Engineering magic number Au19 and Au20 cage structures using electron withdrawing atoms†
Heather M.
Gaebler
,
Julianna R.
Castiglione
and
Ian P.
Hamilton
 *
*
Department of Chemistry and Biochemistry, Wilfrid Laurier University, 75 University Ave W, Waterloo, ON, Canada N2L 3C5. E-mail: ihamilton@wlu.ca
First published on 17th April 2023
Abstract
Gold cages are a subset of gold nanoparticles and these structures are of major interest due to their favourable physiochemical properties. In order for these structures to be useful in applications, they must be chemically stable. The objective of this research is to transform non-magic number cage structures into magic number cage structures by the addition of electron-withdrawing groups on the cages. The electronic properties for Au19X and Au20X2 (X = F, Cl, Br, I) are calculated and observed. It is expected that the electron-withdrawing groups will remove the electron density from the gold cages and leave them positively charged. We first optimize the geometries of the initial gold cages and verify the structures are a local minima. From there, we attach our halogens to the gold cages and optimize the structures to determine the NICS values and HOMO–LUMO gaps. NICS values were found to be more negative when a more electronegative halogen was used. Calculations have found that Au19F and Au20F2 have the most negative NICS values, indicating greater spherical aromaticity. Iodine, being the least electronegative atom, had the most positive NICS value and smallest HOMO–LUMO gap. All calculations are compared to the magic cluster Au18 which satisfies Hirsh's 2(N + 1)2 rule for n = 2.
Introduction
Small gold nanostructures are of considerable interest in the fields of nanoscience and nanotechnology. Viewed as elementary building blocks for the construction of nanoarchitectures, these species have become optimal candidates in a broad range of applications including electrochemistry,1 quantum electronics,2 and heterogeneous catalysis.3 With diameters ranging from sub-nanometer to approximately 2 nm,4,5 they exhibit novel properties resulting from unique atomic packing and strong quantum effects that differ significantly from gold nanostructures in the 5–100 nm range, and from bulk gold.6 Their properties can be fine-tuned by altering characteristics such as the size, shape, and composition of the nanostructure.7–10Compact gold nanostructures can be qualitatively described by the jellium model.11 The discrete orbitals are labeled 1S, 1P, 1D + 2S, 1F + 2P… and have stable configurations of valence electrons (2, 8, 20, 40…) that differs from the atomic series (2, 10, 18, 36…).12–15 In gold nanostructures, each gold atom contributes one 6s valence electron that moves freely throughout the structure16 and it has been shown that compact gold structures with 2, 8, 20, and 40 gold atoms have special electronic and chemical stability.13,17,18 In particular, it is well-established that the minimum energy Au20 structure is the (compact) tetrahedron, Au20(Td).18 We obtained initial Au19 and Au18 compact structures by removing one and two corner gold atoms from Au20(Td).
For cage structures, only the highest angular momentum is relevant. The discrete orbitals are labeled 1S, 1P, 1D, 1F… and have stable configuration of valence electrons (2, 8, 18, 32…) and it has been shown that cage structures with 18 and 32 gold atoms have special electronic and chemical stability.17 Of relevance to our paper, there is strong experimental evidence of (anionic) gold cage structures with 16, 17, and 18 atoms in the gas phase.19 Also of relevance is a computational study which showed that, for Au18, the compact truncated tetrahedral structure transitioned to a cage structure during soft-landing onto a TiO2(110) surface.20
Although used frequently in current scientific literature, aromaticity is a concept with no single, universally accepted definition despite dedicated research into the topic spanning over centuries.21,22 Previously, aromatic compounds were largely defined as planar ring systems that satisfied the 4n + 2π electron rule.23 In this work, aromaticity is defined as electron delocalization in a closed circuit in either two or three dimensions and is associated with extra stability within three-dimensional molecules.24 Experimentally, electronic ring currents in aromatic species are measured through nuclear magnetic resonance (NMR).25–27 A long-standing and widely accepted method for measuring aromaticity theoretically is the nucleus-independent chemical shift (NICS)28 due to its simplicity, efficiency, and easy computability. Specifically, 1H NMR chemical shifts are commonly used for characterizing aromatic (and anti-aromatic) compounds,29 and this method proves useful for three-dimensional structures as long as the chemical shift is calculated at the center of the cage.30 These isotropic NICS values correspond to the negative of the magnetic shielding computed at the center of the cage and substantially negative isotropic NICS values signify the presence of induced ring currents which corresponds to high aromaticity. NICS values therefore serve as a means to quantify the electronic and chemical stability of small spherical gold cage structures. While the correlation between electronic ring currents and stability in aromatic compounds has been questioned regarding organic27,31–33 and inorganic34–36 species, this work focuses on the relationship between NICS values and the stability of spherical gold clusters ranging in size from 18–20 atoms.
