 Open Access Article
Open Access ArticleQuasi-classical trajectory study of the OH− + CH3I reaction: theory meets experiment†
Domonkos A.
Tasi
 *a,
Tim
Michaelsen
b,
Roland
Wester
*a,
Tim
Michaelsen
b,
Roland
Wester
 b and
Gábor
Czakó
b and
Gábor
Czakó
 *a
*a
aInterdisciplinary Excellence Centre and Department of Physical Chemistry and Materials Science, Institute of Chemistry, University of Szeged, Rerrich Béla tér 1, Szeged H-6720, Hungary. E-mail: dtasi@chem.u-szeged.hu; gczako@chem.u-szeged.hu
bInstitut für Ionenphysik und Angewandte Physik, Universität Innsbruck, Technikerstraße 25/3, 6020 Innsbruck, Austria
First published on 20th December 2022
Abstract
Regarding OH− + CH3I, several studies have focused on the dynamics of the reaction. Here, high-level quasi-classical trajectory simulations are carried out at four different collision energies on our recently developed potential energy surface. In all, more than half a million trajectories are performed, and for the first time, the detailed quasi-classical trajectory results are compared with the reanalysed crossed-beam ion imaging experiments. Concerning the previously reported direct dynamics study of OH− + CH3I, a better agreement can be obtained between the revised experiment and our novel theoretical results. Furthermore, in the present work, the benchmark geometries, frequencies and relative energies of the stationary points are also determined for the OH− + CH3I proton-abstraction channel along with the earlier characterized SN2 channel.
I. Introduction
Moving further to larger systems, the investigations of bimolecular nucleophilic substitutions (SN2) have become more and more complex over the last few decades.1–9 The most thoroughly studied seven-atomic SN2 reactions are OH− + CH3Y (Y = F, Cl, Br, I). In these cases, the SN2 reaction profile differs from the traditional X−⋯CH3Y → [X⋯CH3⋯Y]− → XCH3⋯Y− path.10–15 For Y = Cl, Br and I, in the entrance channel, a H-bonded HO−⋯HCH2Y complex and a H-bonded transition state are located, and for Y = I, the typical X−⋯CH3Y ion-dipole pre-reaction complex and the [X⋯CH3⋯Y]− Walden-inversion transition state cannot be found. Moreover, instead of the XCH3⋯Y− post-reaction complex, a H-bonded CH3OH⋯Y− complex is situated in the product channel, which happens to be the global minimum for the OH− + CH3Y reactions. It should be noted that, by front-side attack16 and double-inversion17 mechanisms, retention of the initial configuration may also occur in the OH− + CH3Y SN2 reaction.15In 2002, Hase and co-workers investigated the reaction between OH− and CH3F using direct dynamics classical trajectory simulations, and revealed that the reaction rather takes place through a direct route by avoiding the deep CH3OH⋯F− well in the exit channel.12 The stationary points of the OH− + CH3F Walden-inversion pathway were characterized by Sun et al.13 at HF, MP2 and CCSD(T) levels of theory and by Gonzales et al.18,19 using density functional theory and the focal-point approach. Recently, based on the results of Hase and co-workers, several reaction analysis methods were applied for the reaction of OH− and CH3F.20–24 Furthermore, dynamics simulations uncovered a novel oxide ion substitution for the reaction: in the post-reaction CH3OH⋯F− complex the leaving F− may remove the proton of the hydroxyl group, which leads to the products of HF + CH3O−.25,26 The existence of the oxide ion substitution for OH− + CH3F was also confirmed by Li and co-workers,27 and Ji et al. completed a detailed mapping of the possible proton abstraction-involved channels for the Y−(H2O)n + CH3I (Y = F, Cl, Br, I) reactions, where n = 1 or 2, taking into account an oxide ion substitution leading to the CH3O− + HY + HI + (n − 1)H2O products.28 The OH− + CH3Cl SN2 reaction was examined by various studies as well. The stationary points were identified by Evanseck et al.,10 and later the results were recalculated by Tachikawa et al.,11 Souza et al.,29 Laloo et al.30 and Zhao et al.31 using DFT, HF, MP2 and CCSD(T) methods; moreover, direct dynamics simulations were also conducted at the HF/3-21+G(d) and B3LYP/aug-cc-pVDZ levels of theory.31,32 Wang and co-workers performed QM/MM calculations for the OH− + CH3Y (Y = F, Cl and Br) SN2 reactions in aqueous solution.33–35
For the OH− + CH3I reaction, numerous experimental and theoretical investigations were carried out in the last decade.14,36–43 Cross-beam imaging techniques were employed to analyse the dynamics of the reactions of OH−, OH−(H2O) and OH−(H2O)2 with CH3I at collision energies (Ecoll) of 11.5, 23.1, 34.6 and 46.1 kcal mol−1.37 Considering the I− velocity vector distributions, three different SN2 reaction mechanisms were observed, and their occurrences were dependent on the presence of the water molecules. The stationary points of the latter reactions were studied, as well, using DFT and MP2 methods.14,38,39,41 The crossed-beam and selected ion flow tube experiments were supported by the direct dynamics simulations of Hase and co-workers in order to describe the dynamics and kinetics of the reaction.14,38,40,42 These findings, along with others, showed that the dynamics of the SN2 reactions had to be rethought, because the picture is more complex than the traditional one.44 For that reason, in 2018, we characterized the Walden-inversion, front-side attack and double-inversion mechanisms of the OH− + CH3Y (Y = F, Cl, Br, I) SN2 reactions with high-level ab initio methods.15 In 2020, for the title reaction, several global analytical potential energy surfaces (PESs) were developed utilizing the in-house ROBOSURFER program package.45,46 The final choice of the ab initio method was not evident: quasi-classical trajectory (QCT) simulations on the PESs unveiled that the gold-standard CCSD(T) method provides unphysically negative energies at some regions of the PES, and therefore a composite ab initio method was applied to obtain the energies of the structures.
