Open Access Article
Open Access ArticleOxidation and phase transfer of individual Cr-doped dendritic FeOx particles visualized by full-field nano-XAFS spectroimaging†
Nozomu
Ishiguro
 *abc,
Hirosuke
Matsui
c,
Kohei
Wakamatsu
c,
Yoya
Suzuki
c,
Oki
Sekizawa
d,
Kiyofumi
Nitta
d,
Yasuko
Terada
d,
Tomoya
Uruga
d and
Mizuki
Tada
*ac
*abc,
Hirosuke
Matsui
c,
Kohei
Wakamatsu
c,
Yoya
Suzuki
c,
Oki
Sekizawa
d,
Kiyofumi
Nitta
d,
Yasuko
Terada
d,
Tomoya
Uruga
d and
Mizuki
Tada
*ac
aElement Visualization Team, Materials Visualization Photon Science Group, RIKEN SPring-8 Center, 1-1-1 Kouto, Sayo, Hyogo 679-5148, Japan
bInternational Center for Synchrotron Radiation Innovation Smart (SRIS)/Institute of Multidisciplinary Research for Advanced Materials (IMRAM), Tohoku University, Katahira 2-1-1, Aoba-ku, Sendai 980-8577, Japan. E-mail: nozomu.ishiguro.c1@tohoku.ac.jp; Tel: +81-22-217-5177
cDepartment of Chemistry, Graduate School of Science/Research Center for Materials Science (RCMS)/Integrated Research Consortium on Chemical Sciences (IRCCS)/Institute for Advanced Study, Nagoya University, Furuuchi, Chikusa-ku, Nagoya, Aichi 464-8602, Japan. E-mail: tada.mizuki.u6@f.mail.nagoya-u.ac.jp; Tel: +81-52-788-6200
dJapan Synchrotron Radiation Research Institute, SPring-8, 1-1-1 Kouto, Sayo, Hyogo 679-5198, Japan
First published on 2nd June 2023
Abstract
Iron oxides with various compositions and polymorphs have been widely used as compounds that require reversible redox properties, such as catalysts. However, partial decomposition during phase transitions often causes irreversible degradation of the redox properties of iron oxides. Cr doping into the crystalline framework of iron oxide dendrites improves the stability of the structural transformation of iron oxides. We spatially visualized the FeOx-dendrite phase distribution during oxidation in crystalline dendritic FeOx and Cr-FeOx particles by full-field nano-X-ray absorption fine structure spectroimaging. The spectroimaging visualized propagation in the phase transitions in the individual FeOx particles and changes in the phase transition behaviors of the Cr-FeOx particles. The statistical analysis of the spectroimaging data revealed the phase transition trends in parts of the FeOx and Cr-FeOx particles in three Fe density zones (particle thicknesses) and the probability densities of the phase proportions in the dendrites.
1 Introduction
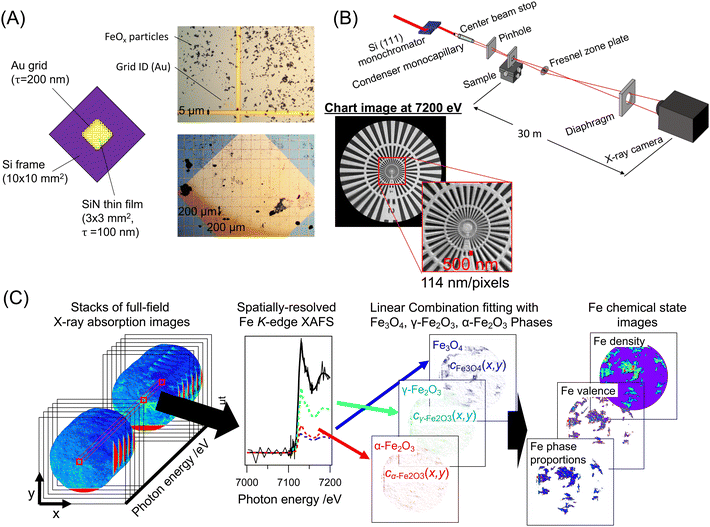
The redox behaviors of solid oxides with oxygen storage and release properties enable these materials to be widely used as cocatalysts in various catalytic systems, such as three-way automobile exhaustion and oxidation. Iron oxides with various stoichiometric compositions and polymorphs show unique chemical and physical properties derived from their crystalline structures. γ-Fe2O3 (maghemite) and Fe3O4 (magnetite) have similar spinel crystalline structures with octahedral Fe sites (Fe2+ and Fe3+) and tetrahedral Fe sites (Fe3+). The topotactic transformation between Fe3O4 and γ-Fe2O3 provides catalytic functions,1 but the low thermal stability of the active γ-Fe2O3 phase results in a phase transition to the corundum α-Fe2O3 phase with an octahedral Fe site.2Many iron oxide particle morphologies have been reported, including nanorods,1,3 nanocubes,4 nanoflowers,5 and dendrites.6 Doping with transition metals, such as Cr,7–9 Co,7,10,11 Ce,7–10 and Ni,7,10,12 increases the thermal stability of the spinel FeOx phases; in particular, Cr-doped iron oxides have been extensively investigated for catalysis.13–16 We have prepared dendritic FeOx and Cr-doped FeOx (Cr-FeOx) and characterized the local structures and structural transformations of the Cr-FeOx dendrites by in situ Fe K-edge X-ray absorption fine structure (XAFS) and X-ray diffraction.16 The Cr doping compressed the lattice strain in the iron oxide spinel structures and extended the redox reaction window of spinel γ-Fe2O3 by suppressing the structural transformation to α-Fe2O3.16 However, the real-space distribution and propagation of the iron oxide phases in individual crystal particles with the same morphological features of redox structural transformation as for iron dendrites are still unclear.