To overcome their high surface energy and prevent agglomeration, nanostructures should be stabilized via electrostatic and/or steric means. Electrostatic stabilization is achieved through the adsorption of ions to the nanostructure surface and results in the repulsion of individual particles.37 Steric stabilization is achieved by encompassing the metal core in layers of materials that are sterically bulky, thus providing a barrier which prevents close contact of the metal cores.37 An added benefit to these stabilization methods is that the number of valence electrons in the nanocluster can be adjusted by selecting the appropriate ligand resulting in tremendous control of the physiochemical properties of the nanocluster.38 It has been shown that, for charged and neutral clusters, the relative stability among 3D isomers can change as a result of the ligand stabilization effect.39–43 Although thiols are the most popular and well-studied ligand for gold clusters,1 other common ligands include amines,44 arynes,45 phosphines,46 and halides.47,48 In this paper, motivated by studies which use electron withdrawing substituents to alter the stability of organic molecules,49,50 we use halides to stabilize small spherical gold cage structures.
Chemical hardness, a measure of the resistance to change or deformation, is another property with an expected relation to structural stability.51–53 It is equal to the difference between the ionization potential and the electron affinity. It has been shown that the negative energy value of the HOMO is approximately equal to the ionization potential, and the negative energy value of the LUMO is approximately equal to the electron affinity.54,55 Therefore, chemical hardness can be associated with the gap between these two frontier orbitals, and it is expected that the larger the HOMO–LUMO gap the more stable the nanocluster.
Here we report increased stability achieved for local minima Au19 and Au20 cage structures through the adsorption of halogens (X = F, Cl, Br, I) on the cage. In Au19X and Au20X2, the gold cage has a formal valence electron count of 18 and we show these halogenated species exhibit stability analogous to Au18, a magic number cage structure that satisfies Hirsch's 2(N + 1)2 rule for n = 2. Specifically, we show that Au19X and Au20X2 have more negative isotropic NICS values and more positive HOMO–LUMO gaps than their unhalogenated counterparts. We also show that, when the electronegativity of the ligand increases (from iodide to fluoride), the stability of the ligated cage structure increases.
All gold local minima isomers presented in this work were adapted from Trombach et al.56 who constructed the spherical gold cages using standard algorithms employed for the construction of fullerenes.57–59 Bulusu et al.19 were successful in identifying and analyzing golden cages via photoelectron spectroscopy.
