Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Elucidating local diffusion dynamics in nickel-rich layered oxide cathodes†
Beth I. J.
Johnston
 *ac,
Innes
McClelland
*ac,
Innes
McClelland
 ac,
Peter J.
Baker
ac,
Peter J.
Baker
 bc and
Serena A.
Cussen
bc and
Serena A.
Cussen
 *ac
*ac
aDepartment of Materials Science and Engineering, The University of Sheffield, Sir Robert Hadfield Building, Sheffield, S1 3JD, UK. E-mail: beth.johnston@sheffield.ac.uk; s.a.cussen@sheffield.ac.uk
bISIS Pulsed Neutron and Muon Source, STFC Rutherford Appleton Laboratory, Harwell Science and Innovation Campus, Didcot, Oxfordshire OX11 0QX, UK
cThe Faraday Institution, Quad One, Harwell Science and Innovation Campus, Didcot, Oxfordshire OX11 0RA, UK
First published on 6th September 2023
Abstract
Elucidating Li-ion transport properties is essential for designing suitable methodologies to optimise electrochemical performance in Ni-rich cathodes for high energy density Li-ion batteries. Here, we report the local-scale Li-diffusion characteristics of a series of nickel-rich layered oxide cathodes, prepared via microwave methods, using muon spin relaxation methods. Our results detail the effects of cation dopants, selected for structure stability, on transport properties in candidate nickel-rich chemistries. We find that the local diffusion properties improve with increasing nickel content. Our results demonstrate that these observations are dependant on substitutional effects.
1. Introduction
Ni-rich layered oxide materials are attractive high energy density cathodes for Li-ion batteries for electric vehicle (EV) applications.1–3 Building on the archetypal transition metal layered oxide cathodes LiCoO2 and LiNi1/3Mn1/3Co1/3O2 (NMC111), the development of Ni-rich layered oxides affords higher specific capacities at stable voltages.4–6 The layered oxides LiMO2 (M = Ni, Mn, Co) adopt the rhombohedral R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group, isostructural with α-NaFeO2. Transition metal and lithium ions are located in octahedral 3b and 3a sites respectively, where oxygen anions occupy the 6c sites to form a cubic close-packed array with M and Li arranged in separate, alternating layers.7 Upon increasing the Ni-content, LiNi0.8Mn0.1Co0.1O2 (NMC811) delivers higher discharge capacities than LiNi0.6Mn0.2Co0.2O2 (NMC622) when cycled under identical conditions, but NMC622 benefits from enhanced stabilities owing to its lower nickel content.8–10
m space group, isostructural with α-NaFeO2. Transition metal and lithium ions are located in octahedral 3b and 3a sites respectively, where oxygen anions occupy the 6c sites to form a cubic close-packed array with M and Li arranged in separate, alternating layers.7 Upon increasing the Ni-content, LiNi0.8Mn0.1Co0.1O2 (NMC811) delivers higher discharge capacities than LiNi0.6Mn0.2Co0.2O2 (NMC622) when cycled under identical conditions, but NMC622 benefits from enhanced stabilities owing to its lower nickel content.8–10
Ni-rich variants require that synthetic parameters be carefully considered to deliver high quality materials.11,12 Co-precipitation from stoichiometric amounts of transition metal salts by addition of base is the typical approach for preparing a metal hydroxide precursor, followed by calcination with a lithium source to deliver the final Li-containing layered oxide.13 Controlled temperatures and treatment times, alongside an oxygen-rich atmosphere, are critical during the calcination process to stabilize Ni3+, failure of which can result in excess Ni2+ and the promotion of anti-site mixing between 3a Li+ and 3b Ni2+ which can be detrimental to the cycling performance.14–16 Doping with electrochemically inactive cations such as Al3+ or Mg2+ is one route to stabilising cycling performance, with dopants predicted to sit in the 3b and 3a sites respectively.17,18 The presence of Al3+ on Ni sites is thought to improve thermal stability and mitigate anti-site mixing while small quantities of Mg2+ occupying sites in the Li layer imparts structural stability through pillaring.19–24 There is also a need to develop more sustainable synthetic approaches, aimed at limiting synthesis energy demands while still producing high-quality materials with tailored particle morphologies. One example of this is the development of microwave-assisted strategies which can afford faster, lower temperature routes to energy storage materials.25–28 These faster synthetic routes can also allow for morphology tailoring which may influence structural stability and Li+ diffusion processes.