Combination of X-ray imaging techniques, such as scanning transmission/fluorescence X-ray microscopy (STXM/SFXM),17–23 full-field transmission X-ray microscopy (FF-TXM),24–34 coherent X-ray diffraction imaging (CXDI) including X-ray ptychography,35–42 with X-ray absorption spectroscopy, has been employed to visualize the chemical state distribution in materials.43 STXM/SFXM or scanning nano-XAFS uses X-ray beams focused by a Fresnel zone plate (FZP) or Kirkpatrick–Baez mirrors on the submicro to nanoscale to obtain X-ray transmission/fluorescence and chemical state images of materials. The resolution is restricted by the specifications of the optical elements, and there is a trade-off between the field-of-view size and the total measurement time.17–20 Coherent X-ray diffraction imaging techniques, such as X-ray ptychography, have also been developed, in which computational image reconstruction replaces the optical lens to achieve high spatial resolution.35–42 FF-TXM techniques, such as projection XAFS imaging, achieve full-field chemical state visualization by irradiating the sample with a large X-ray beam and detecting the transmitted X-rays with an X-ray camera. Real-space images with a wide field-of-view can be visualized directly in real space. However, the resolution is limited to X-ray cameras with a micrometer-order pixel size and the technique is used for visualizing large device samples.25
Here, we report on the visualization of the spatial distribution of FeOx phases in dendritic FeOx single-crystal particles by full-field nano-XAFS imaging with 50 nm spatial resolution. The FF-TXM images using an FZP at the Fe K-edge were recorded for the energy of the Fe K-edge XANES region and the two-dimensional (2D) images of the Fe density, Fe oxidation state, and FeOx phase proportion were obtained for the oxidation of dendritic Cr-FeOx crystal particles. The spatially resolved Fe K-edge XANES spectra showed the distribution of chemical states and phase proportions in a number of single particles in the samples simultaneously, with a statistical amount of data that enabled us to investigate the reactions in the heterogeneous oxide particles.
2 Experimental
2.1 Sample preparation
Dendritic FeOx crystal particles were synthesized by the reported hydrothermal reaction of K4[Fe(CN)6],6,16 and dendritic Cr-FeOx particles containing 10 mol% (Cr/(Cr+Fe)) Cr were also synthesized by a similar hydrothermal reaction from K2Cr2O7 and K3[Fe(CN)6], as reported in our previous paper.16 The as-prepared dendritic FeOx or 10 wt% Cr-FeOx was dispersed in ethanol (Wako Chemicals) by sonication. The dispersion was dropped onto a SiN membrane (NTT-AT; 3 × 3 mm, 100 nm thick) with an Au grid printed by lithography (thickness 200 nm), and then the sample was dried in air (Fig. 1B). The Au grid was used as the marker of the correction of the chromatic aberration of the XAFS absorption images. The SiN membranes with the FeOx crystal particles were observed with an optical microscope to replicate the desired view sight in the full-field XAFS imaging measurements. The as-reduced FeOx and 10 wt% Cr-FeOx samples were first reduced under a H2 flow (99.99%) of 100 mL min−1, heated from 293 to 483 K at 3 K min−1, and then cooled under a He flow (99.999%). The as-reduced FeOx and Cr-FeOx crystal particles on the SiN membranes were oxidized from room temperature to 423, 473, 523, 573, 603, 623, 653, or 673 K at 3 K min−1 under an O2 flow (99.9%) of 100 mL min−1. After the sample reached the target temperature, the reaction was quenched to room temperature under a He flow.2.2 Full-field nano-XAFS imaging measurements
Full-field nano-XAFS imaging measurements were carried out at the BL32B2 or BL37XU beamlines at SPring-8 (Hyogo, Japan; 8.0 GeV, 100 mA) (Fig. 1A). X-rays emitted from either bending magnets (BL32B2) or an in-vacuum undulator (BL37XU) were monochromatized by a Si(111) double-crystal monochromator. Higher harmonics were rejected by two vertical mirrors (BL32B2) or horizontal mirrors (BL37XU) placed at the downstream monochromator at an angle of 4 mrad. The monochromatized X-rays were first focused to approximately 100 μm at the focal point by using two pinholes, a center beam stop, and a quartz glass monocapillary. The sample was placed at the focal point and the transmitted X-rays passed through a FZP (Applied Nanotools Inc.; 300 μm diameter, 50 nm outer ring width, 1 μm-thick Au zone). The X-rays diffracted by the FZP formed the image downstream at the X-ray CMOS camera (ORCA-flash 4.0v3, Hamamatsu Photonics) equipped with a gadolinium aluminium gallium garnet (GAGG) scintillator and optical lens, or a SOPHIAS direct-detection X-ray SOI-CMOS camera.44 The distances from the sample to the FZP (a) and that from the FZP to camera (b) were related to the focal length of the FZP (f) by the lens equation, | (1) |