Methodology
Geometries for the six Au18, Au19, and Au20 cage isomers, as well as the Au18, Au19, and Au20 compact structures, were initially optimized via the CP2K software package60 using density functional theory (DFT) with the Perdew–Burke–Ernzerhof (PBE) exchange correlation functional61 and the double-zeta valence polarized (DZVP) basis set. To include relativistic effects, which are large for gold, we used the Geodecker–Teter–Hutter (GTH) pseudopotential,62 and dispersion corrections were included via the Grimme D3 approach.63 The Mulliken charge64 of each gold atom was calculated to aid in the placement of the ligands. Each charged and ligated cage was also optimized under identical conditions prior to the subsequent steps. CP2K was also used to calculate the structural energies and HOMO–LUMO gap (in eV) of all bare and ligated species. While DFT is known to underestimate HOMO–LUMO gaps, we can confidently compare the computed HOMO–LUMO gaps to one another. Next using the Gaussian 16 software package65 with the PBE functional and the triple-zeta valence polarized (TZVP) basis set, normal mode calculations were performed on all bare and ligated cages to ensure the optimized structures were local minima via the absence of imaginary vibrational frequencies. To account for relativistic effects in Gaussian, the Def2TZVP basis set was selected as it makes use of the Stuttgart Dresden core potentials for elements heavier than Krypton. We then computed the isotropic NICS value (in ppm) for each gold cage structure. All structures were visualized with the aid of Chemcraft software.66Results and discussion
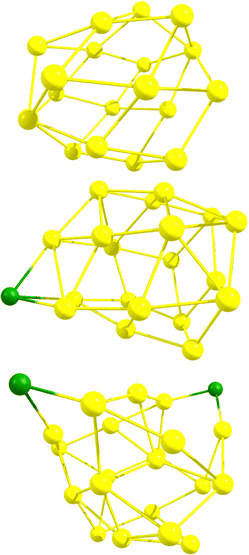
Initially, DFT calculations were performed for six neutral Au18 cage isomers (number in parenthesis denotes the number of different isomers for that particular symmetry group):56 C2–Au18 (2), D2–Au18, D3d–Au18, D3h–Au18, and D3–Au18. Visuals of each optimized structure are located in Fig. S1 of the ESI.† After confirming the isomers were local minima, the Au18 cage structures were determined to have an average energy equal to that of the neutral Au18 compact cluster, as oultined in Table S1 of the ESI.† Next, HOMO–LUMO gaps and isotropic NICS values of the six neutral Au18 cage isomers were computed and are compiled in Table S2 of the ESI† along with the XYZ coordinates. Since Au18 is a closed–shell cage structure with high stability, it was previously found, and confirmed in our calculations, to have a large negative NICS value, averaged at −52.20 ppm. The average HOMO–LUMO gap for the six Au18 isomers was determined to be 1.13 eV. Next, DFT calculations were performed for six Au19 cage isomers:56 C2–Au19 (3), Cs–Au19 (2), and C3v–Au19, each first with a neutral charge and then with +1 charge. Visuals of each optimized neutral structure are located in Fig. S1 of the ESI.† After confirming the neutral isomers were local minima, the Au19 cage structures were determined to be higher in energy by an average of 0.46 eV compared to the neutral Au19 compact cluster, as oultined in Table S1 of the ESI.† Next, the HOMO–LUMO gaps and isotropic NICS values for the six Au19 cage isomers were computed and are compiled in Tables S2 and S3 of the ESI† along with the XYZ coordinates. In contrast to Au18, Au19 (which is an open–shell system) exhibits less spherical aromaticity and stability, observed through the average NICS value of +12.93 ppm and an average HOMO–LUMO gap of 1.05 eV. The Au19+ cage structure (which is isoelectronic to Au18) has an average NICS value of −49.56 ppm and an average HOMO–LUMO gap of 1.19 eV. Finally, DFT calculations were performed for six Au20 cage isomers:56 C2–Au20, D2–Au20 (2), C1–Au20, Cs–Au20, and D2d–Au20, each with a neutral, +1, and +2 charge. Visuals of each optimized neutral structure are located in Fig. S1 of the ESI.† After confirming the neutral isomers were local minima, the Au20 cage structures were determined to be higher in energy by an average of 0.99 eV compared to the neutral Au20 compact cluster, as oultined in Table S1 of the ESI.