In this work, we report a more detailed dynamical study of the OH− + CH3I reaction. At first, for the title reaction, we determine the benchmark geometries, frequencies and energies of the stationary points of the proton-abstraction pathway using the modern explicitly correlated CCSD(T)-F12b method in order to explore the energetics of the competing SN2 and proton-abstraction channels. Then, utilizing our previously constructed PES,45 in total more than half a million QCT computations are run at Ecoll of 11.5, 23.1, 34.6 and 46.1 kcal mol−1 providing the integral cross-sections (ICSs) of the possible reaction mechanisms, the scattering angle distributions and the translational and internal energy distributions of the SN2 and proton-abstraction products, as well. Last but not least, the QCT results are compared with the reanalysed crossed-beam imaging experiments for the OH− + CH3I reaction. The details of the theoretical and experimental methods are given in Section II. The results are described and discussed in Section III, and in Section IV, the summary of the work can be found.
II. Computational and experimental methods
For the ab initio characterization of the OH− + CH3I → H2O + CH2I− reaction, the same process is implemented as for the SN2 channel discussed in ref. 15: the stationary points are searched at the MP2/aug-cc-pVDZ level of theory,47 then, for the more accurate energies, structures and frequencies, the modern explicitly correlated coupled cluster singles, doubles and perturbative triples CCSD(T)-F12b method48 is employed with the correlation-consistent aug-cc-pVDZ and aug-cc-pVTZ basis sets.49 The benchmark classical energies are obtained utilizing the CCSD(T)-F12b method with the aug-cc-pVQZ basis set at the CCSD(T)-F12b/aug-cc-pVTZ geometries, and considering the post-CCSD(T) correlation effects50–52 and the core correlation corrections. Taking account of the zero-point energy (ZPE) of the corresponding stationary point, the adiabatic energy is determined. For I, a small-core relativistic effective core potential53 is applied with the aug-cc-pVnZ-PP (n = 2, 3, 4) and aug-cc-pwCVTZ-PP basis sets. Most of the ab initio calculations are performed using the MOLPRO program package,54 except for CCSDT and CCSDT(Q) where the MRCC program is used.55,56 A more detailed description of the ab initio computations is given in ref. 15.On our previously developed PES, QCT simulations are carried out for the reaction of OH− + CH3I at 11.5, 23.1, 34.6 and 46.1 kcal mol−1Ecoll. In ref. 45, the interested reader may find the details about the PES development. It should be noted that the energy points of the PES are computed at the CCSD-F12b/aug-cc-pVTZ + BCCD(T)/aug-cc-pVDZ − BCCD/aug-cc-pVDZ composite level of theory57 to resolve the failure of the CCSD(T)-F12b method. With regard to the conditions of the simulations, the vibrational ground states of the reactants (ν = 0) are prepared by normal-mode sampling,58,59 and the rotational angular momenta are set to zero. The initial orientations of the reactants are randomly sampled, and the initial distance of OH− and CH3I is 40 bohr with a given impact parameter (b). At each b, 5000 trajectories are propagated and b is scanned from 0 to bmax (where the probability of the reaction becomes 0) with a step size of 0.5 bohr. Each trajectory is run with a time step of 0.0726 fs until the largest interatomic distance becomes larger by 1 bohr than the largest initial one. The opacity function (P(b)) is defined by the reaction probability as a function of b. At each Ecoll, the ICS of each reaction channel is determined by a b-weighted numerical integration of the corresponding P(b):
 | (1) |
The experimental data on the OH− + CH3I reaction have been obtained using crossed-beam velocity map imaging, as described in earlier publications.14,36,37 In brief, the negative ions were produced in a pulsed plasma discharge, mass-selected by time-of-flight, and stored in an octupole ion trap at about 100 K to cool the translational and rotational motion. After extraction from the trap, the ions were crossed with a supersonic neutral jet of CH3I seeded in helium with relative collision energies selected between 11.5 and 46.1 kcal mol−1 (0.5 and 2.0 eV). Product ions were mapped by a velocity map imaging spectrometer onto a position- and time-sensitive detector and for each reactive event the detector information was transformed into a product mass and velocity vector in the centre-of-mass frame. From the three-dimensional velocity distribution, the angular and internal energy distributions are integrated after multiplication with an appropriate Jacobian. During re-evaluation of the data, we noticed that for the integration of the relative translational energy distributions shown in Fig. 6 of ref. 14, an incorrect Jacobian was used, which is corrected in the present work.