The distance from the sample to the camera, (a + b), was constant (2 m for the BL32B2 system and 23 m for the BL37XU system), whereas the focal length of the FZP (f = 85.9 mm @7.1 keV) depended on the X-ray energy. Therefore, the position of the FZP (XYZ) was synchronously adjusted at every energy point with the monochromator by using feedback stages according to the calibration line initially determined by using an X-ray chart (XRESO-50HC, NTT-AT). Full-field nano-XAFS imaging at the Fe K-edge was performed at 150 energy points at 7.0–7.2 keV At each X-ray energy (E), the I0(x,y,E) image with the sample offline and the I1(x,y,E) image with the sample online were recorded with an exposure time of 10 s (Details are given in Table S1, ESI†). To prevent the deterioration of absorption image quality due to instability of the illumination system and the drift of FZP, It and I0 images for each X-ray energy were taken at adjacent time intervals. Using the dark image, Idark(x,y), recorded without X-ray irradiation, the I0(x,y,E) and I1(x,y,E) images were converted to the absorption images, μt(x,y,E), by
 | (2) |
The X-ray absorption of the thin SiN membrane was found to be negligible. To correct the sample drift and magnification rate changes caused by chromatic aberration of the FZP, the μt(x,y,E) image stacks were processed using laboratory-made image registration software.25 Although the contrast of the X-ray absorption of the FeOx and Cr-FeOx particles was changed dynamically by the energy at the Fe K-edge, the absorption of the Au grid on the SiN membranes was almost constant and was used as a reference for the image registration processing. The spatial resolution (pixel resolution) of the μt(x,y,E) images was estimated to be 114 nm/pixel at 7.1 keV in the BL37XU system.
2.3 Analysis of full-field nano-XAFS spectra
Each pixel in the corrected μt(x,y,E) image stacks represents the spatially resolved Fe K-edge XAFS (XANES) spectra. Fe K-edge XANES spectra of dendritic FeOx and Cr-FeOx crystal particles were curve-fitted with a linear combination of background line and reference XANES spectra of the Fe3O4, γ-Fe2O3, and α-Fe2O3 phases (Fig. S1, ESI†). The validity of the linear combination curve-fitting analysis for the three components was investigated using the principal component analysis (PCA) and the multivariate curve resolution alternating least squares (MCR-ALS), as described in Fig. S2,ESI.† From each μt(x,y,E) pixel, the coefficients cFe3O4(x,y), cγ-Fe2O3(x,y), and cα-Fe2O3(x,y) of Fe3O4, γ-Fe2O3, and α-Fe2O3 were estimated and the 2D plots of the coefficients show the 2D maps of the three FeOx phases in the sample (Fig. 1C). Parameters for the Fe chemical states, Fe density ρ(x,y), Fe valence v(x,y), and proportions of the Fe3O4, γ-Fe2O3, and α-Fe2O3 phases, ηFe3O4(x,y), ηγ-Fe2O3(x,y), and ηα-Fe2O3(x,y), respectively, were converted from the obtained cFe3O4(x,y), cγ-Fe2O3(x,y), and cα-Fe2O3(x,y) values (details are provided in Scheme S1, ESI†).2.4 Probability density analysis of Fe phase proportion in dendritic FeOx and Cr–FeOx crystal particles
To investigate the Fe phase proportions in FeOx and Cr–FeOx particles, probability densities were estimated from full-field nano-XAFS imaging data by using the kernel density estimation technique. Linear curve fitting analysis result, μi = (cFe3O4,icγ-Fe2O3,i, cα-Fe2O3,i) = (ηFe3O4,i, ηγ-Fe2O3,i, ηα-Fe2O3,i)ρi = ηiρi for every pixel in the nano-XAFS imaging data was used as an independent and uniformly distributed sample. Kernel pi(μ), expressing the probability density of μ for i-th sample when μi is observed, is calculated using a Gaussian distribution as | (3) |
 | (4) |
 | (5) |
The details of the analyses are summarized in Scheme S1 (ESI†).
3 Results and discussion
3.1 Visualization of Fe chemical states and phase changes in dendritic FeOx and Cr–FeOx particles during oxidation
The as-prepared dendritic FeOx and Cr–FeOx particles consisting of the α-Fe2O3 phase16 were reduced with H2 at temperatures from room temperature to 483 K to obtain the as-reduced samples. Fig. S3A–C (ESI†) show full-field X-ray absorption images at 7.130 keV of dendritic FeOx and Cr–FeOx on a SiN membrane with Au grids and Fe K-edge XANES spectra of all FeOx or Cr–FeOx particles in the full-field image. The linear combination curve-fitting analysis of the Fe K-edge XANES spectra showed that as-reduced FeOx and Cr–FeOx consisted of the Fe3O4 phase (Fig. S3B1 and D1, ESI†). The Fe K-edge XANES spectra of FeOx after oxidation at 473 and 573 K showed that the main phase was γ-Fe2O3, although unreacted Fe3O4 and overreacted α-Fe2O3 phases were present (Fig. S3B2 and B3, ESI†). After oxidation at 623 K, the Fe3O4 phase was consumed and the α-Fe2O3 phase was formed in the FeOx sample without Cr (Fig. S3B4, ESI†).In contrast, Cr–FeOx particles containing 10 wt% Cr showed different oxidation behaviors and the γ-Fe2O3 phase was selectively formed after oxidation at 473 and 573 K. The γ-Fe2O3 phase remained even after oxidation at 673 K and the formation of the α-Fe2O3 phase was negligible (Fig. S3D2–D4, ESI†). The oxidation temperature profiles of the Fe valence and the FeOx phase proportions (Fig. 2) showed that the Cr–FeOx particles were oxidized from Fe3O4 to γ-Fe2O3 at a slightly lower temperature than the FeOx particles without Cr, indicating that the stability of the γ-Fe2O3 phase was higher than in the FeOx particles.