† This was expected as the literature states the minimum energy Au20 structure is the (compact) tetrahedron. Next, the HOMO–LUMO gaps and isotropic NICS values for the six Au20 cage isomers were computed and are compiled in Tables S2 and S3 of the ESI† along with the XYZ coordinates. The neutral Au20 structure exhibits an average NICS value of +2.61 ppm and an average HOMO–LUMO gap of 0.66 eV. For Au20+, a significant increase in stability is observed as the average NICS value decreases to −20.58 ppm and the average HOMO–LUMO gap value increases to 0.71 eV. The Au202+ cage structure (which is isoelectronic to Au18) displays greater aromaticity and stability with an average NICS value of −38.18 ppm and an average HOMO–LUMO gap of 1.06 eV.For the cationic structures, Au19+ and Au202+, electronic stabilization is achieved through the adsorption of halogen anions (X = F−, Cl−, Br−, I−) on the cage. The location of each halogen was decided through analysis of the Mulliken charges for the gold atoms in the bare structures. Specifically, halogen atoms were placed at the most positive gold atoms for each gold cage. Visuals of isomer 1 optimized halogenated structures for Au19X and Au20X2 are located in Fig. S2 of the ESI.† For comparison, the optimized Au18, Au19Cl, and Au20Cl2 structures are displayed in Fig. 1. The HOMO–LUMO gaps and isotropic NICS values for each of the six isomers for Au19X and Au20X2 are compiled in Table S4 of the ESI† along with the XYZ coordinates. The average isotropic NICS values and HOMO–LUMO gaps for Au19X and Au20X2 are displayed in Fig. 2 and given in Table S5 of the ESI.† While all are 18 electron species, each Au19 halogenated structure has greater spherical aromaticity and a larger HOMO–LUMO gap than its Au20 halogenated counterpart. In comparison to the closed–shell Au18 cage structures, with an average NICS value of −52.20 ppm, the average NICS values for the Au19X species are −43.44, −41.46, −40.01, and −38.33 ppm for Au19F, Au19Cl, Au19Br, and Au19I, respectively. The average NICS values for the Au20X2 species are −31.82, −26.53, −26.43, and −25.79 ppm for Au20F2, Au20Cl2, Au20Br2, and Au20I2, respectively. For Au19X and Au20X2, we observe that, as the halogen becomes more electronegative, the spherical aromaticity of the cage structure increases. When cross-referencing the NICS values with the HOMO–LUMO gaps for Au18, Au19X, and Au20X2, it is observed that the species with more negative NICS values have larger HOMO–LUMO gaps. For instance, Au18 with a NICS value of −52.20 ppm has a HOMO–LUMO gap of 1.13 eV, Au19F with a NICS value of −43.44 ppm has a HOMO–LUMO gap of 1.10 eV, and Au20I2 with a NICS value of −25.79 ppm has a HOMO–LUMO gap of 0.86 eV. As stated previously, high spherical aromaticities and large HOMO–LUMO gaps are known characteristics of stable gold cage structures.
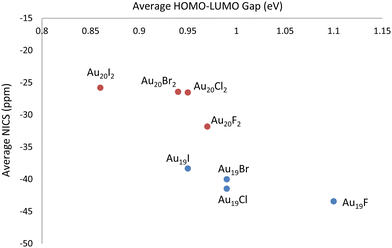 | ||
| Fig. 2 Average (over structural isomers) spherical aromaticity and chemical hardness trends for the halogenated Au19X and Au20X2 cage structures. | ||
Conclusions
We have shown that high spherical aromaticity can be achieved for structures engineered to have a closed–shell “magic number” of electrons. Specifically, we have shown, using nucleus-independent chemical shifts, that Au19X and Au20X2 cage structures have spherical aromaticity comparable to the well-known Au18 cage structure and that their corresponding HOMO–LUMO gaps reflect their position in the stability ranking. Electrostatic stabilization was achieved through the adsorption of halogen anions (X = F−, Cl−, Br−, I−) on the Au19+ and Au202+ cage surface. The trend observed amongst the Au19X and Au20X2 species is that as the halide becomes more electronegative in going from iodide to fluoride, the spherical aromaticity of the cage structure increases. These results support the broader finding that control over the stability of small gold nanostructures is possible using ligands to fine-tune their electronic properties. As noted above, there is strong experimental evidence of an Au17− cage structure in the gas phase and the formation of an Au18 cage structure on a metal oxide surface. We therefore believe that our electronically equivalent Au19X and Au20X2 cage structures are experimentally accessible.Author contributions
All authors have given approval to the final version of the manuscript. Initial calculations were undertaken by HMG. Subsequent calculations were undertaken by HMG and JRC under the supervision of IPH.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge support from NSERC, SOSCIP, Compute Canada, and Wilfrid Laurier University.References
- M.-C. Daniel and D. Astruc, Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology, Chem. Rev., 2004, 104, 293–346 CrossRef CAS PubMed.
