Open Access Article
Open Access ArticleInteraction between carbon dots from folic acid and their cellular receptor: a qualitative physicochemical approach†
Erika
Adhel
a,
Nguyêt-Thanh
Ha Duong
 a,
Thi Huyen
Vu
b,
Dario
Taverna
c,
Souad
Ammar
a,
Thi Huyen
Vu
b,
Dario
Taverna
c,
Souad
Ammar
 a and
Nawal
Serradji
a and
Nawal
Serradji
 *a
*a
aUniversité Paris Cité, CNRS, ITODYS, F-75013 Paris, France. E-mail: serradji@u-paris.fr; Fax: +33 1 57277263; Tel: +33 1 57278883
bUniversity of Engineering and Technology, Vietnam National University, Hanoi (VNUH), Vietnam
cSorbonne Université, CNRS, IMPMC, F-75005 Paris, France
First published on 27th April 2023
Abstract
According to the World Health Organization, the number of cancers (all cancers, both sexes, all ages and worldwide) in 2020 reached a total of 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 292
292![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 789 new cases leading to 9
789 new cases leading to 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 958
958![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 133 deaths during the same period. Many cancers could be cured if detected early. Preventing cancer and detecting it early are two essential strategies for controlling this pathology. For this purpose, several strategies have been described for imaging cancer cells. One of them is based on the use of carbon nanoparticles called carbon dots, tools of physical chemistry. The literature describes that cancer cells can be imaged using carbon dots obtained from folic acid and that the in cellulo observed photoluminescence probably results from the interaction of these nanoparticles with the folic acid-receptor, a cell surface protein overexpressed in many malignant cells. However, this interaction has never been directly demonstrated yet. We investigated it, for the first time, using (i) freshly synthesized and fully characterized carbon dots, (ii) folate binding protein, a folic acid-receptor model protein and (iii) fluorescence spectroscopy and isothermal titration calorimetry, two powerful methods for detecting molecular interactions. Our results even highlight a selective interaction between these carbon made nano-objects and their biological target.
133 deaths during the same period. Many cancers could be cured if detected early. Preventing cancer and detecting it early are two essential strategies for controlling this pathology. For this purpose, several strategies have been described for imaging cancer cells. One of them is based on the use of carbon nanoparticles called carbon dots, tools of physical chemistry. The literature describes that cancer cells can be imaged using carbon dots obtained from folic acid and that the in cellulo observed photoluminescence probably results from the interaction of these nanoparticles with the folic acid-receptor, a cell surface protein overexpressed in many malignant cells. However, this interaction has never been directly demonstrated yet. We investigated it, for the first time, using (i) freshly synthesized and fully characterized carbon dots, (ii) folate binding protein, a folic acid-receptor model protein and (iii) fluorescence spectroscopy and isothermal titration calorimetry, two powerful methods for detecting molecular interactions. Our results even highlight a selective interaction between these carbon made nano-objects and their biological target.
Introduction
Targeted, and therefore selective, drug delivery is now a major therapeutic issue in medicine. Indeed, this strategy enhances the performance of the drugs administered while drastically reducing their side effects, often related to their recognition by multiple possible biological targets.Cancer is an example of a pathology requiring a targeted chemotherapeutic approach. With approximately 10 million deaths per year, cancer is the second leading cause of death in the world. Almost one in six deaths is due to cancer worldwide. It is therefore imperative to offer additional tools to attempt strict control of this pathology. Increasing the efficacy of pre-existing cytotoxic compounds and diminishing induced side effects appear as relevant strategies. To improve drug efficiency, biological therapy,1 photodynamic therapy2 or dietary interventions3 can be used. To reduce side effects, directing drugs to receptors selectively overexpressed by cancer cells can be exploited. The interaction between folic acid receptor (FA-R)4 and folic acid (FA), its natural ligand, is a good illustration of such a therapeutic pathway.5,6 Indeed, FA is highly tolerant to chemical modifications and its recognition by its receptor is seldom altered, making FA-R-overexpressing cancer cell targeting7 and, consequently, drug-conjugate endocytosis and fast cell penetration highly relevant.