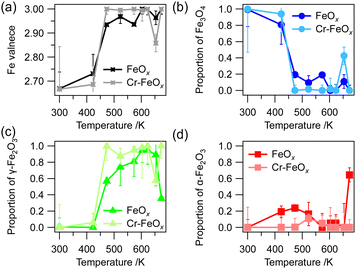 | ||
| Fig. 2 Oxidation temperature profiles of (a) Fe valence and Fe phase proportions of (b) Fe3O4, (c) γ-Fe2O3, and (d) α-Fe2O3, obtained from the integration of full-field XAFS spectra in the particle regions for dendritic FeOx and dendritic 10 wt% Cr–FeOx in Fig. S3 (ESI†). | ||
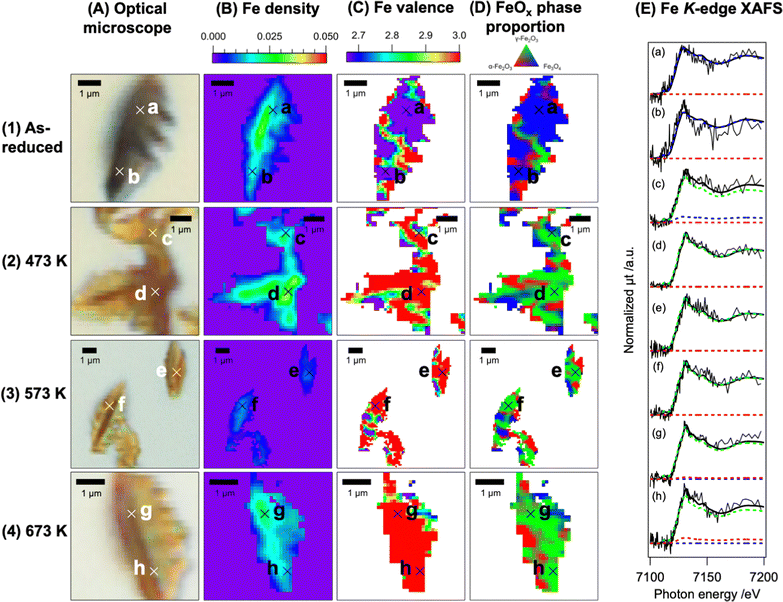
Fig. 3 and 4 show the full-field XAFS images of the dendritic FeOx and Cr–FeOx particles, revealing the structures and reaction trends in the particles. We obtained optical microscopy images (A) and plotted the Fe density images (B), Fe valence images (C), and Fe phase proportion images of the FeOx and Cr–FeOx particles before and after oxidation at different temperatures from linear combination curve fitting of the spatially resolved Fe K-edge XAFS at a resolution of approximately 100 nm. The Fe density images were consistent with the optical microscopy images, suggesting that the full-field X-ray imaging captured the structures of the dendritic particles. The FeOx and Cr–FeOx particles both showed contrasts in the Fe density image, in which the leaf-vein-like stem of the particles had a high Fe density surrounded by lower Fe density regions (Fig. 3B and C).
The 2D mapping images indicated a non-uniform distribution of the Fe valence and Fe phase proportion in the dendritic particles, although their averages followed the trends observed in the Fe K-edge XAFS spectra. A large area of the Fe valence map of the as-reduced FeOx particles was Fe2.67+ (purple) and the phase proportion map showed the Fe3O4 phase (blue), whereas there were oxidized spots of the α-Fe2O3 and γ-Fe2O3 phases, shown as Fe3+ (red) (Fig. 3C1 and D1). After oxidation at 473 K, there was an increase in the oxidized regions, and the oxidation was almost complete at 573 K, where Fe3+ (red) was the main valence state (Fig. 3C2 and C3). Accompanying the changes in the Fe valence state in the dendritic particles, the phase proportion of the particles also changed from Fe3O4 (blue) to γ-Fe2O3 (green) (Fig. 3D2 and D3). After the reaction at 673 K, the phase proportion image suggested a phase transition to α-Fe2O3 in some parts of the particles (red regions in Fig. 3D4).
For dendritic 10 wt% Cr–FeOx, the main region in the Fe density map of the as-reduced sample particles was also Fe2.67+ (purple) and the blue zone in the phase proportion map was assigned to the Fe3O4 phase (Fig. 4C1 and D1), similar to dendritic FeOx without Cr. After oxidation at 473 K, the majority of the Fe valence image was Fe3+ (red) and the phase proportion image suggested the formation of γ-Fe2O3 (green) (Fig. 4C2 and D2). Similar trends were observed for the sample oxidized at 573 K (Fig. 4C3 and D3). The phase transition to α-Fe2O3 was negligible for 10 wt% Cr–FeOx oxidized at 673 K (Fig. 4D4), indicating that Cr–FeOx showed stability against the phase transition to α-Fe2O3 compared with FeOx without Cr. The full-field XAFS images also showed that there were small domains of partially non-uniform Fe valence or Fe phase proportion in the dendritic particles although the majorities of the pixels in the images behaved similarly to the average of the images or the powder assemblies.
