral intensity for the tetrahedrally coordinated Zn species and δ = 0 ppm, CQ = 2.5 MHz and η = 0 with 1/3 integral intensity for the octahedrally coordinated Zn species. No 67Zn MAS NMR signal is observed for the activated Zn2+/ZSM-5 zeolite.
Determination of Zn species concentration in zeolites by 67Zn MAS NMR
For the determination of various Zn species concentration in the 90% 67Zn enriched Zn2+/ZSM-5 and ZnO/H-ZSM-5 samples, we have assumed that both zeolite samples contain similar quantity of the loaded Zn (3.83 wt%). We neglect the weight of the adsorbed molecules in the fused samples. The atomic mass of 67 g mol−1 for the 90% 67Zn enriched zinc was used. In this case, the number of 67Zn species per one gram of Zn-modified zeolite (specific concentration) is cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite = NA × 0.9 × 0.0383/67. The comparison between cs of the Zn-modified zeolites and ZnO powder (see section Reference Samples) gives cs
zeolite = NA × 0.9 × 0.0383/67. The comparison between cs of the Zn-modified zeolites and ZnO powder (see section Reference Samples) gives cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn
Zn![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) zeolite/cs
zeolite/cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 1.037.
ZnO = 1.037.
For the ZnO signal the Topspin integral intensity of the spectrum is 642571386. With a ZnO weight of 0.131 g in the rotor we obtain the specific intensity Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 96 627 per one scan and one gram sample.
ZnO = 96 627 per one scan and one gram sample.
The sample weight of the hydrated Zn2+/ZSM-5 is 0.053 g. The integral intensity of the spectrum (Fig. 4B) is 66975411. The specific intensity per one scan and one gram of sample is Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+/ZSM-5 = 25274. Presuming cs
Zn2+/ZSM-5 = 25274. Presuming cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+/ZSM-5/cs ZnO = Is
Zn2+/ZSM-5/cs ZnO = Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+/ZSM-5/Is
Zn2+/ZSM-5/Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO we have cs
ZnO we have cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Zn2+/ZSM-5/cs
Zn2+/ZSM-5/cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 0.2616.
ZnO = 0.2616.
The sample weight (in the rotor) of the hydrated ZnO/H-ZSM-5 is 0.067 g. The integral intensity of the spectrum C in Fig. 6 is 128258318. Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO/H-ZSM-5 = 38 286. In this case cs
ZnO/H-ZSM-5 = 38 286. In this case cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO/H-ZSM-5/cs
ZnO/H-ZSM-5/cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 0.3962.
ZnO = 0.3962.
The sample weight of the activated ZnO/H-ZSM-5 is 0.020 g in the fused glass ampoule. The integral intensity of the spectrum (Fig. 4D) is 27196740. Is![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO/H-ZSM-5 = 27197. We have cs
ZnO/H-ZSM-5 = 27197. We have cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO/H-ZSM-5/cs
ZnO/H-ZSM-5/cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 0.2815.
ZnO = 0.2815.
The accuracy of our approach to concentration determinations by 67Zn MAS NMR is 20%. We would expect a ratio cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Znzeolite/cs
Znzeolite/cs![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ZnO = 1.037 (see above), if the 67Zn MAS NMR spectrum showed all 67Zn nuclei in the sample according to 3.83 wt% Zn. The lower ratios, estimated above, demonstrate that we see only 38 ± 8% of the expected signal for the hydrated ZnO/H-ZSM-5, 24 ± 5% of the expected signal for the hydrated Zn2+/ZSM-5, 27 ± 5% of the expected signal for the activated ZnO/H-ZSM-5, and less than 5% (detection limit) of the expected signal for the activated Zn2+/ZSM-5 in a glass ampoule. For the hydrated ZnO/H-ZSM-5, the ratio 2
ZnO = 1.037 (see above), if the 67Zn MAS NMR spectrum showed all 67Zn nuclei in the sample according to 3.83 wt% Zn. The lower ratios, estimated above, demonstrate that we see only 38 ± 8% of the expected signal for the hydrated ZnO/H-ZSM-5, 24 ± 5% of the expected signal for the hydrated Zn2+/ZSM-5, 27 ± 5% of the expected signal for the activated ZnO/H-ZSM-5, and less than 5% (detection limit) of the expected signal for the activated Zn2+/ZSM-5 in a glass ampoule. For the hydrated ZnO/H-ZSM-5, the ratio 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 for ZnO4 and ZnO6 intensities implies that we detect 25% and 13% of Zn in tetrahedral and octahedral environments, correspondingly.