- S. Chen, R. S. Ingram, M. J. Hostetler, J. J. Pietron, R. W. Murray, T. G. Schaaff, J. T. Khoury, M. M. Alvarez and R. L. Whetten, Gold Nanoelectrodes of Varied Size: Transition to Molecule-Like Charging, Science, 1998, 280, 2098–2101 CrossRef CAS PubMed.
- G. Li and R. Jin, Atomically Precise Gold Nanoclusters as New Model Catalysts, Acc. Chem. Res., 2013, 46, 1749–1758 CrossRef CAS PubMed.
- H. Qian, M. Zhu, Z. Wu and R. Jin, Quantum Sized Gold Nanoclusters with Atomic Precision, Acc. Chem. Res., 2012, 45, 1470–1479 CrossRef CAS PubMed.
- R. Jin, Atomically Precise Metal Nanoclusters: Stable Sizes and Optical Properties, Nanoscale, 2015, 7, 1549–1565 RSC.
- D. Mollenhauer and N. Gaston, Phosphine Passivated Gold Clusters: How Charge Transfer Affects Electronic Structure and Stability, Phys. Chem. Chem. Phys., 2016, 18, 29686–29697 RSC.
- A. W. Castleman and S. N. Khanna, Clusters, Superatoms, and Building Blocks of New Materials, J. Phys. Chem., 2009, 113, 2664–2675 CrossRef CAS PubMed.
- M. V. Kovalenko, L. Manna, A. Cabot, Z. Hens, D. V. Talapin, C. R. Kagan, X. V. I. Klimov, A. L. Rogach, P. Reiss, D. J. Milliron, P. Guyot-sionnnest, G. Konstantatos, W. J. Parak, T. Hyeon, B. A. Korgel, C. B. Murray and W. Heiss, Prospects of Nanoscience with Nanocrystals, Nanotechnol. Focus, 2015, 9, 1012–1057 CAS.
- Y. Xia, Y. Xiong, B. Lim and S. E. Skrabalak, Shape-Controlled Synthesis of Metal Nanocrystals: Simple Chemistry Meets Complex Physics?, Angew. Chem., Int. Ed., 2009, 48, 60–103 CrossRef CAS PubMed.
- R. Fournier, Theoretical Study of the Structure of Silver Clusters, J. Chem. Phys., 2001, 115, 2165–2177 CrossRef CAS.
- W. D. Knight, K. Clemenger, W. A. de Heer, W. A. Saunders, M. Y. Chou and M. L. Cohen, Electronic Shell Structure and Abundances of Sodium Clusters, Phys. Rev. Lett., 1984, 52, 2141–2143 CrossRef CAS.
- W. A. De Heer, The Physics of Simple Metal Clusters: Experimental Aspects and Simple Models, Rev. Mod. Phys., 1993, 65, 611–676 CrossRef CAS.
- Z. Luo and A. W. Castleman, Special and General Superatoms, Acc. Chem. Res., 2014, 47, 2931–2940 CrossRef CAS PubMed.