3.2 Statistical analysis of FeOx phase proportion trends for the oxidation of dendritic FeOx and Cr–FeOx particles
The full-field XAFS imaging clearly visualized the changes in the FeOx phases after the oxidation of the dendritic FeOx and Cr–FeOx particles and there were differences in the phase proportion between the FeOx and Cr–FeOx particles (Fig. 3 and 4). We performed a statistical analysis of the phase proportions of the FeOx and Cr–FeOx particles by using the 2D XAFS imaging data (Fig. 5 and 6). First, the probability density P(η) of the phase proportion coordinates, η = (ηFe3O4, ηγ-Fe2O3, ηα-Fe2O3), of the FeOx and Cr–FeOx particles was statistically estimated in all effective pixels in the full-field nano-XAFS imaging data by using the kernel density estimation method. The estimated probability densities for the FeOx and Cr–FeOx particles are shown in Fig. 5A and 6A, respectively, in which the probability densities of the phases in the FeOx and Cr–FeOx particles are plotted on ternary diagrams. If P(η) is higher in the right, top, or left corners of the ternary diagram, the Fe3O4, γ-Fe2O3, and α-Fe2O3 phases, respectively, tend to be distributed stochastically in the particle. If P(ηmax) is large and the density distribution is dense, the non-uniformity is small.Table S2 (ESI†) shows the maximum probability densities in the ternary diagrams in Fig. 5 and 6. For the as-reduced FeOx particles, the probability density reached a maximum (P(ηmax) = 7.462) at ηmax = (96%, 0%, and 4%), in the right corner of the ternary diagram (Fig. 5A1). After oxidation at 473 K, the phase density median moved to the top corner (γ-Fe2O3), where P(ηmax) = 2.423 at ηmax = (21%, 68%, and 11%), and the density spread in the (Fe3O4–γ-Fe2O3) direction (Fig. 5A2). This broad distribution indicated that the oxidation from Fe3O4 to γ-Fe2O3 was incomplete. After oxidation at 573 K, where the maximum probability density was almost steady (P(ηmax) = 4.011 at ηmax = (6%, 89%, and 5%)), the probability density spread in the (γ-Fe2O3–α-Fe2O3) direction. This change in direction indicated that the oxidation from Fe3O4 to γ-Fe2O3 was almost complete, and the phase transformation from γ-Fe2O3 to α-Fe2O3 had started (Fig. 5A3). Finally, after oxidation at 673 K, the maximum density moved to near the left corner (α-Fe2O3 side), where P(ηmax) = 2.423 at ηmax = (7%, 28%, and 65%), and the probability density still spread in the (γ-Fe2O3–α-Fe2O3) direction (Fig. 5A4).
For the Cr–FeOx particles, the maximum point for the as-reduced conditions was also in the right corner (Fe3O4), where P(ηmax) = 5.031 at ηmax = (95%, 5%, and 0%) (Fig. 6A1). The Cr–FeOx particles also started to oxidize at 473 K (P(ηmax) = 2.883 at ηmax = (14%, 81%, and 5%)) (Fig. 6A2), and oxidation was complete at 573 K (P(ηmax) = 3.519 at ηmax = (9%, 85%, and 6%)) (Fig. 5A3). Fig. 6A2 and A3 show the probability density spreading in the (Fe3O4−γ-Fe2O3) and (γ-Fe2O3–α-Fe2O3) directions, respectively, similar to the FeOx particles, indicating smooth oxidation to γ-Fe2O3 and a slow phase transformation to α-Fe2O3. The greatest difference between the FeOx and Cr–FeOx particles was observed at 673 K, where the maximum probability density point was near γ-Fe2O3 (P(ηmax) = 4.332 at ηmax = (4%, 88%, and 8%)) and the phase transformation to α-Fe2O3 had not started in the Cr–FeOx particles (Fig. 6A4).
We prepared Fe density histograms of the particles (Fig. 5B and 6B) and classified the histograms into Fe density zones of (i) thin, (ii) medium, and (iii) thick. The posterior probability density of the FeOx phase proportions with pixels belonging to each zone (i)–(iii) was plotted (Fig. 5C and 6C), and the Fe phase proportion belonging to each of zones (i)–(iii) was also imaged (Fig. 5D and 6D). The ternary diagrams of the posterior probability density for zones (i)–(iii) showed distinctive trends in probability density for the phase proportion in the FeOx and Cr–FeOx particles.
The posterior probability densities in zone (i), corresponding to regions that had lower Fe density, showed smaller differences in probability density for both FeOx and Cr–FeOx. The maximum densities were nearer to the left corner (α-Fe2O3) than in the full-field ternary diagram (Fig. 5Ci and 6Ci), regardless of the reaction conditions. The lower contrast in the probability density ternary diagrams of zone (i) indicated that the proportion of either type of Fe phase was uncertain. These pixels assigned to zone (i) were mainly at the edge sites of the particles (Fig. 5Di and 6Di).