In parallel, there is much interest in the use of nanoparticles (NPs) in therapy, due to their numerous advantages: nanometer size, concentration of the drug on their surface, protection of the latter against enzymatic degradation and so on. Therapeutic NPs are of varied natures.8 Among them, Carbon Dots (CDs) are emerging as metal-free NPs with remarkable intrinsic optical properties.9 They were discovered accidentally in 2004 by Xu et al.10 and since then, a variety of easy in-solution synthesis methods have been developed.11 Most CDs exhibit blue-green emissions. Nowadays, they are typically green substitutes for the previous toxic CdSe quantum dots (QDs).12 Their building block is made out of carbon13 with some doped light heteroatoms, creating NPs that are more biocompatible, soluble in water and less toxic than QDs.14 Moreover, surface functionalization of CDs is easy, using their surface sp3 carbon atoms.15 Thanks to these unique properties, the number of related publications about their syntheses, properties and biomedical applications increases exponentially every year.16–18 Despite this huge scientific effervescence, the exact nature of these CDs is still a subject of discussion. For instance, carbon nitride C3N4,19 nanographene, nanographene oxide20 and semiconducting polymeric CDs have been described and named CDs in the literature.21 Furthermore, the understanding of the key factors controlling their physicochemical properties remains challenging. Zhu et al. proposed that some properties, e.g. toxicity, would be dictated primarily by the CD core, while other properties, e.g. dispersibility, would depend primarily on their surface functional groups.22 Zhi et al. reviewed prevalent hypotheses regarding the origin of their photoluminescence, highlighting the role of the in-gap surface group energy levels in the radiative de-excitation processes of CDs assumed as semiconducting nanostructures.23
Their use as carriers for drug delivery, functionalizing their surface by specific biomolecules such as FA, and the mechanism of their interaction with FA-R have been addressed.24–33 Indeed, FA-R is able to interact with its natural ligand, but also with conjugates resulting from the covalent binding of FA to various organic groups. FA attached to larger entities, such as nanovehicles including polymers,34 liposomes, proteins35 or NPs, is also recognized by this receptor, illustrating its wide capacity for ligand recognition. Numerous studies, carried out on cell cultures and using different methods, make it possible to take advantage of the specificity of this ligand–receptor recognition without ever demonstrating a direct interaction. For instance, Narmani et al. reviewed NPs functionalized with FA and successfully prepared as drug delivery systems.36 In addition, Yücel et al. grafted FA to methotrexate (a FA-R antagonist)-loaded gold NPs and demonstrated the receptor specificity of the conjugate by fluorescence microscopy imaging.37 Butzbach et al. synthesized modified dextran NPs and conjugated them with FA in order to increase the uptake of a photosensitizer into tumour cells. A completion assay, using an excess of free FA, demonstrated that these NPs are recognized by FA-R.38 CDs obtained by in-solution FA decomposition (CDsFA) are able to detect or to image cells expressing FA-R, presumably through their interaction with this receptor, but this point was not established unambiguously.39–41 These studies also pointed out the possibility of selectively imaging cancer cells using such CDsFA,42 but did not provide spectroscopic data to validate this biological feature.
Therefore, for the first time in this work and according to the chemist's point of view, we used absorption, emission spectrophotometry methods and isothermal titration calorimetry to evidence the interaction between CDsFA and a soluble form of FA-R, the bovine folate binding protein (bFBP), a surrogate of the human FA-R with 80% homology with the human form and also a high affinity for FA.43 CDsFA were produced by hydrothermal decomposition of FA and characterised. In addition, their putative selectivity was investigated using lactoferrin (LF), another FA target-protein.44,45
Experimental methods
Chemicals
Folic acid (FA), quinine sulfate monohydrate 90%, folate binding protein from bovine milk (bFBP), human lactoferrin (LF) and sodium azide 99% were purchased from Sigma-Aldrich, and potassium hydrogen phosphate trihydrate from Alfa Aesar. PURELAB Classic UV provided 18.2 MΩ cm ultra-pure type I Milli-Q water. Phosphate buffer solution was prepared by dissolving 20 mM of K2HPO4·3H2O (Sigma) in Milli-Q water and adjusting the pH to 7.4 by addition of commercial 37% HCl solution (Sigma). The pH of the solution was measured on a Jenco pH meter, equipped with an Ingold microelectrode and referenced to standard buffers at pH = 7.0 and 10.0 (Sigma).Hydrothermal synthesis and purification of CDsFA
A suspension of FA (0.15 g) in Milli-Q water (50 mL) was placed in a beaker and homogenised by sonication for 20 minutes, in an ultrasonic bath (VWR Ultrasonic Cleaner). The resulting suspension was then transferred to a 120 mL Teflon chamber in a high-pressure reactor autoclave (302 AC T304 030317, Moline, Illinois, USA), and heated in a Serlabo SAV type oven for 6 hours at 180 °C. The autoclave was then cooled to room temperature. The yellow suspension obtained was filtered through a 0.22 μm syringe filter, dialysed 3 times (for 2 hours, overnight, and again for 2 hours) against Milli-Q water (molecular weight cut-off (MWCO) of 100–500 kDa for the Slide-A-Lyser, Thermo Scientific). Freeze-drying (Modulyo freeze dryer) led to 30 mg (20% w/w) of the resulting CDsFA as a yellow powder, and stored in the dark under argon at −20 °C.Preparation of stock solutions
For CDsFA characterisation, the solutions were freshly prepared by dilution of a 1 mg mL−1 stock solution stored at +4 °C.CDsFA characterization
Dynamic light scattering (DLS) and zeta potential (ζ) measurements (Malvern-Zetasizer Nano ZS90) were performed in 3 mL plastic cuvettes with a 1 cm optical path and a folded capillary zeta cell, respectively. UV-Visible absorption spectra were recorded on CDsFA and FA in Milli-Q water, at 25.0 ± 0.5 °C using a Cary 4000 spectrophotometer with a SUPRACIL quartz cuvette (1 × 1 cm). Fluorescence spectra of the same solutions were obtained, at 25.0 ± 0.5 °C, on a Fluorolog spectrophotometer, using a SUPRACIL quartz cuvette (1 × 1 cm). To quantify the quantum yield of CDsFA, the optical absorption and fluorescence spectra of a reference quinine sulfate monohydrate aqueous solution were recorded.Infrared (IR) absorption spectra were recorded on FA and CDsFA (KBr method), using a VariGATRTM, USA, Thermo Scientific spectrometer (Class 1 Laser Product).
Transmission electron microscopy (TEM) and scanning transmission electron microscopy (STEM) data were acquired on a JEOL 2100F microscope, equipped with a Gatan GIF 2001 for electron energy loss spectroscopy (EELS) measurements. In STEM-EELS experiments, images were recorded using the following parameters: spectrum image size: 69 × 98 nm (21 × 30 pixels); spot size: 1 nm; energy dispersion: 0.5 eV per channel; dwell time: 0.3 sec per pixel. To complete these analyses, high-angle annular-dark-field (HAADF) imaging was performed on the same microscope. For all these experiments, a drop of the CDsFA aqueous colloid was deposited on a holey carbon–Cu 300 mesh grid from Oxford Instruments.