- Z. Luo, A. W. Castleman and S. N. Khanna, Reactivity of Metal Clusters, Chem. Rev., 2016, 116, 14456–14492 CrossRef CAS PubMed.
- P. Jena and Q. Sun, Super Atomic Clusters: Design Rules and Potential for Building Blocks of Materials, Chem. Rev., 2018, 118, 5755–5870 CrossRef CAS PubMed.
- M. Faraday and On New, Compounds of Carbon and Hydrogen, and on Certain Other Products Obtained during the Decomposition of Oil by Heat, Philos. Trans. R. Soc. London, 1825, 115, 440–466 CrossRef.
- H. Häkkinen, Atomic and Electronic Structure of Gold Clusters: Understanding Flakes, Cages and Superatoms from Simple Concepts, Chem. Soc. Rev., 2008, 37, 1847–1859 RSC.
- J. Li, X. Li, H.-J. Zhai and L.-S. Wang, Au20: A Tetrahedral Cluster, Science, 2003, 299, 864–867 CrossRef CAS PubMed.
- S. Bulusu, X. Li, L. Wang and X. C. Zeng, Evidence of Hollow Golden Cages, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 8326–8330 CrossRef CAS PubMed.
- L. Li, H. Li and X. C. Zeng, Structure Transition of Au18 from Pyramidal to a Hollow-Cage during Soft-Landing onto a TiO2(110) Surface, Chem. Commun., 2015, 51, 9535–9538 RSC.
- B. J. R. Cuyacot and C. Foroutan-Nejad, [{Th(C8H8)Cl2}3]2—Is Stable But Not Aromatic, Nature, 2022, 603, E18–E20 CrossRef CAS PubMed.
- A. Stanger, What is… Aromaticity: A Critique of the Concept of Aromaticity—Can It Really Be Defined?, Chem. Commun., 2009, 1939–1947 RSC.
- E. Hückel, Quantentheoretische Beiträge zum Problem der Aromatischen und Ungesättigten Verbindungen. III. [Quantum Theoretical Contributions to the Problem of Aromatic and Unsaturated Compounds. III], Z. Phys., 1932, 76, 628–648 CrossRef.
- P. von Ragué Schleyer, Introduction: Aromaticity, Chem. Rev., 2001, 101, 1115–1118 CrossRef PubMed.
- R. H. Mitchell, Measuring Aromaticity by NMR, Chem. Rev., 2001, 101, 1301–1315 CrossRef CAS PubMed.
- R. Islas, T. Heine and G. Merino, The Induced Magnetic Field, Acc. Chem. Res., 2012, 45, 215–228 CrossRef CAS PubMed.
- J. A. N. F. Gomes and R. B. Mallion, Aromaticity and Ring Currents, Chem. Rev., 2001, 101, 1349–1383 CrossRef CAS PubMed.
- P. Von Rague, C. Maerker, A. Dransfeld, H. Jiao, N. J. R. V. E. Hommes, D. Erlangen and R. V. February, Nucleus-Independent Chemical Shifts: A Simple and Efficient Aromaticity Probe, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef PubMed.
- L. M. Elvidge and J. A. Jackman, Studies of Aromaticity by Nuclear Magnetic Resonance Spectroscopy. Part I. 2-Pyridones and Related Systems, J. Chem. Soc., 1961, 859–866 RSC.
- M. Bühl and A. Hirsch, Spherical Aromaticity of Fullerenes, Chem. Rev., 2001, 101, 1153–1183 CrossRef PubMed.
- Z. Badri and C. Foroutan-Nejad, Unification of Ground-State Aromaticity Criteria – Structure, Electron Delocalization, and Energy – In Light of the Quantum Chemical Topology, Phys. Chem. Chem. Phys., 2016, 18, 11693–11699 RSC.