The ternary diagrams of zone (iii) with higher Fe density showed different behaviors from those of zone (i) for oxidation (Fig. 5C-iii). Most of the as-reduced FeOx and Cr–FeOx particles consisted of Fe3O4 (Fig. 5D1-iii and 6D1-iii). After oxidation at 473 K, the red regions of the ternary diagrams were shifted to γ-Fe2O3 from Fe3O4 (Fig. 5C2-iii and 6C2-iii), and the shift in Cr–FeOx was clearer than that in FeOx. Then, after oxidation at 573 K, the ternary diagrams shows that the γ-Fe2O3 phase became dominant (Fig. 5D3-iii and 6D3-iii). There were large differences in the phase proportions in the FeOx and Cr–FeOx particles in the ternary diagrams after oxidation at 673 K (Fig. 5C4-iii and 6C4-iii). The red region with a high probability density zone (iii) in the Cr–FeOx particles was localized at the top (γ-Fe2O3). In contrast, the corresponding region for the FeOx particles was widely distributed between γ-Fe2O3 and α-Fe2O3, and was not localized at the corners of γ-Fe2O3 and α-Fe2O3. These results indicate that the FeOx transition proceeded across the whole of the dendritic particles. Similar trends were observed in zone (ii) with the medium Fe density.
The ternary diagrams of the Fe phase proportion for Fe density zones (i)–(iii) revealed the following trends in the reactivity and phase transition of the dendritic FeOx and Cr–FeOx particles during oxidation.
(1) The α-Fe2O3 phase tended to be produced in the thinner parts (zone (i)) of dendritic FeOx and Cr–FeOx. These parts were mainly located on the outer surface of the dendritic particles, which has low crystallinity compared with the bulk, and these parts showed different behaviors compared with zones (ii) and (iii).
(2) Two modes of oxidation were observed in the probability density diagrams: the Fe3O4 → γ-Fe2O3 transition (from the right corner to the top corner in the diagram) and the γ-Fe2O3 → α-Fe2O3 transition (from the top corner to the left corner). The XAFS analysis of the powder assemblies of the FeOx and Cr–FeOx dendrites suggested the negligible formation of α-Fe2O3 below 550 K, but spatial imaging by full-field imaging XAFS suggested a fine distribution of the minor phases in each dendritic particle, suggesting that the two oxidation modes coexisted during thermal oxidation. The local phase transition to the α-Fe2O3 would structurally interfere with smooth redox propagation between the Fe3O4 and γ-Fe2O3 phases, which tends to create a non-uniform phase distribution within the particles.
(3) The Cr doping in the Cr–FeOx particles led to the appearance of a thick body of dendrites and the strong suppression of the second phase transition from γ-Fe2O3 to α-Fe2O3 was clearly observed in the Cr–FeOx dendrites.
These results demonstrated that the Cr doping in the Cr–FeOx dendrites increased the reactivity for the first oxidation of Fe3O4 → γ-Fe2O3 and improved the stability against the phase transformation to the α-Fe2O3 phase in the body of the dendritic particles. Full-field XAFS chemical imaging revealed the heterogeneous distribution of the structure, oxide phases, and reactive pathways of actual oxide particles.
Conclusions
Full-field XAFS imaging visualized the real-space distribution of the Fe oxidation state and phase proportions of the Fe3O4, γ-Fe2O3, and α-Fe2O3 phases in dendritic FeOx and 10 wt% Cr–FeOx particles. We also visualized the changes in the Fe oxidation state and phase proportions during the oxidation and phase transitions of the dendrites. The statistical analysis of the Fe phase proportion images revealed different phase proportion modes that depended on the Fe density (thickness) of the crystal particles and on the Cr dopant. The suppression of the phase transition from γ-Fe2O3 to α-Fe2O3 was greater in the thick body of the Cr–FeOx particles. These observations suggested that instability in the thin particle region was one cause of the phase transformation to the α-Fe2O3 phase, which suppresses the oxygen storage capacity of FeOx materials and degrades their catalytic performance.Author contributions
N. I.: conceptualization, data curation, methodology, software, funding acquisition, investigation, visualization, project administration and writing – original draft preparation. H.M.: funding acquisition, investigation, methodology, investigation, and resources. K. W. and J. K.: investigation, and resource. O. S., K. N., Y. T. and T. U.: investigation and methodology. M. T.: conceptualization, funding acquisition, methodology, project administration, supervision, and writing – reviewing and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by KAKENHI (Grant No. 16K17863 and 167K18288), JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (26288005, 18H01940, and 22H02031), and RIKEN SPring-8. XAFS measurements were performed at SPring-8 (No. 2015A1125, 2015A1559, 2016B1330, 2017B1852, 2018A1332, and 2018B1343). We thank Ms Noriko Takada (Institute for Molecular Science) for Au grid fabrication on the SiN membranes.Notes and references
- X. Mou, Y. Li, B. Zhang, L. Yao, X. Wei, D. S. Su and W. Shen, Eur. J. Inorg. Chem., 2012, 2684–2690 CrossRef CAS.
- J. Lai, K. V. P. M. Shafi, K. Loos, A. Ulman, Y. Lee, T. Vogt and C. Estournès, J. Am. Chem. Soc., 2003, 125, 11470–11471 CrossRef CAS PubMed.