X-ray photoelectron spectroscopy (XPS) was used to check the surface chemical composition of the particles produced. A K-Alpha+ system (ThermoFisher Scientific, East Grinstead, UK) fitted with a micro-focused, monochromatic Al Kα X-ray source (1486.6 eV, spot size: 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) μm) was used. The survey spectra were collected in steps of 1 eV at a pass energy of 150 eV. They were calibrated against the (C–C/C–H) C 1s component set at 285 eV. High-resolution spectra of separate photoelectron signals (C, O, N) were taken by steps of 0.1 eV at a pass energy of 40 eV. A few milligrams of CDsFA as powder were deposited on carbon tape to form a thick layer.
μm) was used. The survey spectra were collected in steps of 1 eV at a pass energy of 150 eV. They were calibrated against the (C–C/C–H) C 1s component set at 285 eV. High-resolution spectra of separate photoelectron signals (C, O, N) were taken by steps of 0.1 eV at a pass energy of 40 eV. A few milligrams of CDsFA as powder were deposited on carbon tape to form a thick layer.
A high-resolution mass spectrum (HRMS) was recorded at the Small Molecule Mass Spectrometry platform of IMAGIF (Centre de Recherche de Gif – https://www.imagif.cnrs.fr), on a Waters spectrometer using electrospray ionization-TOF (ESI-TOF; M + H+).
FA-R interaction
The reverse titration was also performed with the same automated sequence of injections wherein 50 μL of 100 μM FBP were loaded in the syringe and 8.55 μg mL−1 CDsFA were in the cell. The reverse titration experiments were carried out in duplicate.
Data were collected automatically and analysed, after substitution of the heat produced during CDsFA dilution, using the NanoAnalyze software from TA instruments and an independent binding site model to yield the association constant, stoichiometry, and the thermodynamic parameters (ΔH and ΔS) of binding reactions.
Results and discussion
Synthesis and characterisation of CDsFA
CDsFA were synthesised by a hydrothermal method using FA as the carbon source (Scheme 1).We first tried to reproduce the synthesis proposed by Bhunia et al.42 who described a simple one-step route, in which FA was mixed with sodium hydroxide and heated at 90 °C for 2 hours to induce carbonisation. However, our trials did not provide luminescent objects. We then modified the experimental procedure by increasing the temperature to 180 °C, without however obtaining CDsFA. Finally, removing sodium hydroxide from the reaction medium and heating to 180 °C (Scheme 1) led to a yellow suspension with a fluorescent behaviour which is not observed in free FA under UV irradiation (Fig. 1). The product was purified by filtration through a 0.22 μm filter, dialysed against Milli-Q water and lyophilised to give the desired CDsFA as a yellow powder. Running the reaction for 6 hours increased the yield to 20% whereas it was only 3.3% after 2 hours. We also improved particle purification by filtering before dialysis and by reducing the MWCO of the dialysis bag. CDsFA obtained after a 6 hour reaction were selected for characterisation and interaction studies. Data concerning size, morphology, zeta potential, composition and/or surface analysis of the nanoparticles are presented in the ESI.†
 | ||
| Fig. 1 Solutions of free FA and CDsFA in Milli-Q water at 40 μg mL−1 under white light (left) and under UV irradiation at 365 nm (right). | ||
The complementarity results obtained from EELS, XPS and FTIR experiments, presented in the ESI,† allow us to propose that (i) the sample consists of carbon, nitrogen and oxygen, (ii) CDsFA would appear to be particles with a core of carbon (major) with a very weak nitrogen doping, since oxygen was not identified by the EELS measurements, (iii) these cores are, for sure, decorated by organic N and/or O based hydrocarbon groups, as suggested by XPS and FTIR, (iv) the particles are non-crystalline, according to TEM observations, and (v) are very probably not FA-based polymers, since their core composition does not include oxygen whereas FA contains both N and O heteroatoms in almost the same atomic ratio.
The photoluminescent properties of CDsFA
The optical properties of the CDsFA were characterized by recording the UV-visible absorption (Fig. 2) and emission (Fig. 3) spectra of their aqueous solutions and comparing them to those of free FA. Interestingly, the spectra of both CDsFA and FA present π–π* sp2 carbon UV bands (274 and 283 nm, respectively) with a shoulder attributed to an n–π* transition of a carbonyl function (348 nm), suggesting structural analogies between FA and at least the surface of CDsFA. This also demonstrates that some UV chromophoric organic groups are present in both systems. | ||
| Fig. 2 UV-visible absorption spectra of FA (10 μg mL−1 in Milli-Q water, blue curve) and CDsFA (10 μg mL−1 in Milli-Q water, orange curve). | ||
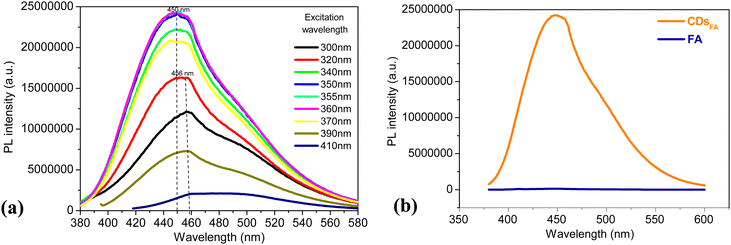
Their optical photoluminescence (PL) response was recorded by exciting them at a wavelength increasing from 300 to 410 nm (Fig. 3a). CDsFA are luminescent, with two main emissions, a stronger one at around 450 nm, corresponding to the blue-green emission observed (Fig. 1) under UV excitation, and a weaker one around 500 nm, assumed to be due to the water Raman signature. Interestingly, whereas FA, as an organic chromophore, requires excitation at a wavelength close to the LUMO–HOMO transition energy involved and relaxes radiatively at a constant energy, slightly smaller than the excitation one, CDsFA can be excited over a wider range of wavelengths, as long as the corresponding energies remain higher than those required for the excitation of the involved emitting surface state levels within the semiconducting core band gap of CDs, as already reported in several works dealing with CDs photoluminescence (Fig. 3b).19
The photoluminescence quantum yield of CDsFA (ΦCD) was then computed using the equation below and quinine sulfate monohydrate (SQ), dissolved in 0.1 M sulfuric acid, as a reference. It expresses the ability of an excited chemical system to emit a photon.