1 for ZnO4 and ZnO6 intensities implies that we detect 25% and 13% of Zn in tetrahedral and octahedral environments, correspondingly.
Discussion
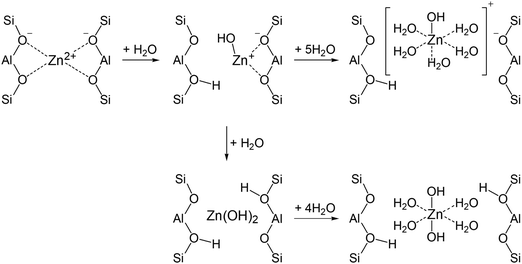
Characterization of the zeolite samples with elemental analysis, TEM, 1H and 27Al MAS NMR allowed us to determine the state of Zn species in the zeolites and calculate the composition of the zeolite unit cell (Table 2). It follows that ZnO/H-ZSM-5 contains both ZnO and Zn2+ cation species. Only the signal of the tetrahedrally coordinated Zn species is detected for the activated ZnO/H-ZSM-5 by 67Zn MAS NMR (Fig. 6D). This implies that highly dispersed ZnO species in the zeolite may represent the small ZnO-like clusters or ZnO-agglomerates in which Zn atoms are surrounded by tetrahedrally disposed oxygen atoms.The zeolite exposed to water vapor from air atmosphere (hydrated ZnO/H-ZSM-5) displays the signal of octahedrally coordinated Zn species besides the signal of ZnO species (Fig. 6C). An appearance of this signal upon zeolite hydration implies hydrolysis of the bonds of Zn2+ cations with the oxygens of the zeolite framework and further hydration of the formed [HOZn]+ cations or Zn(OH)2 species with water molecules (Scheme 1). Presumably, [HOZn(H2O)5]+ cations or [(H2O)4Zn(OH)2] species become detectable by 67Zn MAS NMR.
The activated Zn2+/ZSM-5 zeolite exhibits no 67Zn MAS NMR signal. However, the hydration of the zeolite makes the signal visible (Fig. 6B), e.g., in the form of [HOZn(H2O)5]+ cations, generated according to Scheme 1. Note that a notable part of loaded the Zn species (62–76%) remain invisible by 67Zn MAS NMR even for the hydrated zeolites.
Why we cannot see the majority of zinc species in our 67Zn MAS NMR spectra? 67Zn has the nuclear spin I = 5/2 and the second-order quadrupole broadening of the observed central transition is proportional to the square of the electric field gradient on the location of the nucleus.28CQ is proportional to this gradient. In the reported spectra, the observed species have CQ of 2.5 MHz and 3 MHz for tetrahedral or octahedral coordination, respectively. Quadrupole frequencies above 20 MHz for less symmetric coordination can be found in the literature.27 If CQ is larger, for example, by five times, this results in the line broadening by 25 times. This leads to a decrease in the amplitude of the signal by 25 times. Taking into account the signal-to-noise ratio for the spectra in Fig. 6, which is obtained with an acquisition time of one day, it is clear that the species with such a low symmetry of coordination cannot be observed. In this regard, the signal of Zn2+ cations without a hydration shell, which interacts directly with the oxygens of the zeolite framework is not detected.
Detection of 24% of the expected signal intensity for the hydrated Zn2+/ZSM-5 implies partial hydrolysis (with 25 adsorbed water molecules per u.c.) of the bonds of Zn2+ cations with oxygens of the zeolite framework. Most of the Zn2+ cations retain direct interaction with the oxygens of the zeolite framework and remain invisible in the 67Zn MAS NMR spectrum.