III. Results and discussion
The stationary points of SN2 and proton-abstraction channels of the OH− + CH3I reaction are shown in Fig. 1 with the corresponding benchmark classical relative energies and PES values. The detailed ab initio energies are summarized in Table 1 and the structures of the stationary points are given in Fig. S1 (ESI†). Similar to the back-side attack Walden-inversion (HMIN → HTS → PostHMIN), proton abstraction is also a submerged pathway, although SN2 is much more exothermic with a reaction energy of 66.64 kcal mol−1. In the entrance channel of SN2 the Y⋯OH−-bonded front-side complex is below HMIN and the global minimum of the reaction is in the product channel of SN2 (PostHMIN) with a classical energy of −78.78 kcal mol−1. In the case of proton abstraction, involving the same H-bonded complex (HMIN) in the reactant channel as for SN2, four minima and three transition states can be found: HMIN → TS1′/TS1′′ → MIN1′/MIN1′′ → TS2 → MIN2. As it can be seen in Fig. 1 and Fig. S1 (ESI†), TS1′ and TS1′′, as well as, MIN1′ and MIN1′′ are conformational isomers, due to the torsional motion of OH−: For TS1′ and MIN1′, the torsion angle of I–C⋯O–H− is 180° (trans arrangement), while for TS1′′ and MIN1′′ the value is 0° (cis arrangement). The energies of the isomers are similar, TS1′ is below TS1′′ by 0.98 kcal mol−1 and MIN1′ is above MIN1′′ by 1.32 kcal mol−1, without ZPE corrections (Fig. 1). It should be emphasised that, considering ZPE effects, an energy of −17.45 and −18.74 kcal mol−1 can be observed for TS1′ and TS1′′, respectively, and since the benchmark adiabatic energy is −18.07 kcal mol−1 for HMIN, the barrier of TS1′′ vanishes (Table 1). For the stationary points of proton abstraction, the post-CCSD(T) effects are small, whereas, for SN2, more significant post-CCSD(T) effect can be determined, especially for FSTS (−0.90 kcal mol−1). Generally, the post-CCSD(T) and the core correlation effects cancel each other, however, in some cases, accumulation of the effects still appears with the highest values of 1.06 and 0.60 kcal mol−1 for the SN2 and proton-abstraction products, in order. The ZPE effects are usually between ±0.7 kcal mol−1, except for PostHMIN, TS1′′, I− + CH3OH and CH2I− + H2O, where much higher effects occur: 4.49, −1.93, 3.98 and −1.33 kcal mol−1, respectively. The DFT energies of the stationary points provided by Xie et al.38 are in a qualitative agreement with our benchmark results, although the energy differences for several cases are larger than 2 kcal mol−1, especially for TS1′′, showing a deviation of −2.96 kcal mol−1. This latter energy discrepancy shows that by employing DFT method, TS1′′ happens to be below TS1′, and the barrier heights of TS1′′ and TS1′ remarkably diminish (0.30 and 0.44 kcal mol−1) compared to the benchmark classical values (1.68 and 0.70 kcal mol−1, in order). As seen in Fig. 1, for the SN2 pathway, the PES reproduces the benchmark energies of the stationary points within the chemical accuracy (±1 kcal mol−1), while in the case of proton abstraction, PES provides slightly lower relative energies for MIN1′, MIN1′′, TS2, MIN2 and CH2I− + H2O than the corresponding benchmark values, with differences of 1.16, 1.17, 1.53, 1.39 and 1.62 kcal mol−1, respectively. | ||
| Fig. 1 The schematic potential energy surfaces of the SN2 and proton-abstraction pathways for the OH− + CH3I reaction showing the classical benchmark relative energies (kcal mol−1) of the stationary points with the corresponding PES values, relative to OH−(eq.) + CH3I(eq.). In the case of the SN2 channel, the benchmark energies and PES values are derived from ref. 15 and 45, respectively. | ||
| SN2 | DZa | TZb | QZc | δTd | δ(Q)e | Δcoref | Classicalg | ΔZPEh | Adiabatici |
|---|---|---|---|---|---|---|---|---|---|
