 Open Access Article
Open Access ArticleLithium isotope tracing in silicon-based electrodes using solid-state MAS NMR: a powerful comprehensive tool for the characterization of lithium batteries†
Manon
Berthault
a,
Anton
Buzlukov
 *bc,
Lionel
Dubois
d,
Pierre-Alain
Bayle
b,
Willy
Porcher
a,
Thibaut
Gutel
*bc,
Lionel
Dubois
d,
Pierre-Alain
Bayle
b,
Willy
Porcher
a,
Thibaut
Gutel
 a,
Eric
De Vito
a,
Eric
De Vito
 e and
Michel
Bardet
e and
Michel
Bardet
 *b
*b
aUniv. Grenoble Alpes, CEA, Liten, DEHT, 38000 Grenoble, France
bUniv. Grenoble Alpes, CEA, IRIG, MEM, 38000 Grenoble, France. E-mail: michel.bardet@cea.fr
cIMP UB RAS—M.N. Miheev Institute of Metal Physics of Ural Branch of Russian Academy of Sciences, 620137 Ekaterinburg, Russia. E-mail: buzlukov@mail.ru
dUniv. Grenoble Alpes, CEA, IRIG, SYMMES, 38000 Grenoble, France
eUniv. Grenoble Alpes, CEA, Liten, DTNM, 38000 Grenoble, France
First published on 7th August 2023
Abstract
The introduction of lithiated components with different 7Li/6Li isotopic ratios, also called isotopic tracing, can give access to better understanding of lithium transport and lithiation processes in lithium-ion batteries. In this work, we propose a simple methodology based on high-resolution solid-state NMR for the determination of the 7Li/6Li ratio in silicon electrodes following different strategies of isotopic tracing. The 6Li and 7Li MAS NMR experiments allow obtaining resolved spectra whose spectral components can be assigned to different moieties of the materials. In order to measure the ratio of the 6Li/7Li NMR integrals, a silicon electrode with a natural 7Li/6Li isotope abundance was used as a reference. This calibration can then be used to determine the 7Li/6Li ratio of any similar samples. This method was applied to study the phenomena occurring at the interface between a silicon electrode and a labeled electrolyte, which is an essential step for isotopic tracing experiments in systems after the formation of the solid electrolyte interphase (SEI). Beyond the isotopic exchanges between the SEI and the electrolyte already observed in the literature, our results show that isotopic exchanges also involve Li–Si alloys in the electrode bulk. Within a 52-hour contact, the electrolyte labeling disappeared: isotopic concentrations of the electrolyte and electrode become practically homogenized. However, at the electrode level, different silicides are characterized by rather different isotopic enrichment. In the present work, ToF SIMS and liquid state NMR were also used to cross-check and discuss the solid-state NMR method we have proposed.
1. Introduction
Experiments based on isotope tracing have already been successfully applied in a wide range of domains: biology, medicine, environmental sciences,1 chemistry and physics. However, depending on the questions being addressed, they have not been developed and applied to the same extent. In the field of materials for energy storage, such an approach has been proposed to study Li-ion batteries, though it still remains at a very early stage. Since 2011, only a dozen publications have used it for studying the SEI structure,2 SEI formation3 and Li diffusion.4–10 In this field, carbon and lithium nuclei are the favorite probes. As a matter of fact, lithium plays a crucial role in the operation of Li-ion batteries since it acts as a shuttle from one electrode to the other but it is also involved in many side reactions in various media at different states: the liquid phase of the electrolyte, the solid phase of electrode materials and the solid interphase between the negative electrode and the electrolyte (SEI).During the first lithiation of the anode, part of the electrolyte is decomposed by reduction. Insoluble degradation products remain at the negative electrode surface and give rise to the SEI. The soluble counterpart remains in the electrolyte itself. A portion of lithium appears to be irreversibly trapped in the SEI associated with capacity fading. Carbon as a major element of the electrolyte is detected not only in the liquid phase but also in the SEI solid phase. It is also present in binder or conductive additives. Although it can be considered as a key element of Li-ion batteries, it remains a difficult probe compared with Li, especially for NMR due to its low sensitivity and the huge number of expected chemical shifts. However, carbon has been also used to study the dynamics of organic compounds in the electrolyte or in the SEI. 13C NMR has shown that organic products observed in the SEI of silicon were mostly derived from ethylene carbonate (EC) rather than dimethylcarbonate (DMC) and that the degradation of EC with aging leads to the formation of linear alkylcarbonates-based oligomers with methoxide-end groups, accumulating in the electrolyte.11 Using lithium tracing, the lithium diffusion coefficient in materials such as LiMn2O4, Li3PO4 or LixCoO25,6,12,13 was determined and more general information regarding the lithium dynamics between the different phases of electrode materials was obtained.14–18
Irrespective of the technique used, a large part of the studies focuses on the interactions between the electrode and the electrolyte by monitoring their respective Li isotope contents in these two counterparts. In most of these experiments, an electrode is immersed in an electrolyte. Investigations on a model SEI using Time-of-Flight Secondary Ion Mass Spectrometry (ToF-SIMS) analysis have already shown that Li isotope exchanges take place quickly (∼30 s) between the electrolyte and the SEI.2 Similar isotope exchanges have been also observed on delithiated graphite electrodes via ToF-SIMS with the same types of experiments.10 The results show that the diffusion of Li ions from the electrolyte to the SEI is very fast: the isotopic exchange is almost achieved in less than 20 min. From this, the thickness of the mineral part of the SEI has been estimated. However, while the Li isotope exchanges have been demonstrated between the electrolyte and SEI, no study has been realized considering Li in the active material. For instance, in the experiments carried out by Ilott et al.15 and Gunnarsdóttir et al.,17 they soaked a 6Li metal piece in an electrolyte containing 7Li and then monitored 7Li NMR signals, corresponding to both the metal and the electrolyte during all the prolonged contact. In parallel, a numerical model has been developed to describe the process. It allowed them to quantify the growth of the SEI and the evolution of ion permeability into the Li metal. For isotopic tracing, the solid-state NMR is a promising tool since in addition to the quantitative analysis of each isotope, 7Li and 6Li, it provides information on their chemical environments. For instance, in a silicon-based electrode, the different silicides can be easily distinguished with solid-state 6,7Li NMR.19,20 This technique also enables to detect Li dendrites on graphite21 and lithium metal electrodes.22 Moreover, recent studies on either in situ NMR or in situ MRI raise a lot of expectations, especially to follow Li isotope exchange under operando conditions.22 It is worthwhile to recall that NMR analyzes the whole sample, and some experimental conditions have to be fulfilled to provide quantitative data.