A quantum yield of 39 ± 2% was thus obtained for excitation wavelengths ranging from 300 to 370 nm (Table S1 in the ESI†). These values are significantly greater than that reported by Bhunia et al. (9% at 365 nm).42 This difference may be explained by an improved purification process providing CDsFA.
CDsFA/FA-R interaction study
FA-R are cell membrane-associated proteins responsible for FA transportation within the cells via a specific receptor-mediated endocytosis phenomenon.46 Although expressed at very low levels in most tissues, FA-R are overexpressed in many cancer cells5 and are considered to be tumour-specific target platforms. So, as explained before, folate binding protein (FBP), the soluble form of FA-R with high affinity for FA,47 was used in this study as a surrogate of FA-R. In particular, we evaluated the interaction of CDsFA with a bovine form of FBP (bFBP), isolated from cow milk.48 The high degree of homology with the human milk FBP supports the idea that this protein can serve as an analytical tool43 and a model in ligand–protein interaction studies.49 Indeed, Nygren-Babol et al. described the interaction between folic acid and bFBP with a dissociation constant (Kd) at the pM range, using surface plasmon resonance.50 In what follows, bFBP will be expressed as FBP.To highlight CDsFA/FBP interaction, fluorescence emission spectroscopy, which is a powerful method for detecting molecular interactions,51 was used. We typically followed the photoluminescence (PL) intensity variation of the protein in the 320–400 nm wavelength range after excitation at 280 nm while varying the concentration of the ligand (FA or CDsFA). First, to validate our method, we determined the dissociation constant (Kd) for the interaction between FBP and FA (see ESI†). The value of Kd obtained, (9.2 ± 0.3) × 10−8 M, enables the quantification of this phenomenon and confirms that FA binds efficiently to FBP under our experimental conditions.
In a second step, we transposed this method to study CDsFA/FBP interaction, which can be defined as follows:
 | (1) |
The reverse experiment, i.e. the addition of a solution of FBP to CDsFA in solution, was set up to evaluate the influence of such addition on the PL properties of the NPs. We observed an exaltation of these PL properties when the nanoparticles were excited at 350 nm (Fig. S9 and S10 in the ESI†).
Ideally, to fully characterise this interaction, the constant Kd is calculated and compared to that associated with free FA. The molecular weight of CDsFA is required. An attempt was made to obtain a value by high resolution mass spectroscopy (HRMS). Electrospray ionisation (ESI†) results are presented in the ESI† (Fig. S11). Unfortunately, they only demonstrate that folic acid residues are the main components of this sample. This is an important piece of information for our purpose, but they do not allow any determination of the total molecular weight of our nano-objects. Attempts to determine nanoparticle molecular weights by HRMS have already been described with more or less success. For instance, Hou et al. prepared antibacterial carbon dots from ciprofloxacin and carried out laser desorption/ionization time-of-flight mass spectroscopy, but only ciprofloxacin fragments could be identified.52
The same experimental strategy was applied to appreciate, at least qualitatively, the selectivity of this interaction using lactoferrin (LF), which is not the natural FA receptor even if it is capable of binding it.44
LF is a glycoprotein of the transferrin family initially described as an iron-binding molecule, but it is now known to be a multifunctional protein.53 Tavares et al. investigated the ability of positively charged LF to form complexes with negatively charged FA at pH 5.5, and found a moderate binding constant Ka of 105 M−1.44 Following the previously described methodology, we added increasing amounts of CDsFA to a solution of LF, measured the corresponding fluorescence emission and plot curves presented in Fig. 6a. As expected, the addition of increasing amounts of NPs does not modify the fluorescence emission spectrum of LF, expressed as PL intensity, demonstrating that the interaction is weak compared to that observed when CDsFA are added to a solution of FBP, under the same conditions of concentration, pH and temperature (Fig. 6b). Furthermore, no spectral changes are observed for either LF or the corresponding putative complex when the FI is expressed as a function of the emission wavelength, in accordance with the absence of a strong interaction between these both partners (Fig. S12 in ESI†).