It is interesting, the quantity of tetrahedrally coordinated Zn species visible in the hydrated and activated ZnO/H-ZSM-5 are similar, 25 and 27%, correspondingly. Presuming that Zn2+ is completely invisible for the activated ZnO/H-ZSM-5, it is reasonable to conclude that all Zn sites in the form of ZnO species are visible in both samples. Indeed, the composition of the zeolite unit cell of ZnO/H-ZSM-5 (Table 2) implies that 36% of Zn should be in the form of ZnO. This value is close to the experimentally observed quantity of ZnO species (25–27%), provided that the accuracy of Zn quantification by 67Zn NMR is 20%.
Taking into account that the unit cell composition and the relative intensities of Zn signals in tetrahedral ZnO4 and octahedral ZnO6 coordination is 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, one can infer that 71% of Zn in the form of Zn2+ cations remain invisible for the hydrated ZnO/H-ZSM-5.
1, one can infer that 71% of Zn in the form of Zn2+ cations remain invisible for the hydrated ZnO/H-ZSM-5.
Quantitative 27Al MAS NMR spectroscopy of the hydrated samples can exclude that a significant dealumination takes place during the Zn modifications of the zeolite. The spectra of the Zn2+/ZSM-5 and ZnO/H-ZSM-5 samples show mainly (100 and 94% integral intensity, respectively) AlO4 coordination of framework aluminum atoms as expected for a ZSM-5 zeolite. The loading of Zn does not destroy the zeolite structure, but provides some effect on the structure of AlO4 tetrahedra. Each AlO4 unit is negatively charged and requires a charge compensation by either BAS (H+) or zinc cation (Zn2+). The spectrum of the hydrated Zn2+/ZSM-5 sample does not show AlO6 signal of extra-framework aluminum, whereas, in the hydrated sample ZnO/H-ZSM-5, 0.47 aluminum atoms per unit cell are in AlO6 coordination. A deconvolution of the AlO4 signal of both zeolites shows that it consists of two lines that have the same chemical shift of 60.5 ppm, but different quadrupole coupling constants of 5.2 and 8.2 MHz. The smaller CQ value corresponds to 59% and 79% and the larger one to 35% and 21% of the total intensity of 27Al signal for the hydrated ZnO/H-ZSM-5 and Zn2+/ZSM-5 zeolites, respectively.
For the hydrated ZnO/H-ZSM-5, 29% of Zn2+ in the form, e.g., of [HOZn(H2O)]+ species is visible. Visible cations correspond to 0.625 Zn2+ cations per u.c. Upon hydration of the zeolite, this quantity of the cations together with SiOHAl sites (3.06 H+ site per u.c.) can afford Al species in a tetrahedral oxygen environment (4.31 sites per u.c.) that are visible by 27Al MAS NMR. This quantity of AlO4 corresponds to 58% of the total quantity of AlO4 species in zeolite. This is in good accordance with 59% of AlO4 species measured by 27Al MAS NMR with the signal with smaller CQ = 5.2 MHz. This means that, for the hydrated ZnO/H-ZSM-5 zeolite, a narrower signal with smaller CQ corresponds to AlO4 tetrahedra in 27Al MAS NMR spectrum, which have hydrated [HOZn]+ or/and Zn(OH)2 and H+ cations in the neighborhood, while that with larger CQ belongs to the non-hydrated Zn2+ cations directly interacting with zeolite framework.
The situation seems to be completely different for the hydrated Zn2+/ZSM-5. If we accept that 76% of Zn in hydrated Zn2+/ZSM-5 remains invisible, while all Al in this sample is visible in 27Al MAS NMR, then the larger signal with the relative intensity of 79% and smaller CQ should be assigned to Al in the AlO4 tetrahedra, which have non-solvated Zn2+ cation in the neighborhood, that directly interact with the oxygen of zeolite framework. The broader Al signal with larger CQ should be assigned to Al of the AlO4 tetrahedra with hydrated [HOZn]+ or H+ cations in the neighborhood.