Concerning lithium at natural abundance, it has two isotopes 7Li (I = 3/2) and 6Li (I = 1) at 92.6% and 7.4%, respectively. On non-labelled compounds, the 7Li and 6Li fractions are expected to remain constant, while by introducing a 6Li-enriched component during battery assembly, the evolution of the 7Li/6Li ratio after battery cycling enables to identify various processes and trace lithium pathways. In principle, this can be easily done by acquiring quantitative data for each isotope. However, such an approach would require the use of an internal lithium reference inside the studied sample. This strategy is generally followed in liquid-state NMR. However, for solid materials, it appeared worthwhile to develop a method, without an internal reference, to quantify 7Li and 6Li using high resolution solid-state NMR techniques. It is the objective of this work.
The method we have developed was tested on several samples with unknown 7Li/6Li isotope ratios. These samples consist of lithiated silicon-based electrodes in which isotopic enrichment was performed using an electrochemical method. The main 6Li enrichment setups that were used in the present work are illustrated in Fig. 1.
To validate the methodology based on solid-state NMR, we also carried out the quantification using liquid state 7Li and 6Li NMR. On one hand, it should be easier to implement since internuclear and electron-to-nuclear interactions are averaged due to Brownian motion. On the other hand, the liquid-state 6,7Li NMR requires a complete extraction and dissolution of lithium from electrode samples with a complete loss of information associated with different phases of the corresponding materials. Mass spectrometry analyses were also performed on the residue obtained from these solutions after drying to determine the 7Li/6Li ratio and double cross-check the methodology we have proposed.
2. Results and discussion
2.1. Theoretical background and methodology development
From the experimental point of view, both 7Li and 6Li solid-state NMR can be easily carried out using classical probes dedicated to CPMAS experiments. The 6Li nucleus, having a smaller gyromagnetic ratio and lower quadrupolar moment than 7Li, usually results in better-resolved spectra. However, the low natural abundance of the 6Li nucleus requires longer acquisition times for non-enriched samples. The main problem is to compare the integrals of 7Li and 6Li NMR signals. As a matter of fact, even if the measurements are performed on the same sample and the same NMR probe, at a given magnetic field (11.74 T in our case), the 7Li and 6Li NMR frequencies (Larmor frequencies) are 194.37 MHz and 73.6 MHz, respectively. This means that from an experimental point of view, the NMR probe has to be tuned and matched for each isotope in order to optimize the resonant circuit. It is known that such a circuit cannot deliver a linear response for a whole range of frequencies. Moreover the NMR signal is further filtered and amplified by electronic circuits. They are also not expected to have linear amplification in the whole NMR frequency range. These observations have been rationalized by different formulae describing the dependence on the signal-to-noise ratio, S/N, after a 90° pulse. Among the following options, one that has been very often used23 and extensively discussed is outlined in the excellent review of Doty et al.24 | (1) |
![[h with combining macron]](https://www.rsc.org/images/entities/i_char_0068_0304.gif) is Plank's constant divided by 2π, μ0 is the permeability of free space, kb is Boltzmann's constant, nS is the number of spins at resonance per unit volume, γ is the gyromagnetic ratio, Ix is the spin quantum number,
is Plank's constant divided by 2π, μ0 is the permeability of free space, kb is Boltzmann's constant, nS is the number of spins at resonance per unit volume, γ is the gyromagnetic ratio, Ix is the spin quantum number, 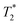 is the effective spin–spin relaxation time, TS is the sample temperature, Tn is the probe noise temperature, TP is the effective preamp noise temperature, ηE is the RF efficiency, ηf is the filling factor of the resonant coil, QL is the quality factor of the matched circuit, VS is the sample volume, and ω is the Larmor frequency, where ω = γB0.24
is the effective spin–spin relaxation time, TS is the sample temperature, Tn is the probe noise temperature, TP is the effective preamp noise temperature, ηE is the RF efficiency, ηf is the filling factor of the resonant coil, QL is the quality factor of the matched circuit, VS is the sample volume, and ω is the Larmor frequency, where ω = γB0.24
The above equation eqn (1) shows that it is impossible to perform direct quantitative comparison of 7Li and 6Li without signal-to-noise (S/N) calibration for each isotope. However, it can be done with sufficient precision by using a reference compound with known isotope abundance and with similar physical properties (magnetic susceptibility, permittivity, etc.). After that, for any samples to be analyzed, provided that the measurements on each Li isotope are carried out under identical conditions, the calculated 7Lis/n and 6Lis/n ratios will depend only on the fraction of each isotope (ns value in eqn (1)). Indeed, as 6Li and 7Li NMR experiments are performed on the same sample using the same spectrometer, then Ts, Vs, B0 and ηf are equivalent for both isotopes. For each isotope, γ and Ix, as well as h, μ0 and kB are constants. Moreover, due to the proximity of physical properties of the reference and studied samples, the QL and 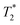 factors also remain similar. Thus, the integral of measured NMR signals (int) of 6Li and 7Li will be, respectively, proportional to the amount (in %) of 6Li and 7Li nuclei:
factors also remain similar. Thus, the integral of measured NMR signals (int) of 6Li and 7Li will be, respectively, proportional to the amount (in %) of 6Li and 7Li nuclei:
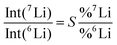 | (2) |
This relation allows calculating the isotopic ratio of any sample on the basis of the measured integrals. Note that the sum of %7Li and %6Li remains constant and is equal to 100%.