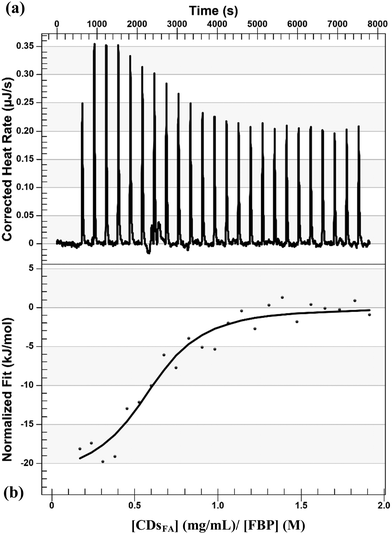
To reinforce the complex formation observed during the spectrofluorimetric study between FBP and CDsFA, we employed isothermal titration calorimetric (ITC). ITC usually provides enthalpy (ΔH) and entropy (ΔS) parameters of the binding reaction but also the corresponding dissociation constant (Kd) and the stoichiometry (n) of the complex. Taking into account the previous limitations we met (no molecular weight determination), only a qualitative study was set up. Our experimental data are presented in Fig. 7–9 and were fitted with an independent-site model. Ligands (CDsFA) and macromolecules (FBP or lactoferrin) were placed in the same buffer to avoid large background heat effects related to the ligand injection into the buffer. These background heats were measured in an experiment wherein the ligand is injected into a buffer (Fig. 9).
ITC is a classical method that intrinsically measures the heat produced (or consumed) when components are interact together, including during protein–nanoparticle interaction.54 In our conditions, the addition of a solution of CDsFA to a solution of FBP (direct titration) led to heat production as presented in Fig. 7, where raw data are presented as a series of peaks measured as power (μJ s−1) versus time. The formation of the CDsFA/FBP complex was further confirmed by the reverse ITC titrations, given in Fig. 8, where a solution of FBP was added to CDsFA in solution. In both experiments, the plot of heat as a function of [ligand]/[macromolecule] ratio provided a sigmoidal curve, indicating a single binding event, demonstrated that binding reactions proceed spontaneously as they are thermodynamically favoured, and confirming the interaction previously observed. The direct and reverse reactions are exothermic (ΔH < 0, Fig. 7 and 8), as also observed during the titration of CDots by HSA or BSA.55 Moreover, as most of the protein–Np interactions are enthalpy-driven (|ΔH| < |TΔS|), we then suggest that the main driving force of the interaction between FBP and the NPs is non-covalent bonding, as van der Waals and/or hydrogen interactions.56 Furthermore, the CDsFA are negatively charged whereas the FBP is positively charged at pH 7, regarding its isoelectric point (pI) value around 8, electrostatic bonds may also contribute to the exothermic reaction.
ITC was also used to study a putative protein selectivity. At pH 7.4, the thermogram profiles of the titration of lactoferrin by CDsFA shows a weak heat production (Fig. 9, right) which is similar to the one observed when CDsFA in solution are added to the buffer (Fig. 9, left), demonstrating the absence of interaction between this protein and the particles in these experimental conditions.
Recently Jin et al. suggested an explanation of the interaction between hydrothermally FA-made CDs and cells overexpressing FA-R.57 They explained that the pterin moiety, also presents in FA's structure, and required to the interaction with the FA-R, is maintained in the produced CDs. Hence, they showed that structural similarities exist between their NPs and FA using UV-visible, PL, FT-IR, XPS and NMR data. We also observed them (see the ESI†), even if the structural properties of CDsFA do not completely match with those of Jin et al.'s particles. Indeed, certain differences can, however, be evidenced between the two set of particles and are certainely related to differences in the operating synthesis conditions (FA concentration, NaOH addition, reaction temperature and so one). Despite these discrepancies, all the CDs obtained from FA bind efficiently to their target demonstrating an important tolerance in their structure. The latter is certainly linked to the previously described tolerance of FA to structural modifications.
Conclusions
Thus, the literature describes a very large variety, in nature and size, of objects capable of being recognized by the FA-R; among these are CDsFA. Several publications, cited in the introduction of this article, demonstrate the capacity of these objects to target FA-R in a cellular medium. The present study is the first to verify this interaction in a direct manner, bringing together the main actors of this interaction in a cell-free system. For this purpose, we synthesised carbon dots from folic acid, as a source of carbon, and characterised them. Using a folate-binding protein, a soluble form of the folic acid receptor with a high affinity for folic acid, its natural ligand, we established the formation of a complex between the two partners and the selectivity of this interaction. To our knowledge, these objects have only been used to visualize cancerous cells. The demonstration of their direct interaction with their target contributes to extending their field of application. Indeed, the possible drug delivery of an anti-cancer compound, for instance, that would have been grafted to them becomes possible.Author contributions
Erika Adhel: investigation, formal analysis, validation, data curation, writing—review and editing, visualization; Nguyêt-Thanh Ha Duong: conceptualization, methodology, formal analysis, validation, resources, data curation, writing—review and editing, funding acquisition, visualization; Thi Huyen Vu: investigation, formal analysis, validation, data curation, visualization; Dario Taverna: investigation, formal analysis, resources, validation, data curation, funding acquisition, visualization; Souad Ammar: formal analysis, writing—review and editing, funding acquisition, visualization and Nawal Serradji: conceptualization, methodology, formal analysis, resources, data curation, writing—original draft preparation, writing—review and editing, supervision, project administration, funding acquisition, visualization. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
This work was supported by PhD grants to E. A. from the Université Paris Cité (ED388) and to T. H. V. from the University of Science and Technology of Hanoi. The ANR (Agence Nationale de la Recherche) and the CGI (Commissariat à l’Investissement d’Avenir) are gratefully acknowledged for financial support of this work through Labex SEAM (Science and Engineering for Advanced Materials and devices), ANR-10-LABX-096 and ANR-18-IDEX-0001. The authors are also grateful to the CNRS for financial support and to ITODYS for NMR, FTIR, XPS facilities (Université Paris Cité, CNRS UMR 7086, Paris, France). We thank Sébastien Bellynck for valuable technical assistance and Delphine Schaming for fruitful scientific discussion. John Lomas is also warmly thanked for proofreading this manuscript.References
- V. Schirrmacher, From chemotherapy to biological therapy: A review of novel concepts to reduce the side effects of systemic cancer treatment (Review), Int. J. Oncol., 2019, 54, 407–419 CrossRef CAS PubMed.