| a CCSD(T)-F12b/aug-cc-pVDZ. b CCSD(T)-F12b/aug-cc-pVTZ. c CCSD(T)-F12b/aug-cc-pVQZ. d CCSDT/aug-cc-pVDZ – CCSD(T)/aug-cc-pVDZ. e CCSDT(Q)/aug-cc-pVDZ – CCSDT/aug-cc-pVDZ. f AE-CCSD(T)/aug-cc-pwCVTZ – FC-CCSD(T)/aug-cc-pwCVTZ. g QZ + δT + δ(Q) + Δcore. h ΔZPE(CCSD(T)-F12b/aug-cc-pVTZ). i QZ + δT + δ(Q) + Δcore + ΔZPE. | |||||||||
| HMIN | −18.89 | −18.64 | −18.48 | −0.02 | 0.00 | 0.02 | −18.49 | 0.42 | −18.07 |
| HTS | −16.74 | −16.69 | −16.57 | −0.04 | −0.04 | 0.01 | −16.65 | 0.50 | −16.15 |
| PostHMIN | −79.30 | −79.08 | −79.43 | 0.09 | 0.27 | 0.29 | −78.78 | 4.49 | −74.29 |
| FSMIN | −24.09 | −24.10 | −24.05 | 0.07 | −0.23 | 0.51 | −23.69 | 0.68 | −23.01 |
| FSTS | 17.19 | 17.03 | 17.10 | −0.11 | −0.79 | 0.42 | 16.62 | 0.58 | 17.20 |
| DITS | −4.20 | −4.13 | −4.05 | 0.07 | −0.01 | 0.15 | −3.85 | 0.18 | −3.67 |
| I− + CH3OH | −67.16 | −67.08 | −67.71 | 0.08 | 0.31 | 0.67 | −66.64 | 3.98 | −62.67 |
| ABS | DZa | TZb | QZc | δTd | δ(Q)e | Δcoref | Classicalg | ΔZPEh | Adiabatici |
|---|---|---|---|---|---|---|---|---|---|
| TS1′ | −18.19 | −17.92 | −17.76 | −0.04 | −0.02 | 0.02 | −17.79 | 0.33 | −17.45 |
| TS1′′ | −17.38 | −17.11 | −16.93 | 0.01 | −0.02 | 0.14 | −16.81 | −1.93 | −18.74 |
| MIN1′ | −19.60 | −19.49 | −19.36 | −0.02 | 0.04 | 0.32 | −19.03 | 0.33 | −18.70 |
| MIN1′′ | −20.89 | −20.80 | −20.69 | −0.03 | 0.04 | 0.32 | −20.35 | 0.53 | −19.82 |
| TS2 | −10.65 | −10.60 | −10.57 | −0.06 | 0.06 | 0.41 | −10.15 | −0.08 | −10.23 |
| MIN2 | −13.14 | −13.13 | −13.07 | −0.06 | 0.05 | 0.35 | −12.73 | 0.56 | −12.16 |
| CH2I− + H2O | −1.77 | −1.96 | −2.08 | −0.07 | 0.11 | 0.56 | −1.49 | −1.33 | −2.82 |
In order to characterize the dynamics of the title reaction, QCT computations are carried out at several Ecoll (11.5, 23.1, 34.6 and 46.1 kcal mol−1) on our earlier constructed analytical PES45 (see Computational and experimental methods). The ICSs of the possible reaction channels at each Ecoll are presented in Fig. 2 and in Table S1 (ESI†). As it is expected for barrierless reactions, with increasing Ecoll, the ICSs of proton abstraction and SN2 are decreasing. The largest ICSs are obtained for proton abstraction at each Ecoll, peaking at a value of 169.8 bohr2 at Ecoll = 11.5 kcal mol−1, and even with soft ZPE restriction, the ICSs of proton abstraction are larger than the corresponding values of the SN2 channel. Using hard ZPE restriction for proton abstraction, the reaction probability suffers a significant decrease, as its ICSs are 23.9 and 14.8 bohr2 at Ecoll = 11.5 and 23.1 kcal mol−1, respectively, while in the case of soft ZPE restriction, ICSs of 100.0 and 40.1 bohr2 are determined. For SN2, no ZPE violation can be obtained. The opacity functions of SN2 and proton abstraction are shown in Fig. S2 (ESI†). Since the four hydrogen atoms can be distinguished during the course of a simulation, two additional pathways can be identified within SN2: besides inversion, retention of the initial CH3I configuration can also take place (SN2 with retention), on the other hand, a proton exchange is feasible between the OH− and CH3I reactants followed by the traditional Walden inversion (SN2 with proton exchange). Within proton abstraction, two other paths can be separated as well: following proton abstraction, a dissociation of the C–I bond may occur (proton abstraction with dissociation), and analogously to SN2 with proton exchange, proton abstraction with proton exchange can proceed. Likewise for the NH2− + CH3I reaction,60 the formation of the products of iodine abstraction ([I⋯OH]− + CH3) is viable, although with a significantly lower probability, and in some cases, as for OH− + CH3F,26 proton exchange between OH− and CH3I may eventuate resulting products apparently similar to reactants: OH− + CH2H′I → HOH′ + CH2I− → OH′− + CH2HI. Regarding the channels involving proton exchange, at lower Ecoll, their ICSs are in the range of 0.3–1.2 bohr2, whereas, at higher Ecoll, these paths are almost negligible. Fig. 3 shows that proton abstraction with dissociation can be separated into four different channels with the largest probability of the CH2 + I− + H2O product formation.