It is essential to remind that such a quantification is possible only if the acquisition conditions are optimized and kept constant: excitation pulse, acquisition delay (fulfilling a complete recovery of nuclear magnetization), tuning and matching of resonance circuit. Additionally, the number of scans (N) and the receiver gain (G) used to acquire NMR spectra with good quality can be easily taken into account: as a matter of fact, signal-to-noise ratios can be normalized by dividing the measured S/N ratio by G and N.25,26 Note that the mass of the material does not need to be taken into account since the 7Li and 6Li measurements are performed in the same MAS rotor.
2.2. Determination of S factor and validation of the NMR method.
For the determination of the S factor, a powder of a lithiated silicon-based electrode was used as a reference. This silicon-based electrode was electrochemically lithiated using a Li metal counter electrode where lithium isotope abundance is around natural (92.7% in 7Li–7.3% in 6Li) in order to reach a lithiation state corresponding to 1000 mA h g−1 (related to active material).Fig. 2 shows the 7Li and 6Li NMR spectra of the reference sample. The differences between 7Li and 6Li spectra are as expected. The signal-to-noise ratio for the 6Li NMR spectrum is lower compared to the 7Li NMR spectrum due to a lower gyromagnetic ratio and natural abundance of the 6Li isotope. Moreover, the 7Li NMR spectrum presents a spinning side band pattern (additional signals shifted by νrot relative to the isotropic lines). It is most likely due to the quadrupolar interactions which are not averaged at the operating 10 kHz spinning rate. In contrast, for 6Li, the quadrupolar moment is lower and almost no spinning side band is observed. These features can be overcome by increasing the spinning rate for 7Li and/or by increasing the number of accumulated transients for 6Li (or otherwise by increasing the magnetic field intensity).
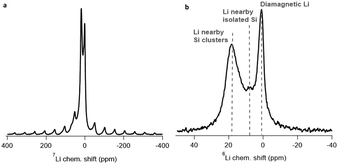 | ||
| Fig. 2 7Li (a) and 6Li (b) NMR spectra of the reference sample (lithiated silicon-based electrode with natural lithium isotopes abundance). The proposed assignments are based on ref. 16, 17 and 24. | ||
Both 6Li and 7Li NMR spectra show the presence of several spectral components. The NMR signal with the chemical shift value of ∼0 ppm is assigned usually to lithium atoms in the SEI. Two NMR lines near 19 and 6 ppm can be attributed to lithium in the Si bulk, namely to Li atoms nearby small Si clusters (stars, rings) and isolated Si, respectively. Indeed, similar signals were observed earlier for the stable alloys Li12Si7 and Li15Si4 with these characteristic local surroundings.19,20 The first lithiation of crystalline Si is accompanied by the breaking of Si–Si bonds. This process requires high energy and leads to the so-called “core–shell” mechanism of lithiation with a shell made of an amorphous LixSi phase (x = 2.8 ± 0.3), surrounding the core of pristine crystalline Si.27,28 Taking it into account, it seems reasonable to associate the observed NMR signals near 19 and 6 ppm with Li atoms which are located in the bulk of the shell and with those found in the highly lithiated “core–shell” front, respectively.
It is of primary importance to check that the lithium isotope abundances in the sample and those in the counter electrode and electrolyte are alike. It has to be noted that there are some hypothetical phenomena, known as isotope fractionation, which could slightly modify this abundance leading to a small isotope enrichment of a component in one specific isotope. The preferential diffusion of one isotope over the other is an example of an isotope effect leading to isotope fractionation. Isotope fractionation generally leads to very small deviations from the natural ratio (parts per thousand)1,29 and can be neglected. In reversible systems like Li-ion cells, there is no opportunity to observe kinetic fractionation; though equilibrium lithium fractionation could exist. On the other hand, some reactions during SEI formation can be irreversible and could cause a slight isotope fractionation at the electrode surface. In order to clarify this point, ToF-SIMS analyses were performed at the electrode surface where SEI is mainly present. Fig. 3 shows the ToF-SIMS profile obtained for the reference electrode. The evolution of the 30Si− fragment reflects that sputtering has been successful in probing the entire depth of the SEI and reaching the beginning of the bulk material as the 30Si− moiety reaches a plateau. 7Li abundance measured is around 93% over the entire depth-profile. No isotope fractionation is observed. Thus, it can be concluded that 7Li isotope abundance in this electrode is 93% and this sample can be used as a reference. Using the normalized integrals of 7Li and 6Li NMR signals, which are 3435 and 26, respectively, and using eqn (2), we calculate the correction factor S to be equal to about 10.5. Note that all the NMR lines shown in Fig. 2 were taken into account, including spinning side bands. In order to go a step further, the deconvolution of the measured NMR spectra has been performed.
As shown in Fig. 4, they can be considered as a superposition of three components: two “bulk” components, corresponding to lithium nearby isolated Si atoms (Li15Si4 in phase denomination) and lithium nearby Si clusters (Li12Si7), and Li atoms in the SEI with the characteristics presented in Table 1. It is clear from Table 1 that the proportions of each component (silicides and SEI) found in 7Li and 6Li NMR are alike within ±3%, which reinforces the idea that there is no isotope fractionation detectable by NMR. In order to verify the consistency of the measurement performed, the lithiation of a silicon-based electrode using a 6Li (95%) metal counter electrode was carried out (Setup A). This simple experiment is the exact opposite of the one performed to get the reference sample. As previously mentioned, since isotopic fractionation is considered negligible, a value of 5% for the 7Li content in the silicon electrode is expected and observed. Using the S factor, calculations give a value of 4% for 7Li abundance. It clearly shows that the method we proposed can be applied for samples for which the isotopic ratio has to be determined. Let's note that here and further, different NMR signals are labeled and discussed as “phases”: Li12Si7, Li7Si3, Li13Si4 and Li15Si4. However, it is done just for the sake of simplicity and we rather refer to their typical local lithium surroundings. It has to be also noted that at this stage of the discussion, we restricted ourselves to only two Li12Si7 and Li15Si4 “phases”, which correspond to the lowest and the highest stages of silicon lithiation, respectively. However, a better fit of the spectra shown in Fig. 4 can be achieved if we include additional lines near 16 and 12 ppm. These signals correspond to lithium atoms with local coordinations consisting of Si dumbbells, and Si pairs and isolated atoms, respectively. They correspond to the “intermediary” Li7Si3 and Li13Si4 phases if one use the above denomination.19,20 For further discussion (see below), we will use a larger set of lines during NMR spectra deconvolution.