- X. Mou, B. Zhang, Y. Li, L. Yao, X. Wei, D. S. Su and W. Shen, Angew. Chem., Int. Ed., 2012, 51, 2989–2993 CrossRef CAS PubMed.
- R. Wang, C. Xu, J. Sun and L. Gao, Sci. Rep., 2014, 4, 7171 CrossRef CAS PubMed.
- B. Wang, J. Sun, M. Abbas, Y. Liu, F. Kong, H. Xiao and J. Chen, Catal. Lett., 2017, 147, 1153–1161 CrossRef CAS.
- V. Polshettiwar, M. N. Nadagouda and R. S. Varma, Chem. Commun., 2008, 6318–6320 RSC.
- A. Khan, P. Chen, P. Boolchand and P. G. Smirniotis, J. Catal., 2008, 253, 91–104 CrossRef CAS.
- G. K. Reddy and P. G. Smirniotis, Ind. Eng. Chem. Res., 2017, 56, 1772–1781 CrossRef.
- G. K. Reddy, K. Gunasekera, P. Boolchand, J. Dong and P. G. Smirniotis, J. Phys. Chem. C, 2011, 115, 7586–7595 CrossRef CAS.
- G. K. Reddy, P. Boolchand, J. Dong and P. G. Smirniotis, J. Phys. Chem. C, 2012, 116, 11019–11031 CrossRef CAS.
- O. Voniuk, C. Bazzo, S. Albonetti, N. Tanchoux, F. Bosselet, J.-M. M. Millet, F. D. Renzo and F. Cavani, ChemCatChem, 2017, 9, 2219–2230 CrossRef.
- D.-W. Lee, M. S. Lee, J. Y. Lee, S. Kim, H.-J. Eom, D. J. Moon and K.-Y. Lee, Catal. Today, 2013, 210, 2–9 CrossRef CAS.
- C. J. Keturakis, M. Zhu, E. K. Gibson, M. Daturi, F. Tao, A. I. Frenkel and I. E. Wachs, ACS Catal., 2016, 6, 4786–4798 CrossRef CAS.
- M. Zhu, T. C. R. Rocha, T. Lunkenbein, A. Knop-Gericke, R. Schlögl and I. E. Wachs, ACS Catal., 2016, 6, 4455–4464 CrossRef CAS.
- M. Zhu and I. E. Wachs, ACS Catal., 2016, 6, 722–732 CrossRef CAS.
- H. Matsui, N. Ishiguro, Y. Suzuki, K. Wakamatsu, C. Yamada, K. Sato, N. Maejima, T. Uruga and M. Tada, Phys. Chem. Chem. Phys., 2020, 22, 28093 RSC.
- M. Tada, N. Ishiguro, T. Uruga, H. Tanida, Y. Terada, S. Nagamatsu, Y. Iwasawa and S. Ohkoshi, Phys. Chem. Chem. Phys., 2011, 13, 14910–14913 RSC.
- N. Ishiguro, T. Uruga, O. Sekizawa, T. Tsuji, M. Suzuki, N. Kawamura, M. Mizumaki, K. Nitta, T. Yokoyama and M. Tada, ChemPhysChem, 2014, 15, 1563–1568 CrossRef CAS PubMed.
- X. Yu, H. Pan, Y. Zhou, P. Northrup, J. Xiao, S. Bak, M. Liu, K.-W. Nam, D. Qu, J. Liu, T. Wu and X.-Q. Yang, Adv. Energy Mater., 2015, 5, 1500072 CrossRef.
- H. Matsui, N. Ishiguro, K. Enomoto, O. Sekizawa, T. Uruga and M. Tada, Angew. Chem., Int. Ed., 2016, 55, 12022–12025 CrossRef CAS PubMed.
- M.-J. Wang, F.-D. Yu, G. Sun, J. Wang, J.-G. Zhou, D.-M. Gu and Z.-B. Wang, J. Mater. Chem. A, 2019, 7, 8302–8314 RSC.
- W. Li, Z. Wang, F. Zhao, M. Li, X. Gao, Y. Zhao, J. Wang, J. Zhou, Y. Hu, Q. Xiao, X. Cui, M. J. Eslamibidgoli, M. H. Eikerling, R. Li, F. Brandys, R. Divigalpitiya, T.-K. Sham and X. Sun, Chem. Mater., 2020, 32, 1272–1280 CrossRef CAS.
- X. Ye, J. E. Schmidt, R. P. Wang, I. K. van Ravenhorst, R. Oord, T. Chen, F. de Groot, F. Meirer and B. M. Weckhuysen, Angew. Chem., Int. Ed., 2020, 59, 15610–15617 CrossRef CAS PubMed.
- L. Li, Y. C. Chen-Wiegart, J. Wang, P. Gao, Q. Ding, Y. S. Yu, F. Wang, J. Cabana, J. Wang and S. Jin, Nat. Commun., 2015, 6, 6883 CrossRef CAS PubMed.
- H. Matsui, N. Ishiguro, T. Uruga, O. Sekizawa, K. Higashi, N. Maejima and M. Tada, Angew. Chem., Int. Ed., 2017, 56, 9371–9375 CrossRef CAS PubMed.