Underpinning the whole cycling process is the motional behavior of Li+ ions through the structure. In NMCs, 2-dimensional Li+ diffusion takes place in the interlayer.29 Here, there are two pathways available for Li+ diffusion represented in Fig. 1. These can be understood by considering a hop of length a from one Li site to a neighboring site. For fully lithiated materials, this pathway is unviable and a second pathway of length a/√3 must be considered where Li+ moves into an interstitial site within the interlayer.
Here, we investigate the influence of dopants on Li+ diffusion properties in NMC622 and NMC811 cathodes prepared by faster microwave methods. Traditional approaches to ion transport measurement includes impedance or titration methods which probe macro-scale diffusional properties but can be susceptible to sample processing conditions and surface effects.30,31 Here, we apply muon spin relaxation (μ+SR) as a local probe of ion diffusion in doped Ni-rich cathodes which offers advantages over traditional approaches and has been successfully applied previously to study battery materials.25,28,32–40 For example, paramagnetic species do not complicate μ+SR due to the fact that electronic and nuclear contributions to the relaxation process can be separated.41 Operating on a time scale of 10−5 to 10−8 s, μ+SR is perfectly primed to probe the consecutive short range jumps between interstitial sites in a structure that characterize Li+ diffusion.34
2. Experimental section
Synthetic procedures
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) was added and the resultant sol was stirred magnetically on a stirrer-hot plate at 150 °C in order to evaporate the solvent, with a viscous gel obtained at the end. The gel was then pre-dried at ≈300 °C in a heating mantle to obtain a black coloured ash. The ash was transferred to an alumina crucible and heated in air at 450 °C for 14 hours to remove carbon species originating from the acetate groups and the citric acid. The precursor was transferred to an alumina crucible and heated in a CEM Phoenix microwave furnace (2.45 GHz) and heated at 850 °C for 2 hours. Black powders of NMC were obtained and stored in an Ar filled glovebox prior to use.
2) was added and the resultant sol was stirred magnetically on a stirrer-hot plate at 150 °C in order to evaporate the solvent, with a viscous gel obtained at the end. The gel was then pre-dried at ≈300 °C in a heating mantle to obtain a black coloured ash. The ash was transferred to an alumina crucible and heated in air at 450 °C for 14 hours to remove carbon species originating from the acetate groups and the citric acid. The precursor was transferred to an alumina crucible and heated in a CEM Phoenix microwave furnace (2.45 GHz) and heated at 850 °C for 2 hours. Black powders of NMC were obtained and stored in an Ar filled glovebox prior to use.