- S. Kwiatkowski, B. Knap, D. Przystupski, J. Saczko, E. Kędzierska, K. Knap-Czop, J. Kotlińska, O. Michel, K. Kotowski and J. Kulbacka, Photodynamic therapy – mechanisms, photosensitizers and combinations, Biomed. Pharmacother., 2018, 106, 1098–1107 CrossRef PubMed.
- R. Abdollahi, S. Najafi, E. Razmpoosh, R. S. Shoormasti, S. Haghighat, M. Raji Lahiji, M. Chamari, M. Asgari, E. Cheshmazar and M. Zarrati, The Effect of Dietary Intervention Along with Nutritional Education on Reducing the Gastrointestinal Side Effects Caused by Chemotherapy Among Women with Breast Cancer, Nutr. Cancer, 2019, 71, 922–930 CrossRef PubMed.
- N. Parker, M. J. Turk, E. Westrick, J. D. Lewis, P. S. Low and C. P. Leamon, Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay, Anal. Biochem., 2005, 338, 284–293 CrossRef CAS PubMed.
- Y. G. Assaraf, C. P. Leamon and J. A. Reddy, The folate receptor as a rational therapeutic target for personalized cancer treatment, Drug Resistance Updates, 2014, 17, 89–95 CrossRef PubMed.
- M. Scaranti, E. Cojocaru, S. Banerjee and U. Banerji, Exploiting the folate receptor alpha in oncology, Nat. Rev. Clin. Oncol., 2020, 17, 349–359 CrossRef PubMed.
- W. Xia and P. S. Low, Folate-Targeted Therapies for Cancer, J. Med. Chem., 2010, 53, 6811–6824 CrossRef CAS PubMed.
- S. Raj, S. Khurana, R. Choudhari, K. K. Kesari, M. A. Kamal, N. Garg, J. Ruokolainen, B. C. Das and D. Kumar, Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy, Semin. Cancer Biol., 2021, 69, 166–177 CrossRef CAS PubMed.
- F. Yan, Z. Sun, H. Zhang, X. Sun, Y. Jiang and Z. Bai, The fluorescence mechanism of carbon dots, and methods for tuning their emission color, Microchim. Acta, 2019, 186, 583 CrossRef PubMed.
- X. Xu, R. Ray, Y. Gu, H. J. Ploehn, L. Gearheart, K. Raker and W. A. Scrivens, Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments, J. Am. Chem. Soc., 2004, 126, 12736–12737 CrossRef CAS PubMed.
- U. A. Rani, L. Y. Ng, C. Y. Ng and E. Mahmoudi, A review of carbon quantum dots and their applications in wastewater treatment, Adv. Colloid Interface Sci., 2020, 278, 102124 CrossRef PubMed.
- V. K. Sharma, T. J. McDonald, M. Sohn, G. A. K. Anquandah, M. Pettine and R. Zboril, Assessment of toxicity of selenium and cadmium selenium quantum dots: A review, Chemosphere, 2017, 188, 403–413 CrossRef CAS PubMed.
- S. N. Baker and G. A. Baker, Luminescent Carbon Nanodots: Emergent Nanolights, Angew. Chem., Int. Ed., 2010, 49, 6726–6744 CrossRef CAS PubMed.
- M. Havrdova, K. Hola, J. Skopalik, K. Tomankova, M. Petr, K. Cepe, K. Polakova, J. Tucek, A. B. Bourlinos and R. Zboril, Toxicity of carbon dots – Effect of surface functionalization on the cell viability, reactive oxygen species generation and cell cycle, Carbon, 2016, 99, 238–248 CrossRef CAS.
- X. Tian and X. Yin, Carbon Dots, Unconventional Preparation Strategies, and Applications Beyond Photoluminescence, Small, 2019, 15, 1901803 CrossRef CAS PubMed.
- J. Pardo, Z. Peng and R. Leblanc, Cancer Targeting and Drug Delivery Using Carbon-Based Quantum Dots and Nanotubes, Molecules, 2018, 23, 378 CrossRef PubMed.
- M. Farshbaf, S. Davaran, F. Rahimi, N. Annabi, R. Salehi and A. Akbarzadeh, Carbon quantum dots: recent progresses on synthesis, surface modification and applications, Artif. Cells, Nanomed., Biotechnol., 2018, 46, 1331–1348 CrossRef CAS PubMed.
- H. Wang, J. Bi, B.-W. Zhu and M. Tan, Multicolorful Carbon Dots for Tumor Theranostics, Curr. Med. Chem., 2018, 25, 2894–2909 CrossRef CAS PubMed.
- A. Sciortino, A. Cannizzo and F. Messina, Carbon Nanodots: A Review—From the Current Understanding of the Fundamental Photophysics to the Full Control of the Optical Response, C, 2018, 4, 67 CAS.
- F. Yuan, T. Yuan, L. Sui, Z. Wang, Z. Xi, Y. Li, X. Li, L. Fan, Z. Tan, A. Chen, M. Jin and S. Yang, Engineering triangular carbon quantum dots with unprecedented narrow bandwidth emission for multicolored LEDs, Nat. Commun., 2018, 9, 2249 CrossRef PubMed.