 | ||
| Fig. 4 7Li (a) and 6Li (b) NMR spectra of the reference sample (lithiated silicon-based electrode with natural lithium isotopes abundances). The proposed assignments are based on ref. 19, 20, 31. | ||
| Line | Simulation parameters | δ (ppm) | Integral (%) | assignment | |
|---|---|---|---|---|---|
| 6Li | #1 | Gaus/Lor | 18.83 | 44 | Li12Si7 |
| #2 | Gaus/Lor | 6.31 | 34 | Li15Si4 | |
| #3 | Gaus/Lor | 0.99 | 22 | SEI | |
| 7Li | #1 | Gaus/Lor + ssb | 18.83 | 47 | Li12Si7 |
| #2 | Gaus/Lor + ssb | 6.31 | 29 | Li15Si4 | |
| #3 | Gaus/Lor + ssb | 0.99 | 24 | SEI | |
2.3. Determination of the isotopic ratio on representative silicon-based electrode samples
In order to illustrate the reliability of the proposed methodology, three representative samples (B, C, D) obtained from experimental setups B, C and D, respectively, have been analyzed ex situ. These setups, as detailed in Fig. 1 and in the Experimental section, lead to different 6Li-enrichment of the electrode components. Moreover, for these three samples, Li was also extracted in a liquid phase from the solid material according to the protocol described in the Experimental section. Liquid-state NMR analyses were performed to determine the isotopic abundances. After liquid-state NMR analyses, the solutions were also deposited on a silicon substrate to determine the isotopic abundances via ToF-SIMS. These two latter experiments were carried out to crosscheck the results obtained with solid-state MAS NMR.Table 2 gathers the values of 7Li enrichment measured by solid-state MAS NMR, liquid NMR and ToF-SIMS. The results obtained by using the three methods are fairly similar, with a variation of 1%.
| #B | #C | #D | |
|---|---|---|---|
| 7Li measured by ss-NMR | 88% | 11% | 74% |
| 7Li measured by liquid-NMR | 88% | 12% | 74% |
| 7Li measured by ToF-SIMS | 88% | 13% | 73% |
2.4. Inputs of Li NMR tracing to study the Li dynamic in battery materials
Many tracing experiments are conceivable: the labelling of different cell components (cathode, anode and electrolyte) and of the components that are expected to be formed during cycling, such as the SEI. Experiments described in experimental setups B, C and D are some examples. In this section, we would like to focus on the study of Li exchange between electrolyte, SEI and Si core particles using isotope tracing and NMR characterization. Li exchange occurring between the SEI and the electrolyte has been first shown in a SEI-model generated on a copper foil through ToF-SIMS experiments conducted by Peng Lu et al.2 Peng Lu and coworkers have interpreted their results as a permeation of the electrolyte in the first nanometers of the SEI combined with isotope exchanges in depth. From these results, the authors could infer the Li diffusion mechanisms in the SEI in their model setup. Beyond this aspect, this finding is important since it suggests that Li isotope exchange can take place between the liquid and solid media even when the system is at equilibrium, i.e. without any electrical potential. Our recent results also demonstrated that Li isotope exchanges can occur between the SEI of a delithiated graphite electrode and an electrolyte without any electric field.10 Such exchanges are fast and have a large amplitude as isotope distribution becomes homogeneous in less than 20 minutes of contact. In both studies, the methodology is the same: a nat-SEI is dipping in an enr-electrolyte. Note that ToF-SIMS allows the evolution of the 7Li/6Li ratio on the surface of the electrode.Our NMR technique is an efficient tool for studying homogeneous samples arranged in successive layers, such as a SEI film on substrate or delithiated graphite electrodes. However, the studied Si electrodes are not layered but rather porous materials, and the SEI can be found not only on the electrodes surfaces but also within their thickness. In this regard, NMR can be considered as a more powerful technique since it is able to investigate the whole sample volume.
To answer these addressed questions, a silicon-based electrode was lithiated against a Li metal counter electrode, where lithium isotope abundance was around natural (92.6% in 7Li–7.4% in 6Li) to a state of charge corresponding to 1000 mA h g−1. The silicon electrode was then recovered and dipped into a 6Li-enriched electrolyte (95% in 6Li) for 52 hours. These procedures correspond to experimental setup D (Fig. 1D). Fig. 5 shows the 7Li (a) and 6Li (b) NMR spectra of electrode powder at the end of the experiment. The results of spectra deconvolution are present in Table S1 in the ESI.† The corresponding isotopic ratios are reported in Table 3. As can be seen from NMR data, for all LixSi phases and SEI, the 7Li abundance deviates from the natural one (Table 3). This confirms that silicide particles are also affected by the isotope exchange phenomenon, and its extent is high enough to be observed by NMR. The mean 7Li abundance determined using our methodology is 74%. This value is close to that obtained from electrochemical results (see the ESI,† calculation used for mass balance), yielding a 7Li concentration of 71% for the complete system, electrolyte + electrode, with the homogenization of a full isotope.