- L. Zhao, R. Grande-Aztatzi, C. Foroutan-Nejad, J. M. Ugalde and G. Frenking, Aromaticity, the Hückel 4n + 2 Rule and Magnetic Current, ChemistrySelect, 2017, 2, 863–870 CrossRef CAS.
- R. Gershoni-poranne and A. Stanger, Magnetic Criteria of Aromaticity, Chem. Soc. Rev., 2015, 6597–6615 RSC.
- Z. Badri, S. Pathak, H. Fliegl, P. Rashidi-Ranjbar, R. Bast, R. Marek, C. Foroutan-nejad and K. Ruud, All-Metal Aromaticity: Revisiting the Ring Current Model among Transition Metal Clusters, J. Chem. Theory Comput., 2013, 9, 4789–4796 CrossRef CAS PubMed.
- C. Foroutan-Nejad, S. Shahbazian, F. Feixas, P. Rashidi-Ranjbar and M. Solà, A Dissected Ring Current Model for Assessing Magnetic Aromaticity: A General Approach for both Organic and Inorganic Rings, J. Comput. Chem., 2011, 32, 2422–2431 CrossRef CAS PubMed.
- J. O. C. Jiménez-Halla, E. Matito, J. Robles and M. Solà, Nucleus-Independent Chemical Shift (NICS) Profiles in a Series of Monocyclic Planar Inorganic Compounds, J. Organomet. Chem., 2006, 691, 4359–4366 CrossRef.
- L. Guerrini, R. A. Alvarez-Puebla and N. Pazos-Perez, Surface Modifications of Nanoparticles for Stability in Biological Fluids, Materials, 2018, 11, 1–28 CrossRef PubMed.
- T. Omoda, S. Takano and T. Tsukuda, Toward Controlling the Electronic Structures of Chemically Modified Superatoms of Gold and Silver, Small, 2020, 2001439, 1–18 Search PubMed.
- G. Shafai, S. Hong, M. Bertino and T. S. Rahman, Effect of Ligands on the Geometric and Electronic Structure of Au13 Clusters, J. Phys. Chem. C, 2009, 113, 12072–12078 CrossRef CAS.
- K. Spivey, J. I. Williams and L. Wang, Structures of Undecagold Clusters: Ligand Effect, Chem. Phys. Lett., 2006, 432, 163–166 CrossRef CAS.
- I. L. Garzon, C. Rovira, K. Michaelian, M. R. Beltran, P. Ordejon, J. Junquera, D. Sanchez-Portal, E. Artacho and J. M. Soler, Do Thiols Merely Passivate Gold Nanoclusters?, Phys. Rev. Lett., 2000, 85, 5250–5251 CrossRef CAS PubMed.
- G. Periyasamy and F. Remacle, Ligand and Solvation Effects on the Electronic Properties of Au55 Clusters: A Density Functional Theory Study, Nano Lett., 2009, 9, 3007–3011 CrossRef CAS PubMed.
- N. T. Wilson and R. L. Johnston, Passivated Clusters: A Theoretical Investigation of the Effect of Surface Ligation on Cluster Geometry, Phys. Chem. Chem. Phys., 2002, 4, 4168–4171 RSC.
- D. V. Leff, L. Brandt and J. R. Heath, Synthesis and Characterization of Hydrophobic, Organically-Soluble Gold Nanocrystals Functionalized with Primary Amines, Langmuir, 1996, 12, 4723–4730 CrossRef CAS.
- P. Maity, H. Tsunoyama, M. Yamauchi, S. Xie and T. Tsukuda, Organogold Clusters Protected by Phenylacetylene, J. Am. Chem. Soc., 2011, 133, 20123–20125 CrossRef CAS PubMed.
- J. Kilmartin, R. Sarip, R. Grau-Crespo, D. Di Tommaso, G. Hogarth, C. Prestipino and G. Sankar, Following the Creation of Active Gold Nanocatalysts from Phosphine-Stabilized Molecular Clusters, ACS Catal., 2012, 2, 957–963 CrossRef CAS.