- W. Yang, F. Liu, R. Li, X. Wang and W. Hao, Multiple Stimuli-Responsive Fluorescent Sensor from Citric Acid and 1-(2-Aminoethyl)piperazine, ACS Appl. Mater. Interfaces, 2018, 10, 9123–9128 CrossRef CAS PubMed.
- S. Zhu, Y. Song, X. Zhao, J. Shao, J. Zhang and B. Yang, The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): current state and future perspective, Nano Res., 2015, 8, 355–381 CrossRef CAS.
- B. Zhi, X. Yao, Y. Cui, G. Orr and C. L. Haynes, Synthesis, applications and potential photoluminescence mechanism of spectrally tunable carbon dots, Nanoscale, 2019, 11, 20411–20428 RSC.
- J. Zhang, X. Zhao, M. Xian, C. Dong and S. Shuang, Folic acid-conjugated green luminescent carbon dots as a nanoprobe for identifying folate receptor-positive cancer cells, Talanta, 2018, 183, 39–47 CrossRef CAS PubMed.
- H. Saljoughi, F. Khakbaz and M. Mahani, Synthesis of folic acid conjugated photoluminescent carbon quantum dots with ultrahigh quantum yield for targeted cancer cell fluorescence imaging, Photodiagn. Photodyn. Ther., 2020, 30, 101687 CrossRef CAS PubMed.
- S. Feng, J. Pan, C. Li and Y. Zheng, Folic acid-conjugated nitrogen-doped graphene quantum dots as a fluorescent diagnostic material for MCF-7 cells, Nanotechnology, 2020, 31, 135701 CrossRef CAS PubMed.
- A. Mewada, S. Pandey, M. Thakur, D. Jadhav and M. Sharon, Swarming carbon dots for folic acid mediated delivery of doxorubicin and biological imaging, J. Mater. Chem. B, 2014, 2, 698–705 RSC.
- R. Lv, G. Li, S. Lu and T. Wang, Synthesis of Multi-Functional Carbon Quantum Dots for Targeted Antitumor Therapy, J. Fluoresc., 2021, 31, 339–348 CrossRef CAS PubMed.
- B. Yang, M. Wu, S. Pang, D. Li, Y. Yang, L. Wang, Z. Li, J. Zhang and X. Yang, One-pot synthesis of folic acid modified carbonized polymer dots with red emittision for selective imaging of cancer cells, Nanotechnology, 2020, 31, 475501 CrossRef CAS PubMed.
- S. Kadian, G. Manik, N. Das and P. Roy, Targeted bioimaging and sensing of folate receptor-positive cancer cells using folic acid-conjugated sulfur-doped graphene quantum dots, Microchim. Acta, 2020, 187, 458 CrossRef CAS PubMed.
- P. Sarkar, S. Ghosh and K. Sarkar, Folic acid based carbon dot functionalized stearic acid-g-polyethyleneimine amphiphilic nanomicelle: Targeted drug delivery and imaging for triple negative breast cancer, Colloids Surf., B, 2021, 197, 111382 CrossRef CAS PubMed.
- A. Nasrin, M. Hassan and V. G. Gomes, Two-photon active nucleus-targeting carbon dots: enhanced ROS generation and photodynamic therapy for oral cancer, Nanoscale, 2020, 12, 20598–20603 RSC.
- R. V. Goreham, K. L. Schroeder, A. Holmes, S. J. Bradley and T. Nann, Demonstration of the lack of cytotoxicity of unmodified and folic acid modified graphene oxide quantum dots, and their application to fluorescence lifetime imaging of HaCaT cells, Microchim. Acta, 2018, 185, 128 CrossRef PubMed.
- R. I. Pinhassi, Y. G. Assaraf, S. Farber, M. Stark, D. Ickowicz, S. Drori, A. J. Domb and Y. D. Livney, Arabinogalactan−Folic Acid−Drug Conjugate for Targeted Delivery and Target-Activated Release of Anticancer Drugs to Folate Receptor-Overexpressing Cells, Biomacromolecules, 2010, 11, 294–303 CrossRef CAS PubMed.
- Y. Sun, Y. Zhao, S. Teng, F. Hao, H. Zhang, F. Meng, X. Zhao, X. Zheng, Y. Bi, Y. Yao, R. J. Lee and L. Teng, Folic acid receptor-targeted human serum albumin nanoparticle formulation of cabazitaxel for tumor therapy, Int. J. Nanomed., 2018, 14, 135–148 CrossRef PubMed.
- A. Narmani, M. Rezvani, B. Farhood, P. Darkhor, J. Mohammadnejad, B. Amini, S. Refahi and N. Abdi Goushbolagh, Folic acid functionalized nanoparticles as pharmaceutical carriers in drug delivery systems, Drug Dev. Res., 2019, 80, 404–424 CrossRef CAS PubMed.
- O. Yücel, A. Sengelen, S. Emik, E. Önay-Uçar, N. Arda and G. Gürdağ, Folic acid-modified methotrexate-conjugated gold nanoparticles as nano-sized trojans for drug delivery to folate receptor-positive cancer cells, Nanotechnology, 2020, 31, 355101 CrossRef PubMed.
- K. Butzbach, M. Konhäuser, M. Fach, D. Bamberger, B. Breitenbach, B. Epe and P. Wich, Receptor-mediated Uptake of Folic Acid-functionalized Dextran Nanoparticles for Applications in Photodynamic Therapy, Polymers, 2019, 11, 896 CrossRef CAS PubMed.