Materials characterisation
 | (1) |
Measurements were carried out over a temperature range of 100 K to 400 K to encapsulate a low temperature baseline where the ionic motion is too slow to be observed and the thermally activated region where Li+ cations overcome the energetic barriers opposing ionic transport and move on timescales the muon can probe. At each temperature, measurements were performed at zero field (ZF) and with applied longitudinal fields (LFs) of 5 G and 10 G. For all samples discussed here, the data were fit using a baseline asymmetry to account for the background (Abg) and an exponentially relaxing signal accounting for initial fast relaxation from localised 3d electrons on the cations multiplying the dynamical form of the Gaussian Kubo–Toyabe (KT) relaxation function (AKT) that describes the dynamic nuclear magnetic fields such that
| A0P(t) = Abg + AKTGKT(Δ, ν, t, HLF)e−λt | (2) |
3. Results and discussion
The time evolution of the decay positron asymmetry from data collected at 300 K for the NMC811, NMC622 and the 1 mol% Al- and Mg-doped NMC811 samples (with respective targeted stoichiometries of LiNi0.79Al0.01Mn0.1Co0.1O2 and Li0.99Mg0.01Ni0.8Mn0.1Co0.1O2) are shown in Fig. 2 with corresponding fits represented by solid lines. All fitting parameters for the samples are shown in Tables S1–S4 (ESI†). From inspection of the asymmetry, all four samples undergo similar behavior described by the product of the two relaxation processes outlined by the applied relaxation function (eqn (2)). The form of the data suggests the convolution of the Gaussian field distribution coming from the nuclear magnetic moments and a Lorentzian field distribution that is likely to come from the paramagnetic moments. The temperature dependent Li+ ionic motion is apparent in the form of the data at longer times. While the possibility of μ+ diffusion cannot be excluded, recent μ−SR studies (where the negatively charged muon is immobile) have shown there is minimal difference between the field fluctuation rates for positive and negative muons to the field fluctuation rate in spinel materials.46,47 Furthermore, the correlation found between the field fluctuation rate from μ+SR and the diffusion coefficient from electrochemical techniques indicates that the predominant nuclear contribution to the muon spin relaxation also originates from Li+ diffusion in layered oxides.31 Therefore, we assume the changes seen for ν in the thermally activated region for all materials studied are a consequence of changes in Li+ diffusion properties.As the temperature increases to 400 K (Fig. S1, ESI†), more dynamic behavior persists for all four samples, indicating faster Li+ diffusion at higher temperatures. This observation is consistent with high temperature μ+SR data reported for other layered oxides.32,38,39,48 When longitudinal fields of 5 G and 10 G are applied, the relaxation decreases with increasing field strength owing to gradual decoupling of the muon spin from the static nuclear environment. We observe that NMC622 shows a less dynamical environment (characterized by the more Kubo–Toyabe like appearance of the fits obtained) than NMC811 and suggests more sluggish Li+ diffusion in these lower Ni-content compositions.
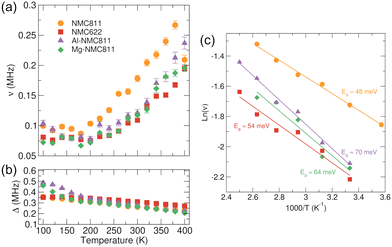
Fig. 3(a) and (b) shows the temperature dependence of the field fluctuation rate (ν) and the static field distribution width (Δ) parameters respectively for each sample. The temperature dependence of ν follow a similar trend where an initial flat (static) region is followed by an increase in ν as the temperature is raised due to the onset and gradual increase of thermally activated Li+ diffusion processes. For NMC811 this occurs at ≈200 K with the fluctuation rate rising sharply to a maximum ≈0.26 MHz. The fall in ν observed at 400 K for NMC811 has been observed previously and is likely due to the Li+ diffusion becoming too fast to detect at these elevated temperatures.25,36 The more sluggish increase in ν seen for NMC622 in Fig. 3(a) suggests that once the energetic barriers for ionic motion are overcome, NMC811 displays a faster frequency of Li+ hopping than NMC622 across the thermally activated temperature range. For Al-NMC811 and Mg-NMC811, ν values obtained suggest that the dopant cations may hinder Li+ ionic diffusion. This effect has been highlighted by Kang and Ceder as a possible concern in designing doped compositions.49
At higher temperatures, all samples display similar behavior for the Δ temperature dependence. The gradual decrease is explained by a motional narrowing effect as a result of more rapid lithium diffusion as the temperature increases.38 Values of Δ for NMC811 are slightly lower than those for NMC622. The muon active isotope of Ni (61Ni, 1.1% abundant, nuclear magnetic moment −0.75 μ μN−1) has a lower nuclear magnetic moment compared to Mn (55Mn, 100%, +3.45 μ μN−1) and Co (59Co, 100%, +4.63 μ μN−1) and resultant internal nuclear fields will thus be weaker for higher Ni-content samples.50 However, larger Δ values at lower temperatures are observed for both doped samples compared to NMC811. Both 27Al (100% and +3.64 μ μN−1) and 25Mg (10% and −0.86 μ μN−1) possess a nuclear magnetic moment which would introduce additional contributions to the local field experienced by the