Factors contributing to halogen bond strength and stretch or contraction of internal covalent bond†
Abstract
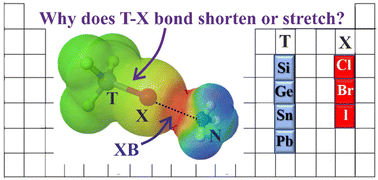
The halogen bond formed by a series of Lewis acids TF3X (T = C, Si, Ge, Sn, Pb; X = Cl, Br, I) with NH3 is studied by quantum chemical calculations. The interaction energy is closely mimicked by the depth of the σ-hole on the X atom as well as the full electrostatic energy. There is a first trend by which the hole is deepened if the T atom to which X is attached becomes more electron-withdrawing: C > Si > Ge > Sn > Pb. On the other hand, larger more polarizable T atoms are better able to transmit the electron-withdrawing power of the F substituents. The combination of these two opposing factors leaves PbF3X forming the strongest XBs, followed by CF3X, with SiF3X engaging in the weakest bonds. The charge transfer from the NH3 lone pair into the σ*(TX) antibonding orbital tends to elongate the covalent TX bond, and this force is largest for the heavier X and T atoms. On the other hand, the contraction of this bond deepens the σ-hole at the X atom, which would enhance both the electrostatic component and the full interaction energy. This bond-shortening effect is greatest for the lighter X atoms. The combination of these two opposing forces leaves the T–X bond contracting for X = Cl and Br, but lengthening for I.
- This article is part of the themed collection: 2023 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...