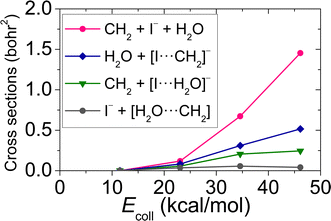 | ||
| Fig. 3 Integral cross-sections for the different types of the proton abstraction with dissociation pathway of the OH− + CH3I reaction as a function of collision energy. | ||
Performing crossed-beam ion imaging experiments for the OH− + CH3I reaction, the following product ions are detected: I−, CH2I− and [I⋯H2O]−/[I⋯OH]−. The latter product ions cannot be resolved individually, because the masses of the ions are almost equivalent (145 u/144 u), thus the time-of-flight peaks of [I⋯H2O]− and [I⋯OH]− overlap. The experimental and theoretical branching ratios of these ions are shown in Table 2 at Ecoll = 11.5, 23.1 34.6 and 46.1 kcal mol−1. In QCT simulations, I− is provided by three different reaction channels: SN2 (I− + CH3OH) and two pathway-types of proton abstraction with dissociation (CH2 + I− + H2O and I− + [CH2⋯H2O]). CH2I− is formed as a result of proton abstraction, and note that, for the branching ratios, the soft ZPE-restricted proton abstraction is taken into account. The product of [I⋯H2O]− is generated by one of the pathways of proton abstraction with dissociation, while [I⋯OH]− occurs as a result of iodine abstraction, as seen in Fig. 2 and 3. At 11.5 kcal mol−1Ecoll, experiment assesses a slightly higher ratio for I− than theory with a difference of ∼1.4%, while at Ecoll = 23.1 kcal mol−1, a reversed situation appears, the formation of the I− is overestimated by theory emerging a ∼4.3% deviation. As a consequence, at low Ecoll, the computed and measured ratios of the CH2I− product ion also differ from each other. Nevertheless, the agreement between theory and experiment is still impressive, almost quantitative, at these low collision energies. Furthermore, at higher Ecoll, an excellent, clearly quantitative, agreement can be identified between the experimental and theoretical branching ratios of I− and CH2I−: the differences of the corresponding ratios are below ∼0.4%. At each Ecoll, experiment reveals a more significant prevalence of the [I⋯H2O]−/[I⋯OH]− product ions, for instance at 46.1 kcal mol−1Ecoll, a value of 2.58% is measured, whilst theory provides only 0.91%. It should be highlighted that, in the case of proton abstraction with dissociation, QCT simulations unveil another product ion, [I⋯CH2]− (see Fig. 3), which may be detected by the crossed-beam technique as I− if the complex dissociates during the time scale of the experiment or as CH2I− if the complex remains stable. Therefore, we do not consider the small fraction of [I⋯CH2]− (0%, 0.1%, 0.8% and 1.8% at Ecoll = 11.5, 23.1 34.6 and 46.1 kcal mol−1, respectively) at the branching ratio computations. Nonetheless, the direct dynamics simulations performed by Xie et al. gave branching ratios of 31%, 38% and 36%, for I−, and 69%, 62% and 63% for CH2I− at Ecoll = 11.5, 34.6 and 46.1 kcal mol−1.14
| E coll (kcal mol−1) | I− | CH2I− | [I⋯H2O]−/[I⋯OH]− | |||
|---|---|---|---|---|---|---|
| Exp. | Sim.b | Exp. | Sim.c | Exp.d | Sim.e | |
| a 100% = ICSSN2 + ICSABS soft + ICSABS dissociation + ICSIodine ABS + ICSProton exch. b I− originates from SN2, and two channels of the proton abstraction with dissociation leading to CH2 + I− + H2O or I− + [CH2⋯H2O]. c CH2I− originates from the soft-restricted proton abstraction. d The [I⋯H2O]− and [I⋯OH]− ions cannot be distinguished from each other experimentally. e The sum of the branching ratios of the [I⋯H2O]− and [I⋯OH]− product ions. [I⋯H2O]− originates from one of the channels of proton abstraction leading to the CH2 + [I⋯H2O]− products, while [I⋯OH]− is generated in iodine abstraction. | ||||||
| 11.5 | 45.38 | 44.01 | 53.79 | 55.76 | 0.84 | 0.03 |
| 23.1 | 32.54 | 36.83 | 66.66 | 62.81 | 0.80 | 0.13 |
| 34.6 | 36.49 | 36.31 | 62.00 | 62.22 | 1.51 | 0.60 |
| 46.1 | 40.49 | 40.75 | 56.93 | 56.57 | 2.58 | 0.91 |