muon. However, as dopant levels are low, the observed effects cannot solely be explained by this. The increased value of Δ and ν below 150 K in both Mg- and Al-doped samples indicates the likelihood of additional electronic contributions from the dopant ions at low temperatures. Magnetic transition effects have been noticed at similar temperatures on commercial NMC811 and have been found to be highly sample dependent.51 During data fitting the relaxing asymmetry is found to decrease noticeably at low temperatures for the doped samples but not NMC622 and NMC811 (Tables S1–S4, ESI†), further indicating the likelihood of a magnetic transition below 150 K as a result of the inclusion of Mg and Al ions.52 Changes to the overall structure caused by dopant incorporation are likely to further explain the differences in Δ observed. Rietveld refinements (Fig. S3 and Table S5, ESI†) reveal changes in the a lattice parameter (indicative of changes to the average oxidation state in the TM layer), the c lattice parameter (indicative of changes in the layer spacings) and the Ni/Li mixing percentages, means that there is likely to be a complex interplay between these parameters that serves to alter the muon stopping site relative to the nuclear moments and induce changes in Δ. However, this effect is lost at higher temperatures, indicating that additional effects from motional narrowing are further likely to have an impact.
Activation energies for thermally activated Li+ diffusion can be calculated from an Arrhenius plot shown in Fig. 3(c). Activation energies for NMC811 and NMC622 of 48 (±2) and 54 (±6) meV respectively compare well with similar studies conducted on LixNi1/3Mn1/3Co1/3O2 materials39 and μ+SR studies of NMC622 and NMC811 samples synthesized by a hydrothermal flow method.53 For higher Ni-content materials, the calculated Li+ diffusion barriers are sensitive to the identity of the transition metal with higher Ni-content found to reduce the activation barrier.54,55 The reasons for enhanced diffusion properties as Ni content increases in NMC materials is likely to stem from structural and electronic changes in the structure as elucidated by Wang et al.56 For example, lattice expansion, particularly along the c-axis, is likely to facilitate easier Li+ hopping along the diffusion pathways, resulting in reduced barriers to Li+ diffusion. Furthermore, additional factors relating to the Li+-TM electrostatic interactions should also be considered. Increasing the Ni-content is achieved by reducing Mn4+ content which in turn decreases the strength of the interactions between the Li+ and the TMO2 layer; a factor which further serves to reduce the Li+ diffusion barriers. It is worth noting that as a volume-averaged probe, μ+SR signals are dominated by intragrain, short-range Li+ diffusion (hops), and impeding contributions from grain boundaries and other extrinsic factors do not play a substantial role as they would in techniques such as electrochemical impedance spectroscopy. As a result, activation energies reported for μ+SR measurements reflect the nature of the unit cell length-scale this technique probes i.e. energies represent the fundamental energy required to motivate singular ionic hops.34,37
To obtain diffusion coefficients, DLi, the ionic hops that enable Li+ motion (Fig. 1) must be considered and eqn (3) is applied.
 | (3) |
Diffusion coefficients at 300 K for NMC811 and NMC622 were calculated as 1.6 ± 0.1 × 10−11 cm2 s−1 and 1.0 ± 0.1 × 10−11 cm2 s−1 respectively, under the assumption of first and second nearest neighbouring jumps Table 1. These values indicate that Li+ diffusion is faster in NMC811 compared to NMC622. For comparison, DLi at 300 K for an NMC111 sample was reported as 3.5 × 10−12 cm2 s−1 by Månsson et al., highlighting further the improvement in ion transport properties with increasing Ni- that we observe. These results further corroborate the enhanced Li+ diffusivity properties of higher Ni-content NMCs previously found through electrochemical methods.54,56 For Al-NMC811 and Mg-NM811, activation energies of 70 (±9) and 64 (±4) meV were estimated respectively. Compared to NMC811 (Ea = 48 ± 2 meV) the results here suggest that the presence of dopant cations can introduce a higher energy barrier for singular ionic hops thus may play a role in hindering Li+ diffusion. Diffusion coefficients were calculated as 1.1 ± 0.1 × 10−11 cm2 s−1 at 300 K for both Al-NMC811 and Mg-NMC811. Higher activation energies coupled with lower diffusion coefficients signal hindered Li+ diffusion in NMC811 upon the introduction of even small quantities of dopants. The presence of Mg2+ within the Li+ containing layer likely block diffusional pathways, with the higher charge density of Mg2+ contributing a further impedance to ionic motion. The contraction in the c parameter observed for the Al-NMC811 could lower the Li+ diffusion through shrinkage of the layer spacing which hinders ionic transport across the layer.49,55 To examine any effect the material microstructure may have on the local scale diffusional properties, we carried out muon studies on commercial NMC811 and NMC622 materials (Fig. S4, S5 and Table S7, ESI†). Our results demonstrate a negligible effect indicating the μ+SR method is not sensitive to differing particle morphology for these samples.