- S. Kuppan, Y. Xu, Y. Liu and G. Chen, Nat. Commun., 2017, 8, 14309 CrossRef CAS PubMed.
- S. Shulda, J. N. Weker, C. Ngo, S. M. Alia, S. A. Mauger, K. C. Neyerlin, B. S. Pivovar and S. Pylypenko, ACS Appl. Nano Mater., 2018, 2, 525–534 CrossRef.
- C. S. Kaira, C. Kantzos, J. J. Williams, V. De Andrade, F. De Carlo and N. Chawla, Acta Mater., 2018, 144, 419–431 CrossRef CAS.
- C. Zhao, T. Wada, V. De Andrade, V. Gürsoy, H. Kato and Y.-C. K. Chen-Wiegart, Nano Energy, 2018, 52, 381–390 CrossRef CAS.
- J. Y. Park, J. P. Singh, J. Lim, K. H. Chae and S. Lee, Mater. Lett., 2020, 261, 126983 CrossRef CAS.
- T. Li, C. Lim, Y. Cui, X. Zhou, H. Kang, B. Yan, M. L. Meyerson, J. A. Weeks, Q. Liu, F. Guo, R. Kou, Y. Liu, V. De Andrade, F. De Carlo, Y. Ren, C.-J. Sun, C. B. Mullins, L. Chen, Y. Fu and L. Zhu, J. Mater. Chem. A, 2020, 8, 750–759 RSC.
- S. Spence, W. Lee, F. Lin and X. Xiao, Nanotechnology, 2021, 32, 442003 CrossRef CAS PubMed.
- Y. Kim and J. Lim, Sci. Rep., 2022, 12, 2894 CrossRef CAS PubMed.
- D. Hou, Z. Xu, Z. Yang, C. Kuai, Z. Du, C.-J. Sun, Y. Ren, J. Liu, X. Xiao and F. Lin, Nat. Commun., 2022, 13, 3437 CrossRef CAS PubMed.
- D. A. Shapiro, Y. S. Yu, T. Tyliszczak, J. Cabana, R. Celestre, W. Chao, K. Kaznatcheev, A. L. Kilcoyne, F. Maia, S. Marchesini, Y. S. Meng, T. Warwick, L. L. Yang and H. A. Padmore, Nat. Photonics, 2014, 8, 765–769 CrossRef CAS.
- Y. S. Yu, M. Farmand, C. Kim, Y. Liu, C. P. Grey, F. C. Strobridge, T. Tyliszczak, R. Celestre, P. Denes, J. Joseph, H. Krishnan, F. R. Maia, A. L. Kilcoyne, S. Marchesini, T. P. C. Leite, T. Warwick, H. Padmore, J. Cabana and D. A. Shapiro, Nat. Commun., 2018, 9, 1–7 CrossRef PubMed.
- M. Farmand, R. Celestre, P. Denes, A. L. D. Kilcoyne, S. Marchesini, H. Padmore, T. Tyliszczak, T. Warwick, X. Shi, J. Lee, Y.-S. Yu, J. Cabana, J. Joseph, H. Krishnan, T. Perciano, F. R. N. C. Maia and D. A. Shapiro, Appl. Phys. Lett., 2017, 110, 063101 CrossRef.
- M. Hirose, N. Ishiguro, K. Shimomura, N. Burdet, H. Matsui, M. Tada and Y. Takahashi, Angew. Chem., Int. Ed., 2018, 57, 1474–1479 CrossRef CAS PubMed.
- M. Hirose, N. Ishiguro, K. Shimomura, D. N. Nguyen, H. Matsui, H. C. Dam, M. Tada and Y. Takahashi, Commun. Chem., 2019, 2, 1–7 CrossRef CAS.
- H. Uematsu, N. Ishiguro, M. Abe, S. Takazawa, J. Kang, E. Hosono, N. D. Nguyen, H. C. Dam, M. Okubo and Y. Takahashi, J. Phys. Chem. Lett., 2021, 12, 5781–5788 CrossRef CAS PubMed.
- M. Abe, F. Kaneko, N. Ishiguro, T. Kubo, F. Chujo, Y. Tamenori, H. Kishimoto and Y. Takahashi, J. Phys. Chem. C, 2022, 126, 14047–14057 CrossRef CAS.
- S. G. Urquhart, X-ray Spectroptychography, ACS Omega, 2022, 7, 11521–11529 CrossRef CAS PubMed.
- B. M. Weckhuysen, Angew. Chem., Int. Ed., 2009, 48, 4910–4943 CrossRef CAS PubMed.
- T. Hatsui, M. Omodani, T. Kudo, K. Kobayashi, T. Imamura, T. Ohmoto, A. Iwata, S. Ono, Y. Kirihara, T. Kameshima, H. Kasai, N. Miura, N. Kuriyama, M. Okihara, Y. Nagatomo, M. Nagasaki, T. Watanabe and M. Yabashi, Proceedings of the 2013 International Image Sensor Workshop (IISW), Snowbird, Utah, USA, June, 2013, Article No. 3.05.
Footnote |
| † Electronic supplementary information (ESI) available: Analysis schemes, Fe K-edge XANES of Fe standard compounds, full-field absorption image, and integrated Fe K-edge XAFS. See DOI: https://doi.org/10.1039/d3cp00907f |
| This journal is © the Owner Societies 2023 |