- W. Guan, W. Gu, L. Ye, C. Guo, S. Su, P. Xu and M. Xue, Microwave-assisted polyol synthesis of carbon nitride dots from folic acid for cell imaging, Int. J. Nanomed., 2014, 9, 5071–5078 Search PubMed.
- H. Liu, Z. Li, Y. Sun, X. Geng, Y. Hu, H. Meng, J. Ge and L. Qu, Synthesis of Luminescent Carbon Dots with Ultrahigh Quantum Yield and Inherent Folate Receptor-Positive Cancer Cell Targetability, Sci. Rep., 2018, 8, 1086 CrossRef PubMed.
- M. Z. Fahmi, N. F. Sholihah, A. Wibrianto, S. C. W. Sakti, F. Firdaus and J. Chang, Simple and fast design of folic acid-based carbon dots as theranostic agent and its drug release aspect, Mater. Chem. Phys., 2021, 267, 124596 CrossRef CAS.
- S. K. Bhunia, A. R. Maity, S. Nandi, D. Stepensky and R. Jelinek, Imaging Cancer Cells Expressing the Folate Receptor with Carbon Dots Produced from Folic Acid, ChemBioChem, 2016, 17, 614–619 CrossRef CAS PubMed.
- L. Nygren-Babol and M. Jägerstad, Folate-Binding Protein in Milk: A Review of Biochemistry, Physiology, and Analytical Methods, Food Sci. Nutr., 2012, 52, 410–425 CAS.
- G. M. Tavares, T. Croguennec, S. Lê, O. Lerideau, P. Hamon, A. F. Carvalho and S. Bouhallab, Binding of Folic Acid Induces Specific Self-Aggregation of Lactoferrin: Thermodynamic Characterization, Langmuir, 2015, 31, 12481–12488 CrossRef CAS PubMed.
- I. Aprodu, L. Dumitrascu, G. Râpeanu, G.-E. Bahrim and N. Stănciuc, Spectroscopic and Molecular Modeling Investigation on the Interaction between Folic Acid and Bovine Lactoferrin from Encapsulation Perspectives, Foods, 2020, 9, 744 CrossRef CAS PubMed.
- M. J. Turk, G. J. Breur, W. R. Widmer, C. M. Paulos, L.-C. Xu, L. A. Grote and P. S. Low, Folate-targeted imaging of activated macrophages in rats with adjuvant-induced arthritis, Arthritis Rheum., 2002, 46, 1947–1955 CrossRef PubMed.
- H. Gary, Folate-binding proteins, Annu. Rev. Nutr., 1990, 10, 319 CrossRef PubMed.
- J. Holm, S. I. Hansen and M. Høier-Madsen, Ionic Charge, Hydrophobicity and Tryptophan Fluorescence of the Folate Binding Protein Isolated from Cow's Milk, Biosci. Rep., 2001, 21, 305–313 CrossRef CAS PubMed.
- I. Svendsen, S. I. Hansen, J. Holm and J. Lyngbye, The complete amino acid sequence of the folate-binding protein from cow's milk, Carlsberg Res. Commun., 1984, 49, 123–131 CrossRef CAS.
- L. Nygren-Babol, Å. Sternesjö, M. Jägerstad and L. Björck, Affinity and Rate Constants for Interactions of Bovine Folate-Binding Protein and Folate Derivatives Determined by Optical Biosensor Technology. Effect of Stereoselectivity, J. Agric. Food Chem., 2005, 53, 5473–5478 CrossRef CAS PubMed.
- S. Deshayes and G. Divita, Fluorescence Technologies for Monitoring Interactions Between Biological Molecules In Vitro, Prog. Mol. Biol. Transl. Sci., 2013, 113, 109–143 CAS.
- P. Hou, T. Yang, H. Liu, Y. F. Li and C. Z. Huang, An active structure preservation method for developing functional graphitic carbon dots as an effective antibacterial agent and a sensitive pH and Al(III) nanosensor, Nanoscale, 2017, 9, 17334–17341 RSC.
- G.-M. Isui Abril, C. Tania Siqueiros, A.-G. Sigifredo and R.-C. Quintín, Lactoferrin a multiple bioactive protein: an overview, Biochim. Biophys. Acta, 2012, 1820, 226–236 CrossRef PubMed.
- D. Prozeller, S. Morsbach and K. Landfester, Isothermal titration calorimetry as a complementary method for investigating nanoparticle–protein interactions, Nanoscale, 2019, 11, 19265–19273 RSC.
- S. Mandal, M. Hossain, P. S. Devi, G. S. Kumar and K. Chaudhuri, Interaction of carbon nanoparticles to serum albumin: elucidation of the extent of perturbation of serum albumin conformations and thermodynamical parameters, J. Hazard. Mater., 2013, 248–249, 238–245 CrossRef CAS PubMed.
- R. Huang and B. L. T. Lau, Biomolecule–nanoparticle interactions: Elucidation of the thermodynamics by isothermal titration calorimetry, Biochim. Biophys. Acta, Gen. Subj., 1860, 2016, 945–956 Search PubMed.
- Y. Jin, Q. Zhang, X. Qin, Z. Liu, Z. Li, X. Zhong, L. Xia, J. He and B. Fang, Carbon dots derived from folic acid attenuates osteoarthritis by protecting chondrocytes through NF-κB/MAPK pathway and reprogramming macrophages, J. Nanobiotechnol., 2022, 20, 469 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cp01277h |
| This journal is © the Owner Societies 2023 |