| Sample | Composition | E a (meV) | D Li at 300 K (cm2 s−1) |
|---|---|---|---|
| NMC811 | LiNi0.8Mn0.1Co0.1O2 | 48 (±2) | 1.6 ± 0.1 × 10−11 |
| NMC622 | LiNi0.6Mn0.2Co0.2O2 | 54 (±6) | 1.0 ± 0.1 × 10−11 |
| Al-NMC811 | LiNi0.79Al0.01Mn0.1Co0.1O2 | 70 (±9) | 1.1 ± 0.1 × 10−11 |
| Mg-NMC811 | Li0.99Mg0.01Ni0.8Mn0.1Co0.1O2 | 64 (±4) | 1.1 ± 0.1 × 10−11 |
4. Conclusions
In conclusion, we demonstrate μ+SR as an excellent technique to study microscopic lithium diffusion in nickel-rich cathodes. We find that even small quantities of structurally and thermally stabilizing dopants can hinder ion diffusion in these materials. These insights are useful for considering the design of new nickel-rich cathodes, where the desired stability introduced by such dopants is considered in tandem with possible implications to electrochemical performance.Author contributions
BIIJ: conceptualisation, investigation, formal analysis, writing – original draft, writing – review and editing, visualisation, funding acquisition. IM: validation, writing – review and editing. PJB: methodology, formal analysis, resources, writing – review and editing, funding acquisition. SAC: conceptualisation, resources, writing – review and editing, supervision, funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the ISIS Neutron and Muon Source and the Facility Access Panel for the award of beamtime (DOI: https://doi.org/10.5286/ISIS.E.RB1920560-1). Electron microscopy was performed in the Sorby Centre for Electron Microscopy at the University of Sheffield. This project was supported through the Faraday Institution projects FutureCat (FIRG017) and Degradation (FIRG001).References
- X. Wang, Y.-L. Ding, Y.-P. Deng and Z. Chen, Adv. Energy Mater., 2020, 10, 1903864 CrossRef CAS.
- S. G. Booth, A. J. Nedoma, N. N. Anthonisamy, P. J. Baker, R. Boston, H. Bronstein, S. J. Clarke, E. J. Cussen, V. Daramalla, M. D. Volder, S. E. Dutton, V. Falkowski, N. A. Fleck, H. S. Geddes, N. Gollapally, A. L. Goodwin, J. M. Griffin, A. R. Haworth, M. A. Hayward, S. Hull, B. J. Inkson, B. J. Johnston, Z. Lu, J. L. MacManus-Driscoll, X. M. D. I. Labalde, I. McClelland, K. McCombie, B. Murdock, D. Nayak, S. Park, G. E. Pérez, C. J. Pickard, L. F. J. Piper, H. Y. Playford, S. Price, D. O. Scanlon, J. C. Stallard, N. Tapia-Ruiz, A. R. West, L. Wheatcroft, M. Wilson, L. Zhang, X. Zhi, B. Zhu and S. A. Cussen, APL Mater., 2021, 9, 109201 CrossRef CAS.