Conclusions
The state of Zn species in Zn- modified ZSM-5 zeolite was characterized by a combination of 67Zn, 27Al, and 1H MAS NMR. Two 67Zn enriched zeolite samples were prepared, by solid-state exchange with metallic 67Zn (Zn2+/ZSM-5 sample) and by ion exchange with a solution of zinc formate [67Zn(HCOO)2] (ZnO/H-ZSM-5 sample), both containing ca. 3.8 wt% Zn. The composition of the zeolite unit cell was established based on the elemental analysis and quantitative BAS and aluminum analyses with 1H and 27Al MAS NMR. It is inferred that Zn2+/ZSM-5 zeolite contains Zn in the form of Zn2+ cations, while BAS are absent in this sample. ZnO/H-ZSM-5 zeolite contains Zn in the form of Zn2+ cations and ZnO species and BAS are also present. 67Zn MAS NMR has detected the signal of Zn in a tetrahedral ZnO4 environment assigned to ZnO species for the activated (dehydrated) ZnO/H-ZSM-5 zeolite. For the hydrated ZnO/H-ZSM-5 zeolite, the signals of tetracoordinated, ZnO4, and octacoordinated, ZnO6, Zn are observed. The signal of Zn in an octahedral environment is assigned to zinc in the form of [HOZn]+ or Zn(OH)2 species surrounded by water molecules. The activated (dehydrated) Zn2+/ZSM-5 zeolite with Zn2+ cations, directly interacting with the oxygens of the framework, exhibits no signal in 67Zn MAS NMR spectrum. Hydration of the sample with 25 water molecules per u.c. makes the signal of Zn in an octahedral oxygen environment visible in the form of [HOZn]+ or Zn(OH)2 species surrounded by water molecules. Quantitative analysis of Zn species detected by 67Zn MAS NMR has shown that only 27 and 38% of zinc quantity loaded in zeolite is visible for the activated and hydrated ZnO/H-ZSM-5 zeolite, respectively. For the hydrated Zn2+/ZSM-5 zeolite, 24% of the loaded Zn is visible by 67Zn MAS NMR, while zinc in the activated sample is not detected at all. Zinc in the form of ZnO species is entirely visible in both the activated and hydrated ZnO/H-ZSM-5 zeolite, while Zn in the form of Zn2+ is not detected at all for the activated sample and only 29% of Zn in a form of Zn2+ cations is visible for the hydrated zeolite. Detection of only a part of Zn2+ cations in the form of [HOZn]+ or Zn(OH)2 species in octahedral environment presumes partial hydrolysis of the bonds of Zn2+ cations with the framework oxygens and further coordination of the Zn sites by adsorbed water molecules. This affords octacoordinated Zn species detectable by 67Zn MAS NMR.Author contributions
M. A.: data curation, formal analysis, investigation; D. F.: methodology, data curation, investigation, writing (original draft); J. H.: funding acquisition; project administration; A. T.: data curation, investigation; S. S. A.: data curation, formal analysis, investigation; A. A. G.: data curation, investigation, visualization; A. G. S. conceptualization, writing (original draft), writing (review & editing), supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
A. G., S. A. and A. S. acknowledge the financial support from Ministry of Science and Higher Education of the Russian Federation within the governmental order for Boreskov Institute of Catalysis (project AAAA-A21-121011390053-4). M. A., D. F., and J. J. acknowledge the support by the Deutsche Forschungsgemeinschaft (grant no. HA 1893/22-1).References
- Y. Ono, Catal. Rev.: Sci. Eng., 1992, 34, 179–226 CrossRef CAS.
- H. Berndt, G. Lietz, B. Lücke and J. Völter, Appl. Catal., A, 1996, 146, 351–363 CrossRef CAS.
- J. A. Biscardi and E. Iglesia, Catal. Today, 1996, 31, 207–231 CrossRef CAS.
- A. Hagen and F. Roessner, Catal. Rev.: Sci. Eng., 2000, 42, 403–437 CrossRef CAS.
- J. Heemsoth, E. Tegeler, F. Roessner and A. Hagen, Microporous Mesoporous Mater., 2001, 46, 185–190 CrossRef CAS.
- Y. G. Kolyagin, V. V. Ordomsky, Y. Z. Khimyak, A. I. Rebrov, F. Fajula and I. I. Ivanova, J. Catal., 2006, 238, 122–133 CrossRef CAS.