- P. Pyykko, Theoretical Chemistry of Gold. II, Inorg. Chim. Acta, 2005, 358, 4113–4130 CrossRef.
- G. M. Berry, M. E. Bothwell, B. G. Bravo, G. J. Cali, J. E. Harris, T. Mebrahtu, S. L. Michelhaugh, J. F. Rodriguez and M. P. Soriaga, Spectroscopic and Electrochemical Studies of Iodine Coordinated to Noble-Metal Electrode Surfaces, Langmuir, 1989, 5, 707–713 CrossRef CAS.
- P. von Ragué Schleyer and L. Nyulászi, Hyperconjugative π-Aromaticity: How To Make Cyclopentadiene Aromatic, J. Am. Chem. Soc., 1999, 121, 6872–6875 CrossRef.
- I. Fernández, J. I. Wu and P. von Ragué Schleyer, Substituent Effects on “Hyperconjugative” Aromaticity and Antiaromaticity in Planar Cyclopolyenes, Org. Lett., 2013, 15, 2990–2993 CrossRef PubMed.
- R. G. Pearson, Hard and Soft Acids and Bases, J. Am. Chem. Soc., 1963, 85, 3533–3539 CrossRef CAS.
- R. G. Parr and K. Chattara, Pratim, Principle of Maximum Hardness, J. Am. Chem. Soc., 1991, 113, 1854–1855 CrossRef CAS.
- R. G. Pearson, Chemical Hardness, 1997 Search PubMed.
- R. G. Parr and R. G. Pearson, Absolute Hardness: Companion Parameter to Absolute Electronegativity, J. Am. Chem. Soc., 1983, 105, 7512–7516 CrossRef CAS.
- R. G. Pearson, Chemical Hardness and Density Functional Theory, J. Chem. Sci., 2005, 117, 369–377 CrossRef CAS.
- L. Trombach, S. Rampino, L. S. Wang and P. Schwerdtfeger, Hollow Gold Cages and Their Topological Relationship to Dual Fullerenes, Chem. – Eur. J., 2016, 22, 8823–8834 CrossRef CAS PubMed.
- P. Schwerdtfeger, L. N. Wirz and J. Avery, The Topology of Fullerenes, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2015, 5, 96–145 CAS.
- P. Schwerdtfeger, L. Wirz and J. Avery, Program Fullerene: A Software Package for Constructing and Analyzing Structures of Regular Fullerenes, J. Comput. Chem., 2013, 34, 1508–1526 CrossRef CAS PubMed.
- L. N. Wirz, R. Tonner, J. Avery and P. Schwerdtfeger, Structure and Properties of the Nonface-Spiral Fullerenes T-C380, D3-C384, D3-C440, and D3-C672 and Their Halma and Leapfrog Transforms, J. Chem. Inf. Model, 2014, 54, 121–130 CrossRef CAS PubMed.
- J. Hutter, M. Iannuzzi, F. Schiffmann and J. Vandevondele, CP2K: Atomistic Simulations of Condensed Matter Systems, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2014, 4, 15–25 CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Generalized Gradient Approximation Made Simple, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- S. Goedecker, M. Teter and J. Hutter, Separable Dual-Space Gaussian Pseudopotentials, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 1703–1710 CrossRef CAS PubMed.
- S. Grimme, Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed.
- R. S. Mulliken, Electronic structures of Molecules XI. Electroaffinity, Molecular Orbitals and Dipole Moments, J. Chem. Phys., 1935, 3, 573–585 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ort and D. J. Fox, Gaussian 09 Revision A.02, 2016 Search PubMed.
- G. A. Andrienko, Chemcraft Molecular Visualization Software V1.8, 2023 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: NICS values and HOMO–LUMO gaps for all structural isomers. See DOI: https://doi.org/10.1039/d3cp00651d |
| This journal is © the Owner Societies 2023 |