- P. S. Grant, D. Greenwood, K. Pardikar, R. Smith, T. Entwistle, L. A. Middlemiss, G. Murray, S. A. Cussen, M. J. Lain, M. J. Capener, M. Copley, C. D. Reynolds, S. D. Hare, M. J. H. Simmons, E. Kendrick, S. P. Zankowski, S. Wheeler, P. Zhu, P. R. Slater, Y. S. Zhang, A. R. T. Morrison, W. Dawson, J. Li, P. R. Shearing, D. J. L. Brett, G. Matthews, R. Ge, R. Drummond, E. C. Tredenick, C. Cheng, S. R. Duncan, A. M. Boyce, M. Faraji-Niri, J. Marco, L. A. Roman-Ramirez, C. Harper, P. Blackmore, T. Shelley, A. Mohsseni and D. J. Cumming, J. Phys.: Energy, 2022, 4, 042006 Search PubMed.
- W. Li, E. M. Erickson and A. Manthiram, Nat. Energy, 2020, 5, 26–34 CrossRef CAS.
- A. Manthiram, J. C. Knight, S.-T. Myung, S.-M. Oh and Y.-K. Sun, Adv. Energy Mater., 2016, 6, 1501010 CrossRef.
- J. Xu, F. Lin, M. M. Doeff and W. Tong, J. Mater. Chem. A, 2017, 5, 874–901 RSC.
- Y. Koyama, N. Yabuuchi, I. Tanaka, H. Adachi and T. Ohzuku, J. Electrochem. Soc., 2004, 151, A1545 CrossRef CAS.
- S.-M. Bak, E. Hu, Y. Zhou, X. Yu, S. D. Senanayake, S.-J. Cho, K.-B. Kim, K. Y. Chung, X.-Q. Yang and K.-W. Nam, ACS Appl. Mater. Interfaces, 2014, 6, 22594–22601 CrossRef CAS PubMed.
- Y. Ding, Z. P. Cano, A. Yu, J. Lu and Z. Chen, Electrochem. Energy Rev., 2019, 2, 1–28 CrossRef CAS.
- R. Jung, M. Metzger, F. Maglia, C. Stinner and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, A1361 CrossRef CAS.
- M. D. Tikekar, S. Choudhury, Z. Tu and L. A. Archer, Nat. Energy, 2016, 1, 16114 CrossRef CAS.
- D. Y. Wang, J. Xia, L. Ma, K. J. Nelson, J. E. Harlow, D. Xiong, L. E. Downie, R. Petibon, J. C. Burns, A. Xiao, W. M. Lamanna and J. R. Dahn, J. Electrochem. Soc., 2014, 161, A1818 CrossRef.
- T. Entwistle, E. Sanchez-Perez, G. J. Murray, N. Anthonisamy and S. A. Cussen, Energy Rep., 2022, 8, 67–73 CrossRef.
- Y. Bi, W. Yang, R. Du, J. Zhou, M. Liu, Y. Liu and D. Wang, J. Power Sources, 2015, 283, 211–218 CrossRef CAS.
- Y. Koyama, H. Arai, I. Tanaka, Y. Uchimoto and Z. Ogumi, Chem. Mater., 2012, 24, 3886–3894 CrossRef CAS.
- H. Yu, Y. Qian, M. Otani, D. Tang, S. Guo, Y. Zhu and H. Zhou, Energy Environ. Sci., 2014, 7, 1068–1078 RSC.
- K. Min, S.-W. Seo, Y. Y. Song, H. S. Lee and E. Cho, Phys. Chem. Chem. Phys., 2017, 19, 1762–1769 RSC.
- T. Weigel, F. Schipper, E. M. Erickson, F. A. Susai, B. Markovsky and D. Aurbach, ACS Energy Lett., 2019, 4, 508–516 CrossRef CAS.
- Z. Huang, Z. Wang, X. Zheng, H. Guo, X. Li, Q. Jing and Z. Yang, Electrochim. Acta, 2015, 182, 795–802 CrossRef CAS.
- H. Li, P. Zhou, F. Liu, H. Li, F. Cheng and J. Chen, Chem. Sci., 2019, 10, 1374–1379 RSC.