- S. M. T. Almutairi, B. Mezari, P. C. M. M. Magusin, E. A. Pidko and E. J. M. Hensen, ACS Catal., 2012, 2, 71–83 CrossRef CAS.
- A. A. Gabrienko, S. S. Arzumanov, A. V. Toktarev, I. G. Danilova, I. P. Prosvirin, V. V. Kriventsov, V. I. Zaikovskii, D. Freude and A. G. Stepanov, ACS Catal., 2017, 7, 1818–1830 CrossRef CAS.
- K. J. R. Rosman and P. D. P. Taylor, Pure Appl. Chem., 1998, 70, 217–235 CrossRef CAS.
- R. K. Harris, E. D. Becker, S. M. C. De Menezes, R. Goodfellow and P. Granger, Pure Appl. Chem., 2001, 73, 1795–1818 CrossRef CAS.
- A. A. Gabrienko, I. G. Danilova, S. S. Arzumanov, D. Freude and A. G. Stepanov, ChemCatChem, 2020, 12, 478–487 CrossRef CAS.
- A. A. Gabrienko, S. S. Arzumanov, Z. N. Lashchinskaya, A. V. Toktarev, D. Freude, J. Haase and A. G. Stepanov, J. Catal., 2020, 391, 69–79 CrossRef CAS.
- S. S. Arzumanov, A. A. Gabrienko, A. V. Toktarev, D. Freude, J. Haase and A. G. Stepanov, J. Phys. Chem. C, 2020, 124, 20270–20279 CrossRef CAS.
- G. D. Qi, Q. Wang, J. Xu, J. Trebosc, O. Lafon, C. Wang, J. P. Amoureux and F. Deng, Angew. Chem., Int. Ed., 2016, 55, 15826–15830 CrossRef CAS PubMed.
- F. H. Larsen, A. S. Lipton, H. J. Jakobsen, N. C. Nielsen and P. D. Ellis, J. Am. Chem. Soc., 1999, 121, 3783–3784 CrossRef CAS.
- C. P. Grey and A. J. Vega, J. Am. Chem. Soc., 1995, 117, 8232–8242 CrossRef CAS.
- E. R. H. van Eck, R. Janssen, W. E. J. R. Maas and W. S. Veeman, Chem. Phys. Lett., 1990, 174, 428–432 CrossRef CAS.
- A. A. Gabrienko, I. G. Danilova, S. S. Arzumanov, L. V. Pirutko, D. Freude and A. G. Stepanov, J. Phys. Chem. C, 2018, 122, 25386–25395 CrossRef CAS.
- A. A. Gabrienko, S. S. Arzumanov, M. V. Luzgin, A. G. Stepanov and V. N. Parmon, J. Phys. Chem. C, 2015, 119, 24910–24918 CrossRef CAS.
- A. A. Gabrienko, S. S. Arzumanov, A. V. Toktarev, D. Freude, J. Haase and A. G. Stepanov, J. Phys. Chem. C, 2019, 123, 27573–27583 CrossRef CAS.
- R. K. Harris, E. D. Becker, S. M. C. De Menezes, R. Goodfellow and P. Granger, Solid State Nucl. Magn. Reson., 2002, 22, 458–483 CrossRef CAS PubMed.
- A. G. Stepanov, in Zeolites and Zeolite-like Materials, ed. B. F. Sels and L. M. Kustov, Elsevier Inc., 2016, pp. 137–188 DOI:10.1016/b978-0-444-63506-8.00004-5.
- A. Petushkov, S. Yoon and S. C. Larsen, Microporous Mesoporous Mater., 2011, 137, 92–100 CrossRef CAS.
- M. Hunger, Catal. Rev.: Sci. Eng., 1997, 39, 345–393 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- G. Wu, Chem. Phys. Lett., 1998, 298, 375–380 CrossRef CAS.
- Y. Huang and A. Sutrisno, Annu. Rep. NMR Spectrosc., 2014, 81, 1–46 CrossRef CAS.
- D. Freude and J. Haase, https://http:/www.quad-nmr.de (accessed 13.09.2022).
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp03136e |
| This journal is © the Owner Societies 2023 |