For the products of OH− + CH3I SN2 and the proton-abstraction reaction, the experimental and theoretical internal energy as well as scattering angle distributions are compared at several Ecoll in Fig. 4 and 5. As we can distinguish the products of proton abstraction in each simulation, the internal energy contributions of CH2I− and H2O can be determined, which is presented in Fig. 6. The theoretical translational energy distributions of the SN2 and proton-abstraction products are shown in Fig. 7. In the case of the products of proton abstraction, with increasing Ecoll, translational energies are more affected, than internal energies and the dominance of the forward-scattering becomes more and more favoured. These tendencies support a prevalence of the direct stripping mechanism at each Ecoll for proton abstraction, and it should also be emphasised that experiment and theory are in accordance with each other. As it can be expected, the internal energy contributions of the CH2I− product ion are more substantial than that of H2O declaring that H2O is more responsible for the hard-restricted proton-abstraction trajectories than CH2I−. In contrast, the dynamics of SN2 has a different character: on the one hand, upon increasing Ecoll, the CH3OH internal energy is more impacted than translational energy showing an indirect feature. On the other hand, the scattering angle distributions of CH3OH display forward and/or backward preference besides the isotropic character signifying direct stripping and/or rebound pathways as well as complex-forming indirect dynamics. As seen in Fig. 4 and 5, the experimental and theoretical CH3OH internal energy distributions are in good agreement at Ecoll = 11.5 and 23.1 kcal mol−1, whilst theory underestimates internal energies at Ecoll = 34.6 and 46.1 kcal mol−1. Regarding the scattering angle distributions of the SN2 products, a pleasant agreement can be identified, as well, except at Ecoll of 23.1 kcal mol−1, where experiment shows the preference of a dual forward-backward scattering; however computations reveal an isotropic feature. Concerning the direct dynamics computations of Xie et al., for proton abstraction and SN2, as well, a forward-scattered distribution is reported at Ecoll = 11.5, 34.6 and 46.1 kcal mol−1.14
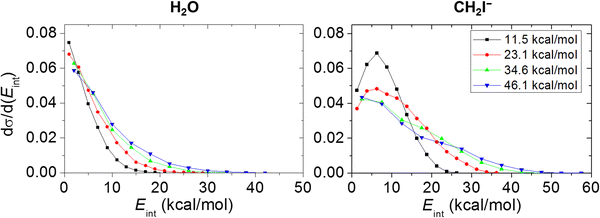 | ||
| Fig. 6 Theoretical ZPE-corrected internal energy distributions of the products of the proton-abstraction channel (H2O and CH2I−) of the OH− + CH3I reaction at different collision energies. | ||
IV. Summary and conclusions
In this paper, the dynamics of the OH− + CH3I reaction has been investigated performing QCT simulations at Ecoll of 11.5, 23.1, 34.6 and 46.1 kcal mol−1 on our high-level ab initio PES.45 Utilizing the explicitly correlated CCSD(T)-F12b method, the stationary points of the proton-abstraction channel have been explored and presented with the previously characterized SN2 channel.15 The theoretical analysis for the title reaction has been compared with the revised crossed-beam ion imaging experiments. Briefly, in the case of the branching ratios of the product ions, the internal energy distributions of the SN2 and proton-abstraction products, as well as the scattering angle distributions of the proton-abstraction products, a pleasant agreement has been found. For proton abstraction, a dominance of the direct stripping mechanism is unveiled, in contrast for SN2, a rather indirect character is found. The most notable difference between theory and experiment is observed for the scattering angle distributions of CH3OH at Ecoll = 23.1 kcal mol−1, where a mixed backward–forward character is measured, while simulations show an isotropic feature. The earlier reported direct dynamics study of Xie et al.14 could not describe the dynamics of the OH− + CH3I reaction as accurate as our present QCT simulations, underlining the importance of the accuracy of the PES to provide well comparable results with experiments.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors at the University of Szeged are thankful for the financial support from the National Research, Development and Innovation Office–NKFIH, Grant No. K-125317; the Ministry of Human Capacities, Hungary, Grant No. 20391-3/2018/FEKUSTRAT; Project No. TKP2021-NVA-19, provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP2021-NVA funding scheme; the National Young Talent Scholarship (Grant No. NTP-NFTÖ-22-B-0050 for D. A. T.); and the Momentum (Lendület) Program of the Hungarian Academy of Sciences. The work at the University of Innsbruck has been supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 885479).References
- S. S. Shaik, H. B. Schlegel and S. Wolfe, Theoretical Aspects of Physical Organic Chemistry: The SN2 Mechanism, Wiley, New York, 1992 Search PubMed.
- A. H. Maulitz, F. C. Lightstone, Y. J. Zheng and T. C. Bruice, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 6591 CrossRef CAS PubMed.
- J. K. Laerdahl and E. Uggerud, Int. J. Mass Spectrom., 2002, 214, 277 CrossRef CAS.
- G. Vayner, K. N. Houk, W. L. Jorgensen and J. I. Brauman, J. Am. Chem. Soc., 2004, 126, 9054 CrossRef CAS PubMed.
- J. M. Gonzales, W. D. Allen and H. F. Schaefer, J. Phys. Chem. A, 2005, 109, 10613 CrossRef CAS PubMed.
- T. A. Hamlin, M. Swart and F. M. Bickelhaupt, ChemPhysChem, 2018, 19, 1315 CrossRef CAS PubMed.
- R. Wester, Mass Spectrom. Rev., 2022, 41, 627 CrossRef PubMed.
- T. Hansen, P. Vermeeren, L. De Jong, F. M. Bickelhaupt and T. A. Hamlin, J. Org. Chem., 2022, 87, 8892 CrossRef CAS PubMed.
- X. Lu, C. Shang, L. Li, R. Chen, B. Fu, X. Xu and D. H. Zhang, Nat. Commun., 2022, 13, 4427 CrossRef CAS PubMed.
- J. D. Evanseck, J. F. Blake and W. L. Jorgensen, J. Am. Chem. Soc., 1987, 109, 2349 CrossRef CAS.
- H. Tachikawa, M. Igarashi and T. Ishibashi, J. Phys. Chem. A, 2002, 106, 10977 CrossRef CAS.
- L. Sun, K. Song and W. L. Hase, Science, 2002, 296, 875 CrossRef CAS PubMed.
- L. Sun, K. Song, W. L. Hase, M. Sena and J. M. Riveros, Int. J. Mass Spectrom., 2003, 227, 315 CrossRef CAS.
- J. Xie, R. Sun, M. R. Siebert, R. Otto, R. Wester and W. L. Hase, J. Phys. Chem. A, 2013, 117, 7162 CrossRef CAS PubMed.
- D. A. Tasi, Z. Fábián and G. Czakó, J. Phys. Chem. A, 2018, 122, 5773 CrossRef CAS PubMed.
- H. B. Schlegel, K. Mislow, F. Bernardi and A. Bottoni, Theor. Chim. Acta, 1977, 44, 245 CrossRef CAS.
- I. Szabó and G. Czakó, Nat. Commun., 2015, 6, 5972 CrossRef PubMed.
- J. M. Gonzales, R. S. Cox, S. T. Brown, W. D. Allen and H. F. Schaefer, J. Phys. Chem. A, 2001, 105, 11327 CrossRef CAS.
- J. M. Gonzales, C. Pak, R. Sidney Cox, W. D. Allen, H. F. Schaefer, A. G. Császár and G. Tarczay, Chem. – Eur. J., 2003, 9, 2173 CrossRef CAS PubMed.
- T. Tsutsumi, Y. Ono, Z. Arai and T. Taketsugu, J. Chem. Theory Comput., 2018, 14, 4263 CrossRef CAS PubMed.
- T. Tsutsumi, Y. Ono, Z. Arai and T. Taketsugu, J. Chem. Theory Comput., 2020, 16, 4029 CrossRef CAS PubMed.
- T. Tsutsumi, Y. Ono and T. Taketsugu, Chem. Commun., 2021, 57, 11734 RSC.
- T. Tsutsumi, Y. Ono and T. Taketsugu, Top. Curr. Chem., 2022, 380, 19 CrossRef CAS PubMed.
- S. R. Hare, L. A. Bratholm, D. R. Glowacki and B. K. Carpenter, Chem. Sci., 2019, 10, 9954 RSC.
- Y. G. Proenza, M. A. F. de Souza and R. L. Longo, Chem. – Eur. J., 2016, 22, 16220 CrossRef CAS PubMed.
- D. A. Tasi and G. Czakó, Chem. Sci., 2021, 12, 14369 RSC.
- J. Qin, Y. Liu and J. Li, J. Chem. Phys., 2022, 157, 124301 CrossRef CAS PubMed.
- X. Ji and J. Xie, Phys. Chem. Chem. Phys., 2022, 24, 7539 RSC.
- A. C. B. De Souza and G. F. Bauerfeldt, J. Braz. Chem. Soc., 2014, 25, 340 CAS.
- J. Z. A. Laloo, L. Rhyman, O. Larrañaga, P. Ramasami, F. M. Bickelhaupt and A. de Cózar, Chem. – Asian J., 2018, 13, 1138 CrossRef CAS PubMed.
- S. Zhao, G. Fu, W. Zhen, L. Yang, J. Sun and J. Zhang, Phys. Chem. Chem. Phys., 2022, 24, 24146 RSC.
- H. Tachikawa and M. Igarashi, Chem. Phys., 2006, 324, 639 CrossRef CAS.
- H. Yin, D. Wang and M. Valiev, J. Phys. Chem. A, 2011, 115, 12047 CrossRef CAS PubMed.
- Y. Xu, T. Wang and D. Wang, J. Chem. Phys., 2012, 137, 184501 CrossRef PubMed.
- J. Chen, Y. Xu and D. Wang, J. Comput. Chem., 2014, 35, 445 CrossRef CAS PubMed.
- R. Otto, J. Xie, J. Brox, S. Trippel, M. Stei, T. Best, M. R. Siebert, W. L. Hase and R. Wester, Faraday Discuss., 2012, 157, 41 RSC.
- R. Otto, J. Brox, S. Trippel, M. Stei, T. Best and R. Wester, Nat. Chem., 2012, 4, 534 CrossRef CAS PubMed.
- J. Xie, S. C. Kohale, W. L. Hase, S. G. Ard, J. J. Melko, N. S. Shuman and A. A. Viggiano, J. Phys. Chem. A, 2013, 117, 14019 CrossRef CAS PubMed.
- J. Xie, R. Otto, R. Wester and W. L. Hase, J. Chem. Phys., 2015, 142, 244308 CrossRef PubMed.
- J. Xie, J. Zhang and W. L. Hase, Int. J. Mass Spectrom., 2015, 378, 14 CrossRef CAS.
- J. Xie, X. Ma, J. Zhang, P. M. Hierl, A. A. Viggiano and W. L. Hase, Int. J. Mass Spectrom., 2017, 418, 122 CrossRef CAS.
- J. Xie, J. Zhang, R. Sun, R. Wester and W. L. Hase, Int. J. Mass Spectrom., 2019, 438, 115 CrossRef CAS.
- X. Ji, C. Zhao and J. Xie, Phys. Chem. Chem. Phys., 2021, 23, 6349 RSC.
- J. Xie and W. L. Hase, Science, 2016, 352, 32 CrossRef CAS PubMed.
- D. A. Tasi, T. Győri and G. Czakó, Phys. Chem. Chem. Phys., 2020, 22, 3775 RSC.
- T. Győri and G. Czakó, J. Chem. Theory Comput., 2020, 16, 51 CrossRef PubMed.
- C. Møller and M. S. Plesset, Phys. Rev., 1934, 46, 618 CrossRef.
- T. B. Adler, G. Knizia and H. J. Werner, J. Chem. Phys., 2007, 127, 221106 CrossRef PubMed.
- T. H. Dunning, J. Chem. Phys., 1989, 90, 1007 CrossRef CAS.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, Chem. Phys. Lett., 1989, 157, 479 CrossRef CAS.
- J. Noga and R. J. Bartlett, J. Chem. Phys., 1986, 86, 7041 CrossRef.
- M. Kállay and J. Gauss, J. Chem. Phys., 2005, 123, 214105 CrossRef PubMed.
- K. A. Peterson, D. Figgen, E. Goll, H. Stoll and M. Dolg, J. Chem. Phys., 2003, 119, 11113 CrossRef CAS.
- H.-J. Werner, P. J. Knowles, G. Knizia, F. R. Manby and M. Schütz, et al., Molpro, version 2015.1, a package of ab initio programs, see http://www.molpro.net Search PubMed.
- M. Kállay, P. R. Nagy, D. Mester, Z. Rolik, G. Samu, J. Csontos, J. Csóka, P. B. Szabó, L. Gyevi-Nagy, B. Hégely, I. Ladjánszki, L. Szegedy, B. Ladóczki, K. Petrov, M. Farkas, P. D. Mezei and Á. Ganyecz, J. Chem. Phys., 2020, 152, 074107 CrossRef PubMed.
- MRCC, a quantum chemical program suite written by M. Kállay, P. R. Nagy, D. Mester, Z. Rolik, G. Samu, J. Csontos, J. Csóka, P. B. Szabó, L. Gyevi-Nagy, B. Hégely, I. Ladjánszki, L. Szegedy, B. Ladóczki, K. Petrov, M. Farkas, P. D. Mezei and Á. Ganyecz. See http://www.mrcc.hu.
- K. A. Brueckner, Phys. Rev., 1954, 96, 508 CrossRef CAS.
- W. L. Hase, Encyclopedia of Computational Chemistry, Wiley, New York, 1998, pp. 399–407 Search PubMed.
- S. Pratihar, X. Ma, Z. Homayoon, G. L. Barnes and W. L. Hase, J. Am. Chem. Soc., 2017, 139, 3570 CrossRef CAS PubMed.
- D. A. Tasi and G. Czakó, J. Chem. Phys., 2022, 156, 184306 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Integral cross sections (bohr2) of the possible pathways, structures of the stationary points for the SN2 and proton-abstraction pathways with the most important bond lengths (Å) and angles (deg) obtained on the PES compared to the CCSD(T)-F12b/aug-cc-pVTZ values, opacity functions of the SN2 and proton-abstraction pathways, Cartesian coordinates (Å) and energies (Eh) of the stationary points for the proton-abstraction channel obtained at the CCSD(T)-F12b/aug-cc-pVTZ level of theory. See DOI: https://doi.org/10.1039/d2cp05553h |
| This journal is © the Owner Societies 2023 |