- S. Madhavi, G. V. Subba Rao, B. V. R. Chowdari and S. F. Y. Li, J. Power Sources, 2001, 93, 156–162 CrossRef CAS.
- S. W. Woo, S. T. Myung, H. Bang, D. W. Kim and Y. K. Sun, Electrochim. Acta, 2009, 54, 3851–3856 CrossRef CAS.
- Q. Xie, W. Li and A. Manthiram, Chem. Mater., 2019, 31, 938–946 CrossRef CAS.
- C. Zhang, J. Wan, Y. Li, S. Zheng, K. Zhou, D. Wang, D. Wang, C. Hong, Z. Gong and Y. Yang, J. Mater. Chem. A, 2020, 8, 6893–6901 RSC.
- T. E. Ashton, J. V. Laveda, D. A. MacLaren, P. J. Baker, A. Porch, M. O. Jones and S. A. Corr, J. Mater. Chem. A, 2014, 2, 6238–6245 RSC.
- J. V. Laveda, V. Chandhok, C. A. Murray, G. W. Paterson and S. A. Corr, Chem. Commun., 2016, 52, 9028–9031 RSC.
- M. Amores, S. A. Corr and E. J. Cussen, J. Electrochem. Soc., 2017, 164, A6395 CrossRef CAS.
- M. Amores, T. E. Ashton, P. J. Baker, E. J. Cussen and S. A. Corr, J. Mater. Chem. A, 2016, 4, 1729–1736 RSC.
- A. Van der Ven, J. Bhattacharya and A. A. Belak, Acc. Chem. Res., 2013, 46, 1216–1225 CrossRef CAS PubMed.
- S. Ohno, T. Bernges, J. Buchheim, M. Duchardt, A.-K. Hatz, M. A. Kraft, H. Kwak, A. L. Santhosha, Z. Liu, N. Minafra, F. Tsuji, A. Sakuda, R. Schlem, S. Xiong, Z. Zhang, P. Adelhelm, H. Chen, A. Hayashi, Y. S. Jung, B. V. Lotsch, B. Roling, N. M. Vargas-Barbosa and W. G. Zeier, ACS Energy Lett., 2020, 5, 910–915 CrossRef CAS.
- I. Mcclelland, S. G. Booth, N. N. Anthonisamy, L. A. Middlemiss, G. E. Pérez, E. J. Cussen, P. J. Baker and S. A. Cussen, Chem. Mater., 2023, 35, 4149 CrossRef CAS PubMed.
- J. Sugiyama, K. Mukai, Y. Ikedo, H. Nozaki, M. Månsson and I. Watanabe, Phys. Rev. Lett., 2009, 103, 147601 CrossRef PubMed.
- J. V. Laveda, B. Johnston, G. W. Paterson, P. J. Baker, M. G. Tucker, H. Y. Playford, K. M. Ø. Jensen, S. J. L. Billinge and S. A. Corr, J. Mater. Chem. A, 2018, 6, 127–137 RSC.
- I. McClelland, B. Johnston, P. J. Baker, M. Amores, E. J. Cussen and S. A. Corr, Annu. Rev. Mater. Res., 2020, 50, 371–393 CrossRef CAS.
- I. McClelland, S. G. Booth, H. El-Shinawi, B. I. J. Johnston, J. Clough, W. Guo, E. J. Cussen, P. J. Baker and S. A. Corr, ACS Appl. Energy Mater., 2021, 4, 1527–1536 CrossRef CAS PubMed.
- J. Sugiyama, H. Nozaki, M. Harada, K. Kamazawa, Y. Ikedo, Y. Miyake, O. Ofer, M. Månsson, E. J. Ansaldo, K. H. Chow, G. Kobayashi and R. Kanno, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 85, 054111 CrossRef.
- P. J. Baker, I. Franke, F. L. Pratt, T. Lancaster, D. Prabhakaran, W. Hayes and S. J. Blundell, Phys. Rev. B: Condens. Matter Mater. Phys., 2011, 84, 174403 CrossRef.