 | ||
| Fig. 5 7Li (a) and 6Li (b) NMR spectra of the reference electrode dipped into a 6Li-electrolyte. The proposed NMR signal assignments are based on ref. 16, 17, 28. | ||
| Assignment | Isotopic abundance in 7Li (%) |
|---|---|
| SEI | 78 |
| Li12Si7 | 49 |
| Li13Si4 | 83 |
| Li15Si4 | 78 |
However, the distribution of lithium isotopes in different phases is highly non-uniform: meanwhile for the SEI, Li13Si4 and Li15Si4 exhibit a 7Li abundance of around 80 ± 3%, while for Li12Si7, it does not exceed 50% (i.e. it is the most enriched in 6Li). The observation of 6Li fractionation in different LixSi silicides can be explained if we assume again that the lithium concentration increases with the shell depth: on the Si grain boundary, the Li environment is similar to that in the poorly lithiated Li12Si7, while for the deepest “core–shell” front, it is rather reminiscent of that for Li15Si4 (while the “intermediary” phase Li13Si4 corresponds obviously to Li atoms in the shell depth). In this case, the difference in 6Li enrichment between different LixSi silicides can be determined by different lithium mobility in these phases. For instance, Dupke et al.8 have shown that lithium ions interacting with silicon clusters or dimers have generally higher mobilities than those surrounded by monomeric silicon atoms. Consequently, Li mobility becomes more and more limited according to the order: Li12Si7 → Li7Si3 → Li13Si4 → Li15Si4.19
At first sight, the most intriguing finding is the high fraction of 7Li in the SEI. Considering that the SEI covers the silicon surface, the 6Li atoms must pass through this domain to reach the Si depth. From this point of view, one would expect a higher fraction of 6Li in the SEI. However, two phenomena have to be taken into account. The first one concerns lithium mobility. Lithium diffusion coefficients are, respectively, estimated to be around 1.6 × 10−16 cm2 s−1 for Li2O; 3.5 × 10−16 cm2 s−1 for LiF; 1.1 × 10−11 cm2 s−1 for ROLi and 7 × 10−12 cm2 s−1 for ROCO2Li31,32 which are typical compounds found in the SEI,33 whereas the lithium diffusion coefficient in silicon is estimated to be 10−11 cm2 s−1.28,34,35 The second aspect to consider is that not all the silicon surface can be covered with the SEI. Indeed, the states of Si powder in electrochemical cycling (pressed and stuck to copper foil) and in our experiment (free powder dipped in a liquid electrolyte) are significantly different. In our case, new regions of the Si surface, previously inaccessible for direct contact with the electrolyte, may open up. Thus, the diffusion of 6Li in the Si depth can occur directly across the silicides grains bypassing the SEI.
In order to better understand the processes involved in lithium isotope exchange, an “in situ” MAS NMR experiment was performed. For this, a few drops of a 6Li-electrolyte were added in a rotor filled with a 7Li-lithiathed electrode. Then, the NMR measurements were performed after only one hour of contact. NMR spectra, which correspond to 48 accumulated transients with a 4 s recycling delay, were acquired continuously with a 900 s delay between each spectrum. Fig. 6 displays the 7Li NMR spectra obtained during the sequential acquisition process.
 | ||
| Fig. 6 Evolution of the 7Li distribution in a soaking experiment between a 6Li 95%-enriched electrolyte and a lithiated silicon-based electrode. 7Li-NMR spectra (48 accumulated transients). | ||
Fig. 7 shows the deconvolution of 7Li MAS NMR spectra focusing solely on the central transition (the ssb parameters have been taken into account but are not shown in the figure for clarity). Fig. 8 presents the evolution of 7Li signal intensities (see Table S2, ESI†) in different phases as a function of the soaking time with the 6Li 95% enriched electrolyte.
 | ||
| Fig. 8 Evolution of the 7Li distribution in a soaking experiment between a 6Li 95%-enriched electrolyte and a lithiated silicon-based electrode. The solid lines are added as the guides for eyes. | ||
It is clear from these experimental data that the fractions of 7Li in the electrolyte do not vary significantly over time. If we consider the electrolyte as the only reservoir of 6Li, then the corresponding NMR signal should be the first to be modified if isotope exchanges take place. Therefore, we have to conclude that either the lithium isotope exchanges involving the liquid electrolyte and solid powder do not occur, or they have already taken place during the first non-monitored hour. To check it, the Tof-SIMS experiments have been performed. These data (see Fig. 9) clearly demonstrate that the “liquid–solid” isotope exchange is completely finished during the first hour of soaking. The percentage of 7Li decreases sharply from 93% to 55% during the first ten minutes over the entire depth probed, reflecting a diffusion of 6Li atoms at the surface of the electrode. Curiously, after 30 minutes of immersion, the percentage of 7Li rises to around 75% in the probed depth and to 65% at the surface (0 s of sputtering). Finally, after 60 minutes of immersion, the percentage of 7Li is around 75% for the entire depth probed. This can be explained by mutual 6,7Li diffusion, when 6Li atoms penetrate to deeper Si layers, while 7Li diffuses from the Si depth to the surface, until reaching the homogenized concentration.
The main changes in the 7Li fraction are found on the silicides surface: during 25 hours, the 7Li12Si7 phase completely disappears (Fig. 6–8), most likely due to the 6Li → 7Li replacement. This confirms our previous results and assumptions (see Fig. 5 and corresponding discussion). Interestingly, the decrease in 7Li12Si7 phase intensity is accompanied by an increase in the 7Li fraction in the SEI, from about 32 to 38%. As in the case of ToF-SIMS data, this result allows assuming that besides 6Li → 7Li replacement, there is a simultaneous inverse process of 7Li → 6Li occurring between silicides and SEI compounds. It has to be noted also that in contrast to the outer part of the LixSi shell, the behavior of 7Li fraction in deeper layers remains almost unchanged with soaking time. For the “core–shell” interface (which is represented as the Li15Si4 phase as shown in Fig. 8), it seems to be obvious. For the intermediary part of the shell, the situation is more complicated. Fig. 7 presents one of the possible fits with two NMR lines corresponding to Li7Si3 and Li13Si4 phases. Within this representation with two separate “intermediary” lines, the tendency to decrease the Li7Si3 phase amount is observed. Thus, 6Li penetration is observed in these layers of the LixSi shell. However, it has to be noted that the fit of NMR spectra in the range of chemical shifts around 15 ppm yields rather contradictory results. Due to their proximity, these NMR lines overlap each other. As a result, their parameters (shift, width, ssb distribution) can be changed in a substantially wide range and, consequently, their intensities also change within the large error bars. Therefore, it appears to be more reasonable to represent them as a whole “Li7Si3 + Li13Si4” phase (as shown in Fig. 8 and in Table S2, ESI†). Then it becomes clear that the 7Li fraction remains stable (within ±2% of relative intensity) during entire soaking time. The center of mass of this merged NMR line moves in the region of lower chemical shifts, indicating, most likely, the 7Li atoms’ diffusion in the deeper layers of the LixSi shell.
Thus, we can propose the “two-stage” scenario of the 6Li–7Li isotope exchange in the 6Li-enriched liquid electrolyte–7Li lithiated Si electrode system. During the first 30 min, the fast “liquid–solid” exchange occurs, involving, most likely, both the silicide surface and the SEI. Then the 6Li atoms start to permeate Si bulk, replacing the 7Li ones, which in turn sink deeper in Si particles and/or diffuse in the SEI. This exchange process is much slower (occurs on the scale of tens of hours) and is most likely limited to only the outer part of the LixSi shell.
3. Conclusions
A simple and easy-to-use methodology of lithium isotope titration by high-resolution magic angle spinning solid-state NMR has been proposed. A key point is the use of a reference sample that allows comparing 7Li and 6Li MAS NMR spectra for all acquisitions under the same conditions without the distortion of spectra. The spectra deconvolution makes it possible to determine the isotopes’ ratio for each local chemical environment of the nucleus-probe. Therefore, in silicon-based electrode samples, it was possible to quantify 7Li/6Li for the SEI, electrolyte and different silicides.In the second step, this method was applied to study the isotope exchanges appearing between a 7Li–Si electrode in a partially lithiated state (1000 mA h g−1) and a 6Li-enriched electrolyte. The NMR results in combination with ToF-SIMS data demonstrate that Li isotope exchanges occur most likely due to a “two-stage” mechanism. During the first hour of the electrode and electrolyte contact, the fast “liquid–solid” 6Li–7Li exchange occurs, involving both the SEI and silicide surface. Then, these 6Li atoms start to permeate into the Si bulk, replacing the 7Li ones. This process is much slower and occurs on a scale of tens of hours.
It is worth noting that although in the present work we have considered the battery electrode materials as an example to illustrate our methodological approach, it can be applied in a wide range of systems, for example, to study the chemical reactions involving atomic exchanges such as those expected between different solid phases or in liquid–solid reactions. The method proposed can be performed on standard MAS NMR equipment without involving any additional techniques.
4. Experimental
4.1. Electrochemistry
Silicon based electrodes were prepared by mixing 70 wt% of silicon nanoparticles, 15 wt% of binder (PAA), 10% of carbon black (Super P) and 5% of carbon fibers (VGCF) in aqueous solution. The classical procedures of coating, drying and compressing resulted in an active material loading of 3.80 mg cm−2 on a copper foil (10 μm).Electrolytes were prepared in a glovebox by dissolving the LiPF6 salt in the ethylene carbonate/dimethylcarbonate (EC/DMC 1/1 wt) mixture containing 10 wt% of fluoroethylene carbonate in order to obtain a concentration of 1 mol L−1. Electrolytes were achieved in small amounts to avoid any degradation of the solution over time. For the 6Li-enriched electrolyte, a 6LiPF6 enriched at 95% (Sigma-Aldrich) was used.
Si//Li half-cells were assembled in a pouch cell by stacking the silicon-based electrode (35 × 35 mm), a separator (Celgard 2400, 40 mm × 40 mm) and a foil of lithium metal deposited on copper. The 6Li metal foil was obtained by flattening chunks enriched at 95% in 6Li. A volume of 150 μL of electrolyte was deposited on the separator, and then the cells were sealed in an argon filled glovebox.
Galvanostatic cycling was carried out in a battery tester (Arbin) with a current of 1.3 mA until reaching a specific capacity of 1000 mA h g−1 during the discharge. The cut-off potentials were limited to 10 mV and 1.2 V. If the galvanostatic cycling failed to reach the targeted capacity, a floating step was carried out at 10 mV. At the end of cycling, the cells were disassembled in an argon filled glovebox. Silicon-based electrodes were taken off and washed by soaking in DMC during 1 minute. One part of the electrode was kept for solid-state NMR and the other used for ToF-SIMS and liquid-NMR characterizations.
4.2. Sample preparation
A powder obtained from a lithiated silicon-based electrode was used as a standard for the development of the methodology. In this sample, lithium isotopes are present in natural abundance. The electrode samples on which the validity of the methodology was tested were enriched in 6Li electrochemically. Four kinds of enrichment were carried out by introducing 6Li in different parts of the accumulator. The preparation conditions of the four samples are summarized in Fig. 1. To make reading easier, terms “nat” and “enr” are used to characterize respectively “natural” and “enriched” components in 6Li. In a nat-component, the lithium isotopic ratio is that of natural abundance i.e. ∼93% in 7Li. In an enr-component, the isotopic enrichment in 6Li is 95%.4.3. Solid-state NMR characterizations
7Li and 6Li NMR spectra have been obtained at 194.37 MHz and 73.6 MHz, respectively, on a Bruker AVANCE III 500 MHz spectrometer equipped with a 4 mm Bruker CPMAS probe-head. Tuning of the probe has to be carried out when changing the observed nucleus. The Magic Angle Spinning method is used by setting the spinning rate at 10 kHz. The acquisition sequence used is direct excitation without proton decoupling using a 30° pulse, 2.5 μs and 2 μs for 6Li and 7Li, respectively. Dead times were set to 40 μs and 0.4 μs and recycling delays to 5.6 s and 0.66 s for 6Li and 7Li, respectively. At first sight, these recycling delays may appear to be too short. Indeed, rough estimates showed that in LixSi phases, the spin–lattice relaxation time, T1, for the 7Li nuclei was in the range of about 0.4 s which is close to the values obtained by Kuhn et al.36 Thus, the recycling delay of 0.66 s is much shorter than the “classical” required 5T1. It has to be noted, however, that we deliberately choose the shortest possible value of repetition time in order to maximize the number of FID accumulations. We made sure that the shape of the 6Li and 7Li NMR spectra at given delays does not differ significantly from that obtained at longer repetition times. Other parameters such as the number of accumulated transients (NS) and receiver gain (RG) have to be taken into account for quantitative treatment (see the Results section).4.4. Liquid NMR characterizations
In order to carry out liquid state NMR analyses, cells were first disassembled in a glovebox. After separation of components, electrodes were washed by soaking in DMC during 1 min. Then they were scratched in order to separate the copper collector from the rest of the electrode. The powder obtained is placed in a crucible in nickel in which one or two tablets of sodium hydroxide (Sigma Aldrich®) are added. Then, the crucible is heated until everything is melted. Few drops of MilliQ® water are added in order to dissolve the solution of sodium hydroxide.7Li NMR and 6Li NMR spectra were obtained, respectively, at 194.37 MHz and 73.6 MHz on the same Bruker AVANCE III 500 MHz spectrometer as for solid-state NMR. An electrolyte consisting of a mixture of EC:PC:DMC + 2% VC (1 M LiPF6) was used to calibrate the 6Li and 7Li ratios, in the same manner as for the solid sample. The one pulse acquisition was used. In this sample, NMR spectra were acquired on a Bruker BBI 5 mm probe. For this sample recycling, delays of 1.23 s and 68 s were used for 7Li and 6Li NMR, respectively. For standard conditions, the number of accumulated transients was typically 128 with recycling delays of 1.23 s and 68 s for 7Li and 6Li, respectively.
4.5. ToF-SIMS characterizations
Few drops of solution were deposited on a silicon wafer and heated in an oven at 50 °C during about 15 minutes. ToF-SIMS analyses were achieved on a ToF-SIMS5 ION-TOF spectrometer by using a Bi1+ beam at 15 keV. For each wafer, three acquisitions were carried out in a positive mode on a 150 × 150 μm2 area.Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
A. L. Buzlukov is grateful to the Ministry of Science and Higher Education of the Russian Federation (Theme No. 122021000035-6) for their generous support. Part of this work, carried out on the Platform for Nanocharacterisation (PFNC), was supported by the “Recherches Technologiques de Base” program of the French National Research Agency (ANR).Notes and references
- M. Tiwari, A. K. Singh and D. K. Sinha, Chemostratigraphy, Elsevier, 2015, pp. 65–92 Search PubMed.
- P. Lu and S. J. Harris, Lithium transport within the solid electrolyte interphase, Electrochem. Commun., 2011, 13, 1035–1037 CrossRef CAS.
- Z. Liu, P. Lu, Q. Zhang, X. Xiao, Y. Qi and L.-Q. Chen, A Bottom-Up Formation Mechanism of Solid Electrolyte Interphase Revealed by Isotope-Assisted Time-of-Flight Secondary Ion Mass Spectrometry, J. Phys. Chem. Lett., 2018, 9, 5508–5514 CrossRef CAS PubMed.
- S. Shi, P. Lu, Z. Liu, Y. Qi, L. G. Hector, H. Li and S. J. Harris, Direct Calculation of Li-Ion Transport in the Solid Electrolyte Interphase, J. Am. Chem. Soc., 2012, 134, 15476–15487 CrossRef CAS PubMed.
- N. Kuwata, M. Nakane, T. Miyazaki, K. Mitsuishi and J. Kawamura, Lithium diffusion coefficient in LiMn2O4 thin films measured by secondary ion mass spectrometry with ion-exchange method, Solid State Ionics, 2018, 320, 266–271 CrossRef CAS.
- N. Kuwata, X. Lu, T. Miyazaki, Y. Iwai, T. Tanabe and J. Kawamura, Lithium diffusion coefficient in amorphous lithium phosphate thin films measured by secondary ion mass spectrometry with isotope exchange methods, Solid State Ionics, 2016, 294, 59–66 CrossRef CAS.
- J. Zheng, M. Tang and Y. Hu, Lithium Ion Pathway within Li7La3Zr2O12-Polyethylene Oxide Composite Electrolytes, Angew. Chem., Int. Ed., 2016, 55, 12538–12542 CrossRef CAS PubMed.
- S. Dupke, T. Langer, F. Winter, R. Pöttgen, M. Winter and H. Eckert, Ionic Pathways in Li13Si4 investigated by 6Li and 7Li solid state NMR experiments, Solid State Nucl. Magn. Reson., 2015, 65, 99–106 CrossRef CAS PubMed.
- E. Hüger, L. Dörrer and H. Schmidt, Permeation, Solubility, Diffusion and Segregation of Lithium in Amorphous Silicon Layers, Chem. Mater., 2018, 30, 3254–3264 CrossRef.
- M. Berthault, J. Santos-Peña, D. Lemordant and E. D. Vito, Dynamics of the 6Li/7Li Exchange at a Graphite–Solid Electrolyte Interphase: A Time of Flight–Secondary Ion Mass Spectrometry Study, J. Phys. Chem. C, 2021, 125, 6026–6033 CrossRef CAS.
- A. L. Michan, M. Leskes and C. P. Grey, Voltage Dependent Solid Electrolyte Interphase Formation in Silicon Electrodes: Monitoring the Formation of Organic Decomposition Products, Chem. Mater., 2016, 28, 385–398 CrossRef CAS.
- N. Kuwata, G. Hasegawa, D. Maeda, N. Ishigaki, T. Miyazaki and J. Kawamura, Tracer Diffusion Coefficients of Li Ions in LixMn2O4 Thin Films Observed by Isotope Exchange Secondary Ion Mass Spectrometry, J. Phys. Chem. C, 2020, 124, 22981–22992 CrossRef CAS.
- G. Hasegawa, N. Kuwata, Y. Tanaka, T. Miyazaki, N. Ishigaki, K. Takada and J. Kawamura, Tracer diffusion coefficients of Li+ ions in c-axis oriented LixCoO2 thin films measured by secondary ion mass spectrometry, Phys. Chem. Chem. Phys., 2021, 23, 2438–2448 RSC.
- M. Diehl, M. Evertz, M. Winter and S. Nowak, Deciphering the lithium ion movement in lithium ion batteries: determination of the isotopic abundances of 6Li and 7Li, RSC Adv., 2019, 9, 12055–12062 RSC.
- A. J. Ilott and A. Jerschow, Probing Solid-Electrolyte Interphase (SEI) Growth and Ion Permeability at Undriven Electrolyte–Metal Interfaces Using 7Li NMR, J. Phys. Chem. C, 2018, 122, 12598–12604 CrossRef CAS.
- M. Murakami, S. Shimizu, Y. Noda, K. Takegoshi, H. Arai, Y. Uchimoto and Z. Ogumi, Spontaneous Lithium Transportation via LiMn2O4/Electrolyte Interface Studied by 6/7Li Solid-State Nuclear Magnetic Resonance, Electrochim. Acta, 2014, 147, 540–544 CrossRef CAS.
- A. B. Gunnarsdóttir, S. Vema, S. Menkin, L. E. Marbella and C. P. Grey, Investigating the effect of a fluoroethylene carbonate additive on lithium deposition and the solid electrolyte interphase in lithium metal batteries using in situ NMR spectroscopy, J. Mater. Chem. A, 2020, 8, 14975–14992 RSC.
- T. Kobayashi, T. Ohnishi, T. Osawa, A. Pratt, S. Tear, S. Shimoda, H. Baba, M. Laitinen and T. Sajavaara, In-Operando Lithium-Ion Transport Tracking in an All-Solid-State Battery, Small, 2022, 18, 2204455 CrossRef CAS PubMed.
- S. Dupke, T. Langer, R. Pöttgen, M. Winter, S. Passerini and H. Eckert, Structural characterization of the lithium silicides Li15Si4, Li13Si4, and Li7Si3 using solid state NMR, Phys. Chem. Chem. Phys., 2012, 14, 6496 RSC.
- B. Key, R. Bhattacharyya, M. Morcrette, V. Seznéc, J.-M. Tarascon and C. P. Grey, Real-Time NMR Investigations of Structural Changes in Silicon Electrodes for Lithium-Ion Batteries, J. Am. Chem. Soc., 2009, 131, 9239–9249 CrossRef CAS PubMed.
- R. E. Gerald, C. S. Johnson, J. W. Rathke, R. J. Klingler, G. Sandí and L. G. Scanlon, 7Li NMR study of intercalated lithium in curved carbon lattices, J. Power Sources, 2000, 89, 237–243 CrossRef CAS.
- H. J. Chang, N. M. Trease, A. J. Ilott, D. Zeng, L.-S. Du, A. Jerschow and C. P. Grey, Investigating Li Microstructure Formation on Li Anodes for Lithium Batteries by in Situ 6Li/7Li NMR and SEM, J. Phys. Chem. C, 2015, 119, 16443–16451 CrossRef CAS.
- D. I. Hoult and N. S. Ginsberg, The Quantum Origins of the Free Induction Decay Signal and Spin Noise, J. Magn. Reson., 2001, 148, 182–199 CrossRef CAS PubMed.
- F. D. Doty, G. Entzminger, J. Kulkarni, K. Pamarthy and J. P. Staab, Radio frequency coil technology for small-animal MRI, NMR Biomed., 2007, 20, 304–325 CrossRef PubMed.
- H. Mo, J. S. Harwood and D. Raftery, A quick diagnostic test for NMR receiver gain compression, Magn. Reson. Chem., 2010, 48, 782–786 CrossRef CAS PubMed.
- H. Mo, J. S. Harwood and D. Raftery, Receiver gain function: the actual NMR receiver gain: receiver gain function, Magn. Reson. Chem., 2010, 48, 235–238 CrossRef CAS PubMed.
- E. Radvanyi, E. De Vito, W. Porcher, J. Danet, P. Desbois, J.-F. Colin and S. J. Si Larbi, Study of lithiation mechanisms in silicon electrodes by Auger Electron Spectroscopy, J. Mater. Chem. A, 2013, 1, 4956 RSC.
- J. W. Wang, Y. He, F. Fan, X. H. Liu, S. Xia, Y. Liu, C. T. Harris, H. Li, J. Y. Huang, S. X. Mao and T. Zhu, Two-Phase Electrochemical Lithiation in Amorphous Silicon, Nano Lett., 2013, 13, 709–715 CrossRef CAS PubMed.
- E. A. Schauble, Applying Stable Isotope Fractionation Theory to New Systems, Rev. Mineral. Geochem., 2004, 55, 65–111 CrossRef CAS.
- D. Massiot, F. Fayon, M. Capron, I. King, S. Le Calvé, B. Alonso, J.-O. Durand, B. Bujoli, Z. Gan and G. Hoatson, Modelling one- and two-dimensional solid-state NMR spectra: modelling 1D and 2D solid-state NMR spectra, Magn. Reson. Chem., 2002, 40, 70–76 CrossRef CAS.
- P. Guan, L. Liu and X. Lin, Simulation and Experiment on Solid Electrolyte Interphase (SEI) Morphology Evolution and Lithium-Ion Diffusion, J. Electrochem. Soc., 2015, 162, A1798–A1808 CrossRef CAS.
- M. B. Pinson and M. Z. Bazant, Theory of SEI Formation in Rechargeable Batteries: Capacity Fade, Accelerated Aging and Lifetime Prediction, J. Electrochem. Soc., 2013, 160, A243–A250 CrossRef CAS.
- P. Verma, P. Maire and P. Novák, A review of the features and analyses of the solid electrolyte interphase in Li-ion batteries, Electrochim. Acta, 2010, 55, 6332–6341 CrossRef CAS.
- N. Ding, J. Xu, Y. X. Yao, G. Wegner, X. Fang, C. H. Chen and I. Lieberwirth, Determination of the diffusion coefficient of lithium ions in nano-Si, Solid State Ionics, 2009, 180, 222–225 CrossRef CAS.
- G. A. Tritsaris, K. Zhao, O. U. Okeke and E. Kaxiras, Diffusion of Lithium in Bulk Amorphous Silicon: A Theoretical Study, J. Phys. Chem. C, 2012, 116, 22212–22216 CrossRef CAS.
- A. Kuhn, P. Sreeraj, R. Pöttgen, H.-D. Wiemhöfer, M. Wilkening and P. Heitjans, Li Ion Diffusion in the Anode Material Li12Si7: Ultrafast Quasi-1D Diffusion and Two Distinct Fast 3D Jump Processes Separately Revealed by 7 Li NMR Relaxometry, J. Am. Chem. Soc., 2011, 133(29), 11018–11021 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp02646a |
| This journal is © the Owner Societies 2023 |