- J. Sugiyama, Y. Ikedo, K. Mukai, H. Nozaki, M. Månsson, O. Ofer, M. Harada, K. Kamazawa, Y. Miyake, J. H. Brewer, E. J. Ansaldo, K. H. Chow, I. Watanabe and T. Ohzuku, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 82, 224412 CrossRef.
- J. Sugiyama, K. Mukai, M. Harada, H. Nozaki, K. Miwa, T. Shiotsuki, Y. Shindo, S. R. Giblin and J. S. Lord, Phys. Chem. Chem. Phys., 2013, 15, 10402–10412 RSC.
- B. I. J. Johnston, P. J. Baker and S. A. Cussen, J. Phys. Mater., 2021, 4, 044015 CrossRef CAS.
- R. S. Hayano, Y. J. Uemura, J. Imazato, N. Nishida, T. Yamazaki and R. Kubo, Phys. Rev. B: Condens. Matter Mater. Phys., 1979, 20, 850–859 CrossRef CAS.
- B. H. Toby and R. B. Von Dreele, J. Appl. Crystallogr., 2013, 46, 544–549 CrossRef CAS.
- S. J. Blundell, Contemp. Phys., 1999, 40, 175–192 CrossRef CAS.
- L. Nuccio, L. Schulz and A. J. Drew, J. Phys. D: Appl. Phys., 2014, 47, 473001 CrossRef.
- F. L. Pratt, Phys. B, 2000, 289–290, 710–714 CrossRef.
- I. Umegaki, K. Ohishi, T. Nakano, S. Nishimura, S. Takeshita, A. Koda, K. Ninomiya, M. K. Kubo and J. Sugiyama, J. Phys. Chem. C, 2022, 126, 10506–10514 CrossRef CAS.
- J. Sugiyama, K. Ohishi, O. K. Forslund, M. Månsson, S. P. Cottrell, A. D. Hillier and K. Ishida, Z. Phys. Chem., 2022, 236, 799–816 CrossRef CAS.
- M. Månsson, H. Nozaki, J. M. Wikberg, K. Prša, Y. Sassa, M. Dahbi, K. Kamazawa, K. Sedlak, I. Watanabe and J. Sugiyama, J. Phys.: Conf. Ser., 2014, 551, 012037 CrossRef.
- K. Kang and G. Ceder, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 094105 CrossRef.
- N. J. Stone, At. Data Nucl. Data Tables, 2005, 90, 75–176 CrossRef CAS.
- P. Mukherjee, J. A. M. Paddison, C. Xu, Z. Ruff, A. R. Wildes, D. A. Keen, R. I. Smith, C. P. Grey and S. E. Dutton, Inorg. Chem., 2021, 60, 263–271 CrossRef CAS PubMed.
- C. T. Kaiser, V. W. J. Verhoeven, P. C. M. Gubbens, F. M. Mulder, I. De Schepper, A. Yaouanc, P. Dalmas De Réotier, S. P. Cottrell, E. M. Kelder and J. Schoonman, Phys. Rev. B: Condens. Matter Mater. Phys., 2000, 62, R9236–R9239 CrossRef CAS.
- T. E. Ashton, P. J. Baker, C. Sotelo-Vazquez, C. J. M. Footer, K. M. Kojima, T. Matsukawa, T. Kamiyama and J. A. Darr, J. Mater. Chem. A, 2021, 9, 10477–10486 RSC.
- H.-J. Noh, S. Youn, C. S. Yoon and Y.-K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef CAS.
- Y. Wei, J. Zheng, S. Cui, X. Song, Y. Su, W. Deng, Z. Wu, X. Wang, W. Wang, M. Rao, Y. Lin, C. Wang, K. Amine and F. Pan, J. Am. Chem. Soc., 2015, 137, 8364–8367 CrossRef CAS PubMed.
- S. Wang, M. Yan, Y. Li, C. Vinado and J. Yang, J. Power Sources, 2018, 393, 75–82 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp02662k |
| This journal is © the Owner Societies